Abstract
OBJECTIVES
Cholestasis affects 50% of extremely low birthweight (ELBW) infants. Its etiology remains poorly understood and the extent of liver injury in these infants unclear. The premature baboon model provides an opportunity to study neonatal liver disease. We characterize hepatic histopathologic changes in this model.
METHODS
Archival tissue and data were obtained from the Southwest Foundation for Biomedical Research Primate Center, San Antonio, Texas. Animals were selected based on history of antenatal steroid therapy and absence of sepsis or necrotizing enterocolitis with a protocol duration of at least 21 days and no early death (n=45). Baboons had been treated per protocol in the NICU. At necropsy, liver tissue was harvested and stored. Tissues from fetal gestational controls at similar ages were used for comparison (n=28). Histologic changes were scored by consensus of two pathologists blinded to treatment group. Descriptive and comparative statistics were performed.
RESULTS
Control fetal livers had extramedullary hematopoiesis (EMH) that decreased across the gestational range. There was evidence of hepatocyte iron storage and ongoing portal tract development. Livers of NICU treated baboons had increased Kupffer cell hypertrophy and hemosiderosis. There was a shift away from erythroid EMH toward increased myeloid EMH. There was increased cholestasis, ductular proliferation, portal tract fibrosis and steatosis in treated animals.
CONCLUSIONS
We found pathologic changes in NICU- treated baboons comparable to findings reported in human infants. The baboon model of prematurity may be a useful tool to explore cholestasis and liver dysfunction in ELBW infants.
Keywords: Cholestasis; Infant, Extremely Low Birthweight; Liver
INTRODUCTION
Liver dysfunction, clinically manifested by direct hyperbilirubinemia, is common in neonatal intensive care patients, affecting as many as 50% of extremely low birthweight (ELBW) infants. (1, 2) Suggested causes have included hypoxia/ischemia, hepatotoxicity of parenteral nutrition components, delayed enteral feeding, infection and fetal growth restriction, but the pathogenesis remains poorly understood.(3–5) Infants with short gut syndrome or intestinal failure requiring long-term parenteral nutrition are at highest risk to develop cholestasis and associated liver failure.(6) However, in this era of increased survival of ELBW infants, cholestasis has become common in the non-short gut syndrome population and the pathogenesis and significance of hepatic dysfunction in this setting remain largely unknown.(2) While this liver dysfunction often reverses with time and increased enteral feeding, in some cases it may progress to liver failure and concomitant transplantation or death.(7–9) Infants with early onset liver disease have later shown evidence of intellectual impairment, raising concern for the long term ramifications of cholestatic disease in ELBW infants and highlighting the need for better understanding of and effective treatments for this disorder.(10)
Histopathologic studies of premature infants with cholestasis have generally involved autopsies or biopsies from the sickest infants and have described early steatosis, cholestasis, bile duct proliferation, periportal inflammation and fibrosis.(11–16) Biopsies are seldom performed in less severely affected ELBW infants, and consequently there is very little information on the early stages that might provide clues to the understanding of the pathophysiology of cholestatic liver disease in this population.
The baboon model of premature delivery and neonatal intensive care, with its similarities to human treatments, has advanced our understanding of the pathogenesis of conditions found in ELBW populations.(17–19) Liver tissues were collected in the course of these investigations and provide us with the opportunity to conduct a preliminary study of hepatic histopathology in this model of neonatal intensive care. The major goal of this pilot study in the baboon model was to characterize histopathologic changes so as to have a foundation for future hypothesis-driven studies to investigate the impact of premature delivery and neonatal intensive care on hepatic dysfunction in this animal model. The secondary goal was to determine whether the histologic findings might provide clues to the pathogenesis of liver dysfunction in ELBW human infants.
