Abstract
OBJECTIVES
To examine the characteristics of hospice enrollees with dementia who were discharged alive because their condition stabilized or improved and predictors of death in the year after discharge.
DESIGN
Cross-sectional analysis of clinical and administrative data.
SETTING
For-profit hospice provider.
PARTICIPANTS
Hospice enrollees aged 65 and older with an admission diagnosis of dementia who died or were discharged alive because their condition stabilized or improved between January 1, 1999, and December 31, 2003.
MEASUREMENTS
Demographic variables and hospice length of stay; data did not include functional status or comorbidities.
RESULTS
Of 24,111 enrollees with dementia, 1,204 (5.0%) were discharged alive because their condition stabilized or improved; the remainder died while receiving hospice. The median length of stay for those who died was 12 versus 236 days for those discharged alive. Those discharged alive were more likely to be female or have a length of stay exceeding 180 days and less likely to be in the oldest age group (≥85), be African American, or reside in a nursing home. In a subgroup of 303 patients discharged alive, 75.5% were still alive at 1 year; none of the demographic variables were associated with death after hospice discharge.
CONCLUSION
A small proportion of hospice enrollees with dementia was discharged alive. Most died shortly after enrollment. Future research should examine other factors that may predict which hospice enrollees with dementia are likely to be discharged alive and their subsequent trajectory, such as functional status, comorbidities, and preferences for care.
Keywords: hospice, dementia, end-of-life care
Dyspnea, pain, agitation, and burdensome interventions are common in the last months of life in individuals with dementia.1–3 Hospice is associated with improvements in end-of-life care for these individuals, including better symptom management, fewer hospitalizations, and greater caregiver satisfaction.4–10 Although hospice enrollment in individuals with dementia is increasing, these individuals are still referred to hospice at lower rates than those with some other life-limiting illnesses.11–14 For example, in a study of Medicare beneficiaries, 41% of those who died of dementia used hospice, compared with 65% of those who died of cancer, which is the single most common admission diagnosis of hospice enrollees.11,14
Hospice providers commonly cite difficulty with prognostication due to variability in disease progression as a barrier to hospice referral for individuals with dementia.15–17 National Hospice and Palliative Care Organization (NHPCO) Guidelines for determining prognosis in dementia are based on the Functional Assessment Staging (FAST), a seven-step staging system that identifies progressive cognitive and functional decline. These guidelines suggest that an appropriate cutoff for enrolling persons with dementia in hospice is stage 7C (completely dependent in all activities of daily living, nonambulatory, limited or no speech) along with the presence of one or more dementia-related comorbidities (e.g., aspiration pneumonia, urinary tract infection, impaired nutritional status).18,19 A number of studies suggest that these criteria do not accurately predict 6-month mortality, which is a requirement for certification under the Medicare Hospice Benefit.19–23 These criteria also do not include other factors associated with poorer survival in individuals with dementia, including older age, male sex, and comorbidities such as diabetes mellitus and heart disease.19,23,24
In the absence of accurate tools for prognostication, not surprisingly, individuals with dementia enrolled in hospice have longer lengths of stay than individuals with cancer, who tend to have a more-predictable trajectory of decline in the last months of life.25–27 In 2005, the median length of stay for Medicare beneficiaries with dementia who enrolled in hospice was 27 versus 20 days for those with cancer, and one-quarter of those with dementia had lengths of stay exceeding 180 days, compared with fewer than 10% of individuals with cancer.28 In addition to longer lengths of stay, hospice enrollees with dementia are also more likely than those with cancer to be discharged from hospice alive because their condition stabilizes or improves and they no longer meet eligibility criteria.29,30 In 2008, Medicare beneficiaries with dementia or other neurological conditions who were discharged alive made up 18% to 41% of all hospice discharges, whereas those with cancer who were discharged alive made up only 10% to 24% of hospice discharges.13
Although longer lengths of stay, female sex, better functional status, and having a noncancer diagnosis have been associated with being discharged alive from hospice,30 little is known about which individuals with dementia are likely to be discharged because they stabilize and no longer meet prognostic eligibility criteria or about what happens to them after they are discharged. This information would be valuable in the current regulatory environment with greater scrutiny of hospice providers to identify fraud and misuse of the Medicare Hospice Benefit related to enrollment of individuals who have prognoses exceeding 6 months.13,31,32 Given the longer lengths of stay of enrollees with dementia than for those with other diagnoses, utilization review and fraud investigators may tend to focus on the charts of these individuals and on hospice providers whose enrollees include a significant proportion diagnosed with dementia. Because of concerns about allegations of fraud and difficulties with accurate prognostication, hospice providers may be cautious about admitting or retaining individuals with dementia.31,32
Using data from a large national hospice provider, the purpose of this study was to compare the characteristics of individuals with dementia who died while receiving hospice with the characteristics of those who were discharged alive because their condition stabilized or improved and to identify factors associated with death in the year after discharge from hospice in individuals who were discharged alive. Understanding which individuals with dementia are likely to be discharged alive from hospice and, of those, which are likely to die in the year after discharge may improve prognostication in hospice enrollees with dementia and inform the development of other services that may contribute to quality of life for those who are no longer eligible for hospice care.