MATERIALS AND METHODS
Baboon Model of Prematurity
Liver tissues were obtained from studies of the baboon model of prematurity, performed in the Neonatal Intensive Care Unit (NICU) at the Southwest Foundation for Biomedical Research Primate Center (SFBRPC), San Antonio, Texas. The model and studies were approved by the Institutional Animal Care and Use Committee at the SFBRPC. Data were obtained from electronic and hardcopy records kept at the SFBRPC. Paraffin blocks for hepatic histology were obtained from tissue stored at the time of necropsy and maintained by the SFBRPC and the lab of Jacqueline Coalson, University of Texas Health Sciences Center Department of Pathology. The model has been described in detail.(17, 20, 21) In brief, baboon dams (Papio papio) were treated with betamethasone (6 mg intramuscular, 48 and 24 hours prior to scheduled delivery) then delivered by scheduled cesarean section at approximately 125 days (67% gestation, term = 185 days). After delivery the infant baboons were endotracheally intubated, treated with surfactant, placed on mechanical ventilation, instrumented with umbilical arterial and percutaneous central venous lines and provided respiratory and nutritional support as previously described for the intended duration of their protocol (anywhere from 6 to 28 days). Transfusions of small volumes of packed red blood cells were given on an ongoing basis as needed to maintain predefined hematocrit level. At the end of their prescribed protocol the animals were euthanized and tissues harvested and stored for future analysis. Specific to nutritional support, within 24 hours of delivery animals were started on parenteral nutrition of content and volume similar to that used in human NICU patients including amino acids (Trophamine, B.Braun Medical, Bethlehem, PA), glucose, multivitamins, trace elements and intralipids, advanced gradually as tolerated per guidelines specific to the individual protocol. Depending on protocol the animals were given small volume enteral feedings (Similac, Abbott Nutrition, Columbus GA or Primalac, Bio-Serv Inc, Frenchtown NJ) by orogastric gavage; these were begun by day of life 7 and gradually advanced as tolerated per specific protocol guideline. There was a gestational control subset of the model who were also delivered by elective cesarean at prescribed timepoints; necropsy was performed after delivery and tissues stored for future study. Gestational control animals were not subjected to NICU treatments in the manner of study animals. There were a number of prescribed gestational ages for these controls, designed to be able to compare to treatment animals both at birth and at the different lifespans.
For the present study 125 day-delivered, NICU- treated baboon liver tissue samples were selected from the cohort based on the (positive) history of antenatal steroid treatment, absent diagnosis of sepsis or necrotizing enterocolitis (NEC), priori protocol length of at least 21 days and no early/unanticipated death (prior to prescribed protocol end). There were 45 animals that fulfilled these criteria; 37 that had been treated for 21 days and 8 for 28 days. Gestational controls were selected from available samples over a gestational time span from 125 to 160 days in an attempt to encompass fetal development that occurs during the lifespan of the NICU treated animals. Additional liver tissue samples from four baboons delivered at term and fed for 72 hours prior to necropsy were also evaluated along with the gestational controls. The following groups were studied: 10 animals delivered at 125 days (67% gestation), 10 at 140 days (76% gestation), 4 at 153 days (83% gestation),4 at 160 days (86% gestation) and 4 term animals. The controls were selected from limited, preplanned specific gestational timepoints available in order to compare to the lifespan of the 125 day animals treated in the NICU for 21–28 days.
Histology and Histology Scoring
Liver tissue specimens were obtained at necropsy from both treated and gestational control animals. They were fixed in 10% formalin for 24 hours at room temperature, then dehydrated in alcohol and embedded in paraffin. Sections were cut at 4µm, and stained with hematoxylin and eosin, Masson trichrome and Perl’s iron stains. All slides were evaluated by consensus of two hepatic pathologists (ZG, AM) who were blinded to group or clinical condition of the source animal (no information about gestational control vs. study animal category, clinical or nutritional data regarding study animals). After an initial observational review, the following histological characteristics appeared noteworthy and were evaluated in detail: Extramedullary hematopoiesis (EMH) was prominent in most of the specimens and was scored according to the method of Miranda.(22) Erythroid hematopoiesis was scored from 0–2, with 0= no areas of hematopoietic foci, 1= widely spaced hematopoietic foci and 2= closely opposed hematopoietic foci. Portal and Lobular Myelopoiesis were scored from 0–3 with 0= absent myelopoiesis, 1= isolated myeloid cells, 2= scattered cell clusters and 3= frequent clusters or confluent myeloid cells in portal spaces. Acinar Bile Ducts were noted to be present or absent and expressed as a percent of portal tracts containing at least one duct, with 20 portal tracts counted per animal. Other semiquantifiable parameters were scored from 0–3 with 0= none, 1= mild/few, 2 = moderate and 3= many/marked including Kupffer Cell Hypertophy, Hemophagocytosis, Hepatocyte Macrovesicular and Microvesicular Steatosis, Apoptotic Bodies, Lobular atrophy, Bile Stasis, Ductular Proliferation, Portal and Acinar Fibrosis (Masson’s trichrome), Kupffer cell iron and hepatocyte iron (Perl’s iron stain). Other pathologic findings were noted when present but not quantified.
Statistical Analysis
Data were described using mean/standard deviation, median/IQR or frequency of score category depending on distribution. For comparison of acinar ducts one-way Anova with Bonferroni correction was used. Between groups comparison of histology scores were made using Fisher’s exact test or Kruskal-Wallis with posthoc testing. Significance was accepted at an alpha of < 0.05 for primary testing and p< .002 for Kruskal-Wallis post hoc testing. All analysis was performed using Stata IC10 (Stata, College Station, TX).
RESULTS
Treatment Groups and Animals
We evaluated the necropsy liver samples from 45 baboons delivered at 125 days and treated in the NICU for 21–28 days. We compared the treated specimens to livers from 28 gestational controls, delivered at 125 (n=10), 140 (n=10), 153 (n=4) and 160 (n-4) days. We also evaluated specimens from 4 animals delivered at term and necropsied on day of life 3. There were no NICU-treated animals with sepsis (documented or suspected), NEC or early death (unanticipated death prior to scheduled end of treatment protocol). The mean birthweight of treated animals was 388 ± 58 grams and the median lifespan was 21 days (IQR 21–21). All NICU-treated animals received TPN including trophamine and intralipids, attempts at enteral feeds and ≥2 packed red blood cell transfusion in their lifespan.
Gestational Controls
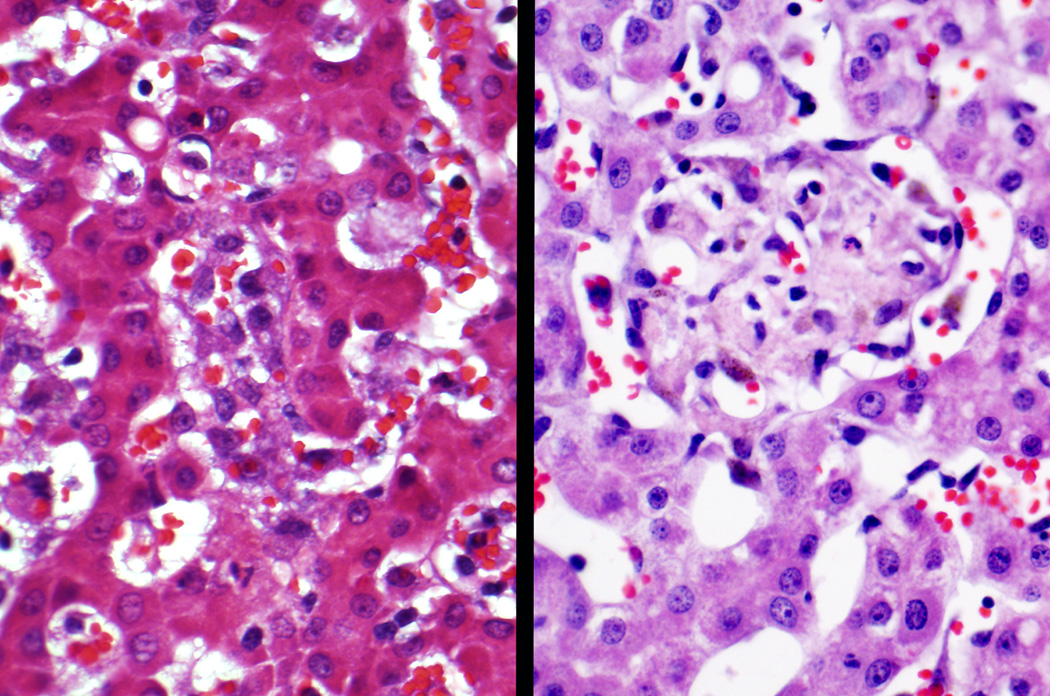
Histologic observations in all animals and semiquantitative scoring were performed without knowledge of treatment group. The most noteworthy findings in the gestational controls, assumed to represent normal histology of later gestation baboon liver development, involved EMH, hepatocyte iron storage, and ongoing bile duct/portal tract development. Some preterm control animals had minor degrees of steatosis, Kupffer Cell hypertrophy, hemophagocytosis or occasional hepatocytes undergoing apoptosis. EMH, both erythroid and myeloid, was