METHODS
Data Source
Data were obtained from VITAS, a large, national, for-profit hospice provider. After obtaining approval from the hospice provider and the Duke University Health System institutional review board, patient-level files were used to create the study database. The data were abstracted from the central administrative and clinical database of the hospice provider.
Study Sample
The sample included hospice enrollees aged 65 and older with a primary admission diagnosis of dementia who died while receiving hospice or were discharged alive because their condition stabilized or improved between January 1, 1999, and December 31, 2003. Enrollees received hospice care from VITAS programs in eight states—California, Florida, Illinois, New Jersey, Ohio, Pennsylvania, Texas, and Wisconsin. International Classification of Diseases, Ninth Revision codes 290.40—290.43 (vascular dementia); 290.0, 290.2, 290.21, and 290.3 (senile dementia); 331.7, 331.9 (cerebral degeneration); 294.1 (dementia in conditions classified elsewhere); 290.10 to 290.13 (presenile dementia); 331.0 (Alzheimer’s disease); and 331.1, 331.11 (frontotemporal dementia) were used to identify those with an admission diagnosis of dementia. During the study period, 25,445 older adults (≥65) with a primary diagnosis of dementia were discharged from VITAS programs included in the analysis, representing 13% of all discharges. Enrollees (n = 1,334) who revoked hospice, moved, or transferred to a different hospice or facility were excluded. This study included the remaining 24,111 enrollees (95% of the total).
Outcome
The outcome for the primary analysis was hospice discharge disposition, which included two groups: those who died while receiving hospice care and those who were discharged alive because their condition stabilized or improved and therefore were believed no longer to meet eligibility criteria of a prognosis of 6 months or less. The discharge disposition was listed in the database of the hospice provider.
Covariates
Variables for the final model were chosen based on a review of the literature describing factors associated with prognosis in dementia (age, sex),19 predictors of live hospice discharge (length of stay, marital status, caregiver, location of care),30,33 and factors associated with variation in hospice enrollment that may lead to differences in the characteristics of individual admitted (race, income, payment source, enrollment in health maintenance organization, region).14,34,35 The final model included age (65–74, 75–84, ≥85), sex, race (white, African American, other); marital status (married or other), relationship of caregiver to enrollee (spouse, child, other), and median household income. Because income was not included in the data, median household income was generated by matching enrollees’ ZIP codes to 2000 U.S. Census Tract Data. This method has been used in other research, but there are potential limitations based on the degree of individual income heterogeneity within ZIP codes.36,37 The income measure was divided into three groups, each containing one-third of the income distribution of the sample (low, ≤$37,280; middle, $37,281–48,541; and high, ≥$48,542). The model also included variables related to use of hospice: admission level of care (routine home care in a private residence, routine home care in a nursing home, inpatient hospice care, or continuous care), payment source (Medicare or other), health maintenance organization enrollment (yes or no); length of stay (<180 or ≥180 days to reflect prognostic eligibility criteria of the Medicare Hospice Benefit), and location of hospice program by region (South, West, Midwest, Northeast).
Analyses
In the bivariate analyses, chi-square tests were used for categorical variables and nonparametric Wilcoxon tests for continuous variables to compare enrollees with dementia who were discharged alive with those who died while receiving hospice. A nonparametric median test was used to examine differences in median length of stay between the two groups.
In the multivariate analysis, logistic regression was used to identify predictors of discharge disposition (alive because of stabilization or improvement vs dead). As noted above, all variables believed to be possible predictors of hospice discharge disposition based on a review of the literature were included in the model. The final model included only main effects. The c-index was used to test the discrimination of the model.
Subgroup Analysis
Survival was examined at 1 year in a subgroup of enrollees with dementia discharged alive because their condition stabilized or improved. Based on the available data, this analysis included hospice enrollees discharged from three Florida VITAS hospice programs between January 1, 1999, and December 31, 2001. Death was determined using a search of the Florida death registry for 1999 through 2002 using enrollees last name and date of birth.
A Cox proportional hazards model was used to examine predictors of death in the year after discharge from hospice. As in the primary analysis, the final model included variables chosen after a review of the literature based on their potential relevance as predictors of death in individuals with advanced dementia: age (<85 vs ≥85), sex, race (white or other), and admission level of care (routine home care in private residence, routine home care in nursing home, or inpatient/continuous care), and their association with risk of mortality in general (median household income—lower third of income distribution of sample—<$37,380 vs other).19,24,33,35,38 To avoid overfitting the model, the number of predictors and categories of each predictor was limited. All analyses were performed using SAS statistical software, version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Between 1999 and 2003, 24,111 older adults (≥65) with a primary hospice admission diagnosis of dementia were discharged alive because their condition stabilized or improved or died while receiving hospice from the VITAS programs included in the analysis. The most common cause of dementia was Alzheimer’s disease, which was the diagnosis for 37.6% of the sample.