present in all animals and tended to be less in animals with longer duration of gestation (Figures 1, 2). EMH was absent/minimal in the term animals. The term animals did show increased microvesicular steatosis compared to controls, presumably due to 3 days of enteral feedings prior to necropsy (control histology data presented in Table s1, http://links.lww.com/MPG/A217). As in humans(23, 24) bile ducts develop from the periportal ductal plate (Figure 3) during the third trimester, and in these gestational control animals, the majority of small portal tracts lacked an acinar bile duct (Figure s1, http://links.lww.com/MPG/A219 [Portal tract in a 125-day gestational control contains blood vessels and hematopoietic cells but no bile duct. Note extramedullary hematopoiesis in surrounding lobule.]). There was a tendency for the percentage of portal tracts that contained bile duct to increase with gestational age through to term. (Table s2, http://links.lww.com/MPG/A218) Some of the ducts in gestational control animals were surrounded by densely cellular fibrous connective tissue. Overall, the amount of fibrous tissue in portal areas was quite variable, with occasional portal tracts having a mild (1+) or moderate (2+) degree of fibrosis (Figure s2, http://links.lww.com/MPG/A220 [Portal fibrous tissue in a 125-day gestational control {Masson trichrome stain}. Small portal tract on left has minimal blue-staining collagenous fibrous tissue. Similar size portal tract on right has more fibrous tissue and was scored as 1+ {mild} fibrosis in the blinded review.]). Erythrophagocytosis was generally mild or absent, and none of the animals had hemosiderin accumulation in hepatocytes, but hepatocellular iron storage was nearly always present and occasionally quite prominent (Figure s3, http://links.lww.com/MPG/A221 [Hepatocellular and Kupffer cell iron storage {Prussian blue stain}. Photomicrograph on left shows 3+ {marked} hepatocellular hemosiderin accumulation without Kupffer cell iron in a 160-day gestational control. On right is photograph of liver biopsy in a 21-day NICU-treated animal, showing 2+ {moderate} Kupffer cell hemosiderin accumulation in addition to 1+ {mild} hepatocellular iron granules.]).
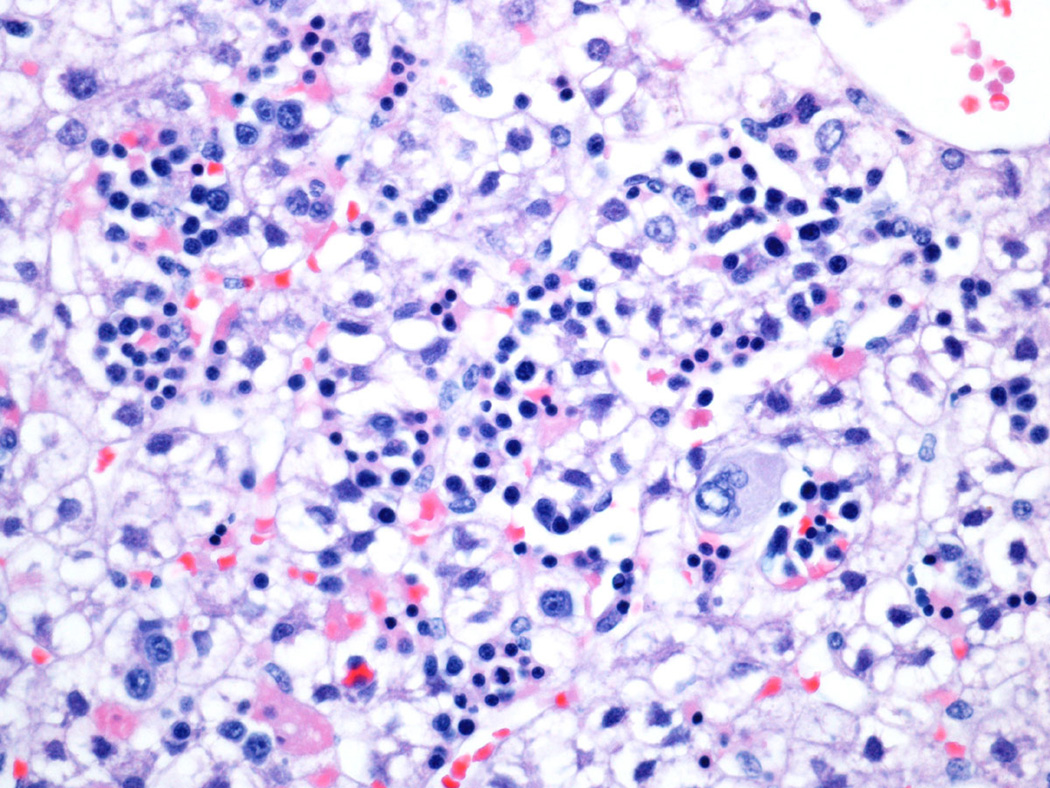
Fig 1.
Lobular extramedullary hematopoiesis in a 125 day gestational control. This liver was scored 2+ for lobular extramedullary hematopoiesis (closely apposed hematopoietic foci) and 3+ for lobular myelopoiesis (frequent >3 cell clusters).
Fig 2.
Portal Myelopoiesis in a 125 day gestational control. This portal tract has 3+ myelopoiesis (confluent myeloid cells) with several eosinophilic myelocytes in the center of the field.
Fig 3.
The ductal plate. This structure exists in the embryo and fetus as a dual-layer of biliary type cells between the portal connective tissue and the surrounding hepatocytes. As the liver develops, the ductal plate remodels to form the acinar bile duct incorporated into the portal tract, and the ductules that connect the bile canaliculi to the acinar bile duct.
Histopathologic Changes in NICU Treated Animals
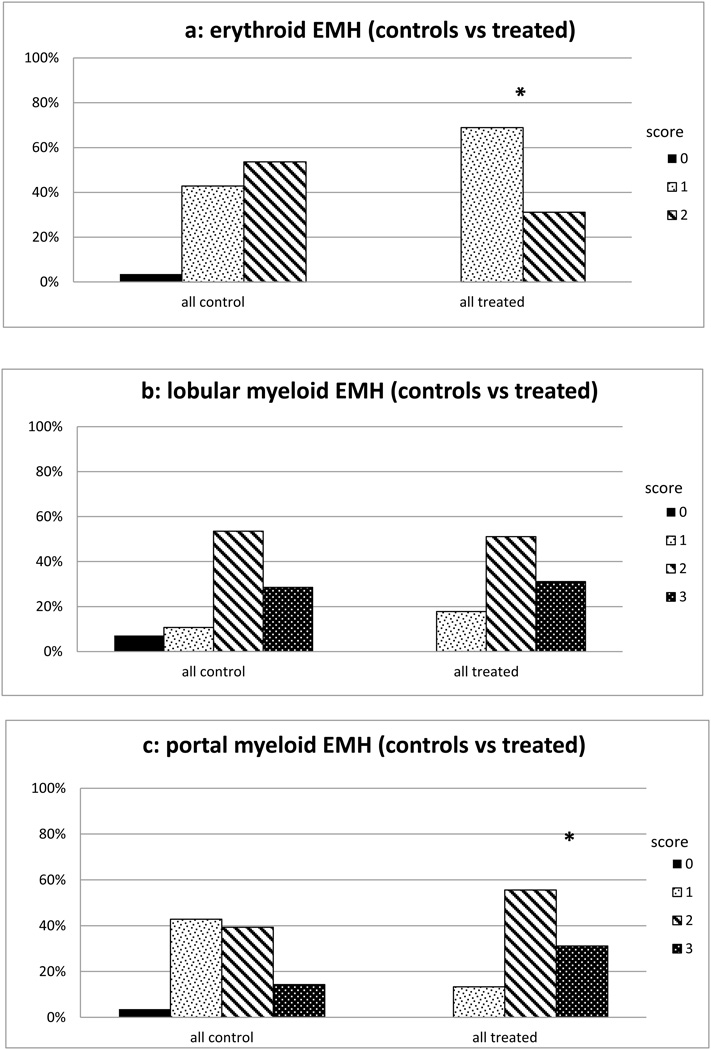
Histopathologic findings in NICU treated animals are presented in Table 1a–b. Similar to gestational controls, NICU treated animals had prominent EMH and hepatocellular iron storage, along with ongoing bile duct development. In addition, they had prominent hemophagocytosis (Figure s4, http://links.lww.com/MPG/A222 [Hemophagocytosis. Phagocytosed erythrocytes are readily identified in hypertrophied Kupffer cells {arrows} in a 21-day NICU-treated animal.]) and a greater degree of hypertrophy and hemosiderosis in the Kupffer cells (Figure s3, http://links.lww.com/MPG/A221). EMH was present to some degree in nearly all animals (Table 1b). Compared to gestational controls, NICU treated animals had lower scores for hepatic erythropoiesis, and