Sample characteristics are listed in Table 1. Of the 24,111 enrollees included in these analyses, 1,204 (5%) were discharged alive because their condition stabilized or improved. Those who were discharged alive were slightly younger (mean age 84.2 vs 86.1, P < .001), more likely to be female (79% vs 70.7%), more likely to have an admission level of care of routine home care in a private residence (35.1% vs 19.5%), and more likely to have a length of stay exceeding 180 days (63.4% vs 10.2%). Those who died were more likely to have a length of stay of 1 week or less (37.4% vs 0.7%). The median length of stay of enrollees who were discharged alive was 236 versus 12 days for those who died (P < .001).
Table 1.
Sample Characteristics According to Discharge Disposition of Hospice Enrollees with Dementia.
| Characteristic | Discharged Alive n = 1,204 (5.0%)a | Died While Receiving Hospice, n = 22,907 (95.0%) | P-Value |
|---|---|---|---|
| n (%) | |||
| Age | <.001 | ||
| 65–74 | 119 (9.9) | 1,447 (6.3) | |
| 75–84 | 478 (39.7) | 7,380 (32.3) | |
| ≥85 | 607 (50.4) | 14,032 (61.4) | |
| Race | .05 | ||
| White | 999 (83.0) | 18,491 (80.7) | |
| Black | 75 (6.2) | 1,861 (8.1) | |
| Other | 130 (10.8) | 2,555 (11.2) | |
| Female | 951 (79.0) | 16,205 (70.7) | <.001 |
| Married | 314 (26.1) | 5,807 (25.4) | .57 |
| Income, $ | .09 | ||
| Low (≤ 337,280) | 344 (28.8) | 6,921 (30.4) | |
| Middle (37,281–48,541) | 400 (33.5) | 7,963 (35.0) | |
| High (≥348,542) | 449 (37.6) | 7,861 (34.6) | |
| Payment source | <.001 | ||
| Medicare | 1,113 (92.4) | 22,389 (97.7) | |
| Other | 91 (7.6) | 518 (2.3) | |
| Health maintenance organization enrollment | 320 (26.58) | 5,710 (24.93) | .20 |
| Caregiver | .48 | ||
| Spouse | 258 (21.4) | 4,757 (20.8) | |
| Child | 710 (59.0) | 13,334 (58.2) | |
| Other | 236 (19.6) | 4,816 (21.0) | |
| Admission level of care | <.001 | ||
| Routine care in private residence | 422 (35.1) | 4,467 (19.5) | |
| Routine care in nursing home | 687 (57.1) | 11,384 (49.7) | |
| Inpatient care | 80 (6.7) | 5,808 (25.4) | |
| Continuous care | 14 (1.2) | 1,244 (5.4) | |
| Length of stay, days | |||
| ≤7 | 8 (0.7) | 8,575 (37.4) | <.001 |
| >180 | 763 (63.4) | 2,335 (10.2) | <.001 |
| Region | .01 | ||
| Midwest | 266 (18.8) | 4,880 (21.3) | |
| Northeast | 42 (3.5) | 605 (2.6) | |
| South | 739 (61.4) | 13,253 (57.9) | |
| West | 197 (16.4) | 4,169 (18.2) | |
Enrollees were discharged alive because their condition stabilized or improved.
The results of the multivariate analysis are shown in Table 2. After controlling for demographic and hospice use variables, those discharged alive because of stabilization or improvement were less likely than those who died to be aged 85 and older than aged 65 to 74 (OR = 0.54, 95% confidence interval (CI) = 0.43–0.69), less likely to be African American than white (OR = 0.67, 95% CI = 0.51–0.87), and less likely to have an admission level of care of routine home care in a nursing home (OR = 0.67, 95% CI = 0.58–0.78), inpatient care (OR = 0.29, 95% CI = 0.23–0.38), or continuous care (OR = 0.24, 95% CI = 0.14–0.41) than routine home care in a private residence. Enrollees discharged alive were more likely to be female (OR = 1.22, 95% CI = 1.03–1.44) or use a payment source other than Medicare (OR = 4.37, 95% CI = 3.30–5.76). The single greatest predictor of a discharge status of alive because of stabilization or improvement was length of stay. Those enrolled for longer than 180 days had 12 times higher odds of being discharged alive (OR = 12.59, 95% CI = 11.03–14.37).
Table 2.