higher scores for portal myelopoiesis (Figure 4a–c). These differences persisted whether comparing all gestational controls (125–160 days) combined or just age-adjusted to match age of treated animals (140 days-plus) (data not presented). Similarly, hypertrophy of Kupffer cells, often with hematophagocytosis and hemosiderin accumulation was frequent in the NICU treated animals, and many histopathologic findings were much more frequent and more severe than in the gestational controls (Table 2). Almost all samples from treated animals had KC hypertrophy and occasional treated animals had granulomatoid Kupffer cell aggregates and/or macrophagic granulomas (Figure 5). No gestational control had evidence of bile stasis or ductular proliferation, but 14 (31%) of NICU treated animals had at least mild histologic cholestasis (Figure 6), 15 (33%) had some degree of ductular proliferation (Figure 7), and three had cholangitic abscesses (Figure s5, http://links.lww.com/MPG/A223 [Cholangitic abscess in 21-day NICU-treated animal. A severely damaged large bile duct is filled with purulent exudate.]), suggesting mechanical biliary obstruction. Bile ducts were occasionally observed apparently developing from ductal plates (Figure 8), and large bile ducts with mitotic figures suggesting active ductal prolifertion, were also occasionally observed (Figure 9). Acinar bile ducts were present in 53% of portal tracts on average (range 15–100%), significantly more than in the gestational controls (Table s2, http://links.lww.com/MPG/A218). Similarly fibrosis of portal tracts was more frequent and more severe than in the gestational controls (Table 2). Mild degrees of macrovesicular and microvesicular steatosis were frequent in gestational controls but more severe steatosis (Figure s6, http://links.lww.com/MPG/A224 [Moderate {2+} macrovesicular steatosis in a 21-day NICU-treated animal.]) was found in the 31% of NICU treated animals.
Table 1.