Multivariate Analysis of Hospice Discharge Disposition of Enrollees with a Primary Diagnosis of Dementia (Alive Because of Stabilization or Improvement vs Died While Receiving Hospice)
| Variable | Adjusted Odds Ratio (95% Confidence Interval) |
|---|---|
| Age (reference 65–74) | |
| 75–84 | 0.81 (0.64–1.03) |
| ≥85 | 0.54 (0.43–0.69) |
| Race (reference white) | |
| African American | 0.67 (0.51–0.87) |
| Other | 0.80 (0.64–1.00) |
| Female | 1.22 (1.03–1.44) |
| Not married | 1.06 (0.81–1.40) |
| Income (reference low, ≤$37,280) | |
| Middle ($37,281–48,541) | 1.04 (0.88–1.23) |
| High (≥$48,542) | 1.16 (0.98–1.37) |
| Payment source other than Medicare | 4.37 (3.30–5.76) |
| Health maintenance organization | 1.15 (0.98–1.34) |
| Caregiver (reference spouse) | |
| Child | 0.93 (0.69–1.24) |
| Other | 0.93 (0.68–1.29) |
| Admission level of care (reference routine home care in a private residence) | |
| Routine home care in a nursing home | 0.67 (0.58–0.78) |
| Inpatient care | 0.29 (0.23–0.38) |
| Continuous care | 0.24 (0.14–0.41) |
| Length of stay > 180 days | 12.59 (11.03–14.37) |
| Region (reference South) | |
| Midwest | 0.77 (0.64–0.92) |
| Northeast | 1.50 (1.05–2.15) |
| West | 0.70 (0.58–0.84) |
C-index for the model was 0.84; the model explained 85% of the variance in discharge disposition.
Subgroup Analysis
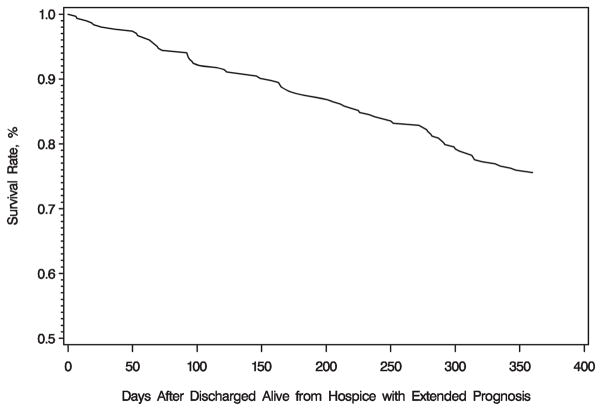
Between January 1, 1999, and December 31, 2001, 303 enrollees with a diagnosis of dementia were discharged alive from a subgroup of three Florida hospice programs because their condition stabilized or improved. Figure 1 shows the survival curve for this subgroup of enrollees. Of those discharged alive, 228 (75.5%) were still alive 1 year after discharge. Of those who died, median time to death was almost 6 months (179.5 days; range 6–360 days). Characteristics of the subgroup are listed in Table 3. There were no significant differences between the characteristics of those who were alive and those who died in the year after discharge from hospice. The results of the Cox proportional hazards model are listed in Table 4. Similar to the bivariate findings, none of the variables were significant predictors of greater risk of death 1 year after being discharged alive from hospice in those whose condition stabilized or improved.
Figure 1.
Survival of hospice enrollees with dementia in the year after discharge from hospice because of stabilization or improvement.
Table 3.
Characteristics of Subgroup of Hospice Enrollees with Dementia Discharged Alive Because Their Condition Stabilized or Improved According to Vital Status 1 Year After Discharge
| Characteristic | Alive at 1 Year, n = 229 (75.5%) | Dead at 1 Year, n = 74 (24.5%) | P-Value |
|---|---|---|---|
| n (%) | |||
| Age | .50 | ||
| 65–84 | 128 (77.1) | 38 (22.9) | |
| ≥85 | 101 (73.3) | 36 (26.3) | |
| Race | .18 | ||
| White | 168 (73.4) | 60 (81.1) | |
| Other | 61 (26.6) | 14 (18.9) | |
| Female | 180 (78.6) | 55 (74.3) | .44 |
| Median household income ($) | .50 | ||
| Low (≤37,280) | 101 (44.1) | 27 (36.5) | |
| Middle (37,281–48,541) | 79 (34.5) | 28 (37.8) | |
| High (≥48,542) | 49 (21.4) | 19 (25.7) | |
| Payment source Medicare | 215 (93.9) | 69 (93.2) | .84 |
| Admission level of care | .98 | ||
| Routine home care in private residence | 100 (43.7) | 32 (43.2) | |
| Routine home care in nursing home | 103 (45.0) | 33 (44.6) | |
| Other | 26 (11.34) | 9 (12.2) | |
| Length of stay before hospice discharge, days | .22 | ||
| ≤180 | 102 (44.5) | 49 (52.7) | |
| >180 | 127 (55.5) | 35 (47.3) | |
Table 4.
Cox Proportional Hazards Model of Risk of Death 1 Year After Discharge from Hospice Because of Stabilization or Improvement in Enrollees with Primary Diagnosis of Dementia
| Variable | Hazard Ratio (95% Confidence Interval) |
|---|---|
| Aged ≥ 85 | 1.22 (0.76–1.96) |
| White race | 0.69 (0.38–1.24) |
| Female | 0.80 (0.46–1.40) |
| Median household income > $37,280 | 1.30 (0.81–2.10) |
| Payment source other than Medicare | 1.23 (0.48–3.16) |
| Admission level of care (reference routine home care in private residence) | |
| Routine home care in nursing home | 1.09 (0.66–1.81) |
| Other | 1.10 (0.52–2.34) |
DISCUSSION
In this analysis of hospice enrollees with a primary diagnosis of dementia, only 5% were discharged alive because their condition stabilized or improved. The remaining 95% died while receiving hospice, and the median time to death was 12 days. Older age, nonwhite race, and higher level of care at admission were associated with a lower likelihood of being discharged from hospice alive, and female sex and a payment source other than Medicare were associated with a greater likelihood of being discharged alive. The single greatest predictor of being discharged alive because of disease stabilization or improvement was a length of stay exceeding 6 months. In the subgroup analysis, three-fourths of those discharged alive were still alive 1 year after discharge. None of the variables included in the analyses were associated with risk of death in the year after discharge. The results of this analysis provide information about factors associated with prognosis and discharge disposition in individuals with dementia who are admitted to hospice.
Although the proportion of hospice programs serving individuals with dementia is increasing (21% in 1995 survey vs 94% in 2008 survey), hospice providers continue to cite difficulty with determining prognosis as a major barrier to caring for individuals with dementia.15,17,19–22 The findings of this study may address the concerns of some hospice providers regarding the potential for long lengths of stay for individuals with dementia. In this sample, only a small minority of individuals (13%) had lengths of stay exceeding 180 days. The vast majority of them died shortly after hospice enrollment, long before the 6-month prognostic cutoff. The absence of accurate tools for determining prognosis may result in late hospice referrals for many individuals with dementia. Providers may feel more comfortable certifying the 6-month prognostic cutoff when they are confident that individuals are very close to death, but such late referrals may not allow individuals and families time to take advantage of the full range of services that hospice provides, including pain and symptom management and psychosocial, spiritual, and bereavement support. Caregivers of those who believed their loved ones were referred to hospice too late have reported greater unmet needs for emotional support and communication and lower satisfaction with care.39,40
Longer time enrolled in hospice has been associated with being discharged before death.30 In this study, those enrolled for longer than 180 days had 12 times greater odds of being discharged alive. Hospice providers may feel more comfortable discharging individuals who are still alive after a long period of observation given the unpredictable course of dementia. Although the optimal period of time needed to determine stability is not known, and individuals may be recertified as long as they continue to meet eligibility criteria, many providers may choose to consider individuals for discharge after 6 months because this time period is included in the prognostic eligibility criteria of the Medicare Hospice Benefit. This seems appropriate because only 13% of individuals with dementia in the sample were enrolled for longer than 180 days. Despite concerns about long lengths of stay, hospice enrollment for many of these individuals may have reduced use of costly acute care services while providing needed palliative care and support.4–10
In the subgroup analysis, 75.5% of enrollees who were discharged alive were still alive 1 year later, and none of the variables included in the analyses were significant predictors of death, which suggests that most enrollees with dementia who improve or stabilize after hospice admission and are therefore discharged are not likely to die in the short term. Given the large symptom burden and uncertain course of dementia, individuals discharged from hospice before death may benefit from other supportive services along with close follow-up for evidence of decline to determine when they should be considered for hospice re-enrollment if desired.1–3 A previous study identified a report that the individual’s condition had worsened as the strongest predictor of death after hospice discharge.33 Information on functional status at admission or after discharge was not available for the current study.
Enrollees who were older and male and had a higher level of care on admission were more likely to die. These variables have been associated with greater risk of death in individuals with dementia in other analyses,19,22,24 but they are not currently included in hospice guidelines for determining prognosis.18 Incorporating these factors into hospice admission criteria for individuals with dementia whose goals of care are consistent with the hospice philosophy of care may increase access to hospice for these individuals and lead to earlier referrals.
African Americans and those with Medicare were also more likely to die while receiving hospice. Because of greater preferences for the use of life-prolonging therapies at the end of life,41,42 African Americans may be more likely to enroll later in the course of their disease, when there are clearly no additional options for life-prolonging therapies. Concerns about potential allegations of Medicare fraud related to enrolling individuals who are likely to live longer than 6 months may have influenced the timing of referral based on payment source.31,32,43 Providers may be reluctant to certify the 6-month prognostic cutoff required in the Medicare Hospice Benefit for those whose payment source is Medicare (vs other) until later in the course of dementia, when they feel more confident that the individual is likely to die in 6 months or less.
This study has a number of limitations. A major limitation is the absence of information about functional status and comorbidities of enrollees. Dependence in activities of daily living and the presence of comorbidities, such as cancer and congestive heart failure, are among the most important predictors of prognosis in individuals with advanced dementia and of live hospice discharges.19,23,24,30 Because this information was not included in the database, the extent to which individuals met NHPCO guidelines for admission at the time of enrollment or how functional status at admission or changes over time were related to the primary outcome (death vs discharge because of stabilization or improvement) or to survival after discharge cannot be determined. There was also no information about criteria that the hospice provider used to determine stabilization or improvement. Future research should include measures of functional status at hospice admission and changes over time, as well as other factors that may be associated with hospice discharge disposition, such as goals of care, individual and family preferences, and management of dementia-related complications (e.g., infections, poor nutrition).
Another limitation is that the data were drawn entirely from a for-profit hospice and included programs in only eight states. Given the heterogeneity of individuals with dementia, differences in physician referral practices, and hospice provider enrollment practices, analyses of other hospice providers, including nonprofit providers, in other states may yield different results. For example, in a recent study, for-profit hospices admitted a higher proportion of individuals with dementia (17.2% vs 8.4%) than nonprofit hospices, and the individuals with dementia admitted to for-profit hospices had longer median lengths of stay (43 vs 26 days).44 Some believe that the potential for greater profit associated with the care of individuals who may require fewer skilled services, live in nursing homes, or have longer lengths of stay because the Medicare hospice benefit pays hospice providers at a fixed per diem drive these differences.44 In this study, individuals with dementia made up 13% of all admissions, but the median length of stay for the sample was only 13 days. No information was available on functional status or care provided to enrollees.
The sample was drawn from those who enrolled in hospice between 1999 and 2003. These findings may differ from an analysis of more-recent data. For example, in 2005, 25% of Medicare beneficiaries admitted to hospice with dementia had stays of longer than 180 days, compared with 13% in this sample. The median length of stay for individuals with dementia in the national sample was 27 days for those with dementia, versus 13 days in the current study’s sample.28 The differences may be due to an increase in the numbers of individuals with dementia who are accessing hospice, the timing of referral, or screening criteria used by the hospice provider to determine eligibility for admission.
The findings of this study suggest that, in the absence of accurate tools for determining prognosis in dementia, healthcare providers tend to err on the side of referring most individuals with dementia to hospice when they are very close to death. Despite concerns about potential allegations of Medicare fraud related to long lengths of stay, only a small minority of enrollees are still alive after 6 months. Most individuals who stabilize or improve and no longer meet eligibility criteria are not likely to die within the first several months after discharge. Future research should focus on improving prognostication in dementia and more fully elucidating factors that may be associated with stabilization after hospice admission and survival after live hospice discharge.
Acknowledgments
This analysis was supported by K08AG028975 —Beeson Career Development Award in Aging Research and Geriatric Research, Education and Clinical Center, Veterans Affairs Medical Center, Durham, North Carolina.
Sponsor’s Role: Although the analysis used data from the VITAS Healthcare Corporation, the study was not funded by VITAS and does not reflect the views of the VITAS Healthcare Corporation. Vitas had no role in the study design, analysis, interpretation of data, preparation, or approval of the manuscript.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Kimberly S. Johnson, Katja Elbert-Avila, and James A. Tulsky: Study concept, design, analysis, interpretation, manuscript preparation. Maragatha Kuchibhatla: Analysis, interpretation, manuscript preparation.
Related Presentations and Published Abstracts: Elbert-Avila, K, Johnson KS, Kuchibhatla, M, Tanis, D, Tulsky, JA. Predictors of extended prognosis in hospice patients with dementia. Oral Presentation, Annual Meeting of the American Geriatrics Society, 2005. Abstract Only—J Am Geriatr Soc 2005;53(Suppl):S9.
References
- 1.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell SL, Morris JN, Park PS, et al. Terminal care for persons with advanced dementia in the nursing home and home care settings. J Palliat Med. 2004;7:808–816. doi: 10.1089/jpm.2004.7.808. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell SL, Kiely DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med. 2004;164:321–326. doi: 10.1001/archinte.164.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Kiely DK, Givens JL, Shaffer ML, et al. Hospice use and outcomes in nursing home residents with advanced dementia. J Am Geriatr Soc. 2010;58:2284–2291. doi: 10.1111/j.1532-5415.2010.03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shega JW, Hougham GW, Stocking CB, et al. Patients dying with dementia: Experience at the end of life and impact of hospice care. J Pain Symptom Manage. 2008;35:499–507. doi: 10.1016/j.jpainsymman.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell SL, Kiely DK, Miller SC, et al. Hospice care for patients with dementia. J Pain Symptom Manage. 2007;34:7–16. doi: 10.1016/j.jpainsymman.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Bekelman DB, Black BS, Shore AD, et al. Hospice care in a cohort of elders with dementia and mild cognitive impairment. J Pain Symptom Manage. 2005;30:208–214. doi: 10.1016/j.jpainsymman.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Miller SC, Mor V, Teno J. Hospice enrollment and pain assessment and management in nursing homes. J Pain Symptom Manage. 2008;35:499–507. doi: 10.1016/s0885-3924(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 9.Gozalo PL, Miller SC. Hospice enrollment and evaluation of its causal effect on hospitalization of dying nursing home residents. Health Serv Res. 2007;42:587–610. doi: 10.1111/j.1475-6773.2006.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teno JM, Gozalo PL, Lee IC, et al. Does hospice improve quality of care for persons dying from dementia? J Am Geriatr Soc. 2011;59:1531–1532. doi: 10.1111/j.1532-5415.2011.03505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Hospice and Palliative Care Organizations. NHPCO Facts and Figures: Hospice Care in America. Alexandria, VA: 2010. [Accessed March 28, 2011]. [on-line]. Available at www.nhpco.org/research. [Google Scholar]
- 12.Miller SC, Lima JC, Mitchell SL. Hospice care for persons with dementia: The growth of access in US nursing homes. Am J Alzheimers Dis Other Demen. 2010;25:666–673. doi: 10.1177/1533317510385809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medicare Payment Advisory Commission. 2E-Beneficiaries access to care: Use of hospice continues to increase. Report to Congress: Medicare Payment Policy; Washington, DC. 2010. [Accessed March 28, 2011]. [on-line]. Available at www.medpac.gov/documents/mar10_entirereport.pdf. [Google Scholar]
- 14.Connor SR, Elwert F, Spence C, et al. Geographic variation in hospice use in the United States in 2002. J Pain Symptom Manage. 2007;34:277–285. doi: 10.1016/j.jpainsymman.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Hanrahan P, Luchins DJ. Access to hospice programs in end-stage dementia: A national survey of hospice programs. J Am Geriatr Soc. 1995;43:56–59. doi: 10.1111/j.1532-5415.1995.tb06243.x. [DOI] [PubMed] [Google Scholar]
- 16.McCarty CE, Volicer L. Hospice access for individuals with dementia. Am J Alzheimers Dis Other Demen. 2009;24:476–485. doi: 10.1177/1533317509348207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torke AM, Holtz LR, Hui S, et al. Palliative care for patients with dementia: A national study. J Am Geriatr Soc. 2010;58:2114–2121. doi: 10.1111/j.1532-5415.2010.03141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Hospice Organization. Medical Guidelines for Determining Prognosis in Selected Noncancer Diseases. 2. Arlington, VA: National Hospice Organization; 1996. [DOI] [PubMed] [Google Scholar]
- 19.Doberman DJ, Yasar S, Durso SC. Would you refer this patient to hospice? An evaluation of tools for determining life expectance in end-stage dementia. J Palliat Med. 2007;10:1410–1419. doi: 10.1089/jpm.2007.9838. [DOI] [PubMed] [Google Scholar]
- 20.Schonwetter RS, Han B, Small BJ, et al. Predictors of six-month survival among patients with dementia: An evaluation of hospice Medicare guidelines. Am J Hosp Palliat Care. 2003;20:105–113. doi: 10.1177/104990910302000208. [DOI] [PubMed] [Google Scholar]
- 21.Luchins DJ, Hanrahan P, Murphy K. Criteria for enrolling dementia patients in hospice. J Am Geriatr Soc. 1997;45:1054–1059. doi: 10.1111/j.1532-5415.1997.tb05966.x. [DOI] [PubMed] [Google Scholar]
- 22.Hanrahan P, Raymond M, McGowan E, et al. Criteria for enrolling dementia patients in hospice: A replication. Am J Hosp Palliat Care. 1999;16:395–400. doi: 10.1177/104990919901600110. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell SL, Kiely DK, Hamel MB. Estimating prognosis for nursing home residents with advanced dementia. JAMA. 2004;291:2734–2740. doi: 10.1001/jama.291.22.2734. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell SL, Miller SC, Teno JM, et al. The advanced dementia prognostic tool: A risk score to estimate survival in nursing home residents with advanced dementia. J Pain Symptom Manage. 2010;40:639–651. doi: 10.1016/j.jpainsymman.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Covinsky KE, Eng C, Lui L, et al. The last 2 years of life: Functional trajectories of frail older people. J Am Geriatr Soc. 2003;51:492–498. doi: 10.1046/j.1532-5415.2003.51157.x. [DOI] [PubMed] [Google Scholar]
- 26.Teno JM, Weitzen S, Fennell ML, et al. Dying trajectory in the last year of life: Does cancer trajectory fit other diseases? J Palliat Med. 2001;4:457–464. doi: 10.1089/109662101753381593. [DOI] [PubMed] [Google Scholar]
- 27.Sachs GA, Shega JW, Cox-Hayley D. Barriers to excellent end-of-life care for patients with dementia. J Gen Intern Med. 2004;19:1057–1063. doi: 10.1111/j.1525-1497.2004.30329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medicare Payment Advisory Commission. Chapter 8: Evaluating Medicare’s hospice benefit. Longer hospice stays consistent with growth in noncancer diagnoses; Report to Congress: Reforming the delivery system; Washington, DC. 2008. [Accessed March 28, 2011]. [on-line]. Available at www.medpac.gov/documents/jun08_entirereport.pdf. [Google Scholar]
- 29.Haupt BJ. Characteristics of hospice care discharges and their length of service: United States, 2000. [Accessed March 28, 2011];Vital Health Stat 13. 2003 154:1–36. [on-line]. Available at www.cdc.gov/nchs/data/series/sr_13/sr13_154.pdf. [PubMed] [Google Scholar]
- 30.Kutner JS, Blake M, Meyer S. Predictors of live hospice discharge: Data from the National Home and Hospice Care Survey (NHHCS) Am J Hosp Palliat Care. 2002;19:331–337. doi: 10.1177/104990910201900510. [DOI] [PubMed] [Google Scholar]
- 31.Hospice Compliance Network. HCL special report on hospice’s eligibility challenge. [Accessed June 30, 2012];Hospice Compliance Letter. 2011 12:1–8. Available at www.hospicecompliance.net/images/pdf/Vol12No1.pdf. [Google Scholar]
- 32.Jennings B. Hospice and Alzheimer disease: A study in access and simple justice. Access to hospice care: Expanding boundaries, overcoming barriers. Hastings Cent Rep Special Supplement. 2003;33:S24–S526. [PubMed] [Google Scholar]
- 33.Kutner JS, Meyer SA, Beaty BL, et al. Outcomes and characteristics of patients discharged alive from hospice. J Am Geriatr Soc. 2004;52:1337–1342. doi: 10.1111/j.1532-5415.2004.52365.x. [DOI] [PubMed] [Google Scholar]
- 34.Virnig BA, Kind S, McBean M, et al. Geographic variation in hospice use prior to death. J Am Geriatr Soc. 2000;48:117–125. doi: 10.1111/j.1532-5415.2000.tb04789.x. [DOI] [PubMed] [Google Scholar]
- 35.Connor SR, Elwert F, Spence C, et al. Racial disparity in hospice use in the United States in 2002. Palliat Med. 2008;22:205–213. doi: 10.1177/0269216308089305. [DOI] [PubMed] [Google Scholar]
- 36.Krieger N. Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geronimus AT, Bound J. Use of census-based aggregate variables to proxy for socioeconomic group: Evidence from national samples. Am J Epidemiol. 1998;148:475–486. doi: 10.1093/oxfordjournals.aje.a009673. [DOI] [PubMed] [Google Scholar]
- 38.Dowd JB, Albright J, Raghunathan TE, et al. Deeper and wider: Income and mortality in the USA over three decades. Int J Epidemiol. 2011;40:183–188. doi: 10.1093/ije/dyq189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schockett ER, Teno JM, Miller SC, et al. Late referral to hospice and bereaved family member perception of quality of end-of-life care. J Pain Symptom Manage. 2005;30:400–407. doi: 10.1016/j.jpainsymman.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Teno JM, Shu JE, Casarett D, et al. Timing of referral to hospice and quality of care: Length of stay and bereaved family members’ perceptions of the timing of hospice referral. J Pain Symptom Manage. 2007;34:120–125. doi: 10.1016/j.jpainsymman.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Barnato AE, Anthony DL, Skinner J, et al. Racial and ethnic differences in preferences for end-of-life-treatment. J Gen Intern Med. 2009;24:695–701. doi: 10.1007/s11606-009-0952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hopp FP, Duffy SA. Racial variations in end-of-life care. J Am Geriatr Soc. 2000;48:758–763. doi: 10.1111/j.1532-5415.2000.tb04724.x. [DOI] [PubMed] [Google Scholar]
- 43.Rau J. Concerns about costs rise with hospices’ use. [Accessed December 7, 2011];New York Times. 2011 Jun 27; [on-line]. Available at www.nytimes.com/2011/06/28/health/28hospice.html.
- 44.Wachterman MW, Marcantonio ER, Davis RB. Association of hospice agency profit status with patient diagnosis, location of care, and length of stay. JAMA. 2011;305:472–479. doi: 10.1001/jama.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]