| a: Liver EMH Findings in NICU-Treated Baboons | ||
|---|---|---|
| score | Treated n=45 |
|
| Erythroid EMH | 1 | 69% |
| 2 | 31% | |
| Lobular myeloid EMH | 1 | 18% |
| 2 | 51% | |
| 3 | 31% | |
| Portal myeloid EMH | 1 | 13% |
| 2 | 56% | |
| 3 | 31% | |
| b: Liver Histology Findings in NICU Treated Baboons | ||
|---|---|---|
| score | all treated n=45 |
|
| KC Hypertrophy (0–3) | 0 | 0.0% |
| 1 | 2.2% | |
| 2 | 37.8% | |
| 3 | 60.0% | |
| KC Hemophagocytosis (0–3) | 0 | 8.9% |
| 1 | 33.3% | |
| 2 | 22.2% | |
| 3 | 35.6% | |
| HC Steatosis (0–3) (macrovesicular) | 0 | 6.7% |
| 1 | 62.2% | |
| 2 | 31.1% | |
| 3 | 0.0% | |
| HC Steatosis (0–3) (microvesicular) | 0 | 13.3% |
| 1 | 60.0% | |
| 2 | 26.7% | |
| 3 | 0.0% | |
| Bile stasis (0–3) | 0 | 68.9% |
| 1 | 11.1% | |
| 2 | 15.6% | |
| 3 | 4.4% | |
| Ductular proliferation (0–3) | 0 | 66.7% |
| 1 | 31.1% | |
| 2 | 0.0% | |
| 3 | 2.2% | |
| Portal fibrosis (0–3) | 0 | 3.0% |
| 1 | 45.0% | |
| 2 | 52.0% | |
| KC iron (0–3) | 0 | 15.8% |
| 1 | 23.7% | |
| 2 | 47.4% | |
| 3 | 10.5% | |
| HC iron (0–3) | 0 | 21.1% |
| 1 | 42.1% | |
| 2 | 34.2% | |
| 3 | 2.6% | |
Liver EMH Findings in 125 day-delivered, NICU-Treated Baboons. EMH = Extramedullary hematopoiesis. Data is presented as percentage of samples with score 0–3 (see methods for scoring parameters). If no tissues had a particular score that category is not presented for the histology parameter. All baboons were delivered at 125 days and treated in the NICU for 21–28 days.
Liver Histology Findings in NICU Treated Baboons. KC = Kupffer Cell. HC = Hepatocyte. Data is presented as percentage of samples with score 0–3 (see methods for scoring parameters). If no tissues had a particular score that category is not presented for the histology parameter. All NICU-treated baboons were delivered at 125 days gestation and survived for 21–28 days.
Fig 4.
a: Frequency of Erythroid EMH scores in treated animal livers. Degree of erythroid EMH was less in treated animals. Hepatic erythropoiesis scored 0–2 where 0= no areas of hematopoietic foci, 1= widely spaced hematopoietic foci and 2= closely opposed hematopoietic foci. * p< 0.05 when compared to 125 day group
b: Frequency of Myeloid (lobular) EMH scores in treated animal livers. Myelopoiesis was scored from 0–3 with 0= absent myelopoiesis, 1= isolated myeloid cells, 2= scattered cell clusters and 3= frequent clusters or confluent myeloid cells. Degree of hepatic lobular myelopoiesis was no different vs controls.
c: Frequency of Myeloid (portal) EMH scores in treated animal livers. Myelopoiesis was scored from 0–3 with 0= absent myelopoiesis, 1= isolated myeloid cells, 2= scattered cell clusters and 3= frequent clusters or confluent myeloid cells. Portal myelopoisesis scores were higher in treated animals vs gestational controls * p< 0.01
Table 2.
Liver Histopathology in NICU-Treated Baboons Versus Gestational Controls
| [histology score ≥ moderate (2)] | ||||
|---|---|---|---|---|
| Gestational control n=28 |
NICU- treated n=45 |
p | ||
| KC Hypertrophy | 7.1% | 97.8% | <0.01 | |
| KC iron | 0.0% | 57.9% | <0.01 | |
| Hemophagocytosis | 0.0% | 57.8% | <0.01 | |
| Portal fibrosis | 17.9% | 46.7% | <0.01 | |
| HC iron | 64.3% | 36.8% | 0.05 | |
| HC Steatosis (macro) | 10.7% | 31.1% | 0.05 | |
| HC Steatosis (micro) | 7.1% | 26.7% | 0.06 | |
| Bile stasis | 0.0% | 20.0% | 0.01 | |
| Ductular proliferation | 0.0% | 2.2% | NS | ** |
Liver histopathology (moderate or greater) in treated baboons. KC= Kupffer Cell. HC = Hepatocyte. Data are presented as percentage of treated baboons with tissue scores ≥ 2 (moderate pathology, see methods for scoring parameters) compared to complete range of gestational control ages.
while moderate or greater ductular proliferation was absent in controls and rare in treated samples, there was mild proliferation in 31% of treated animals vs 0% controls (P< 0.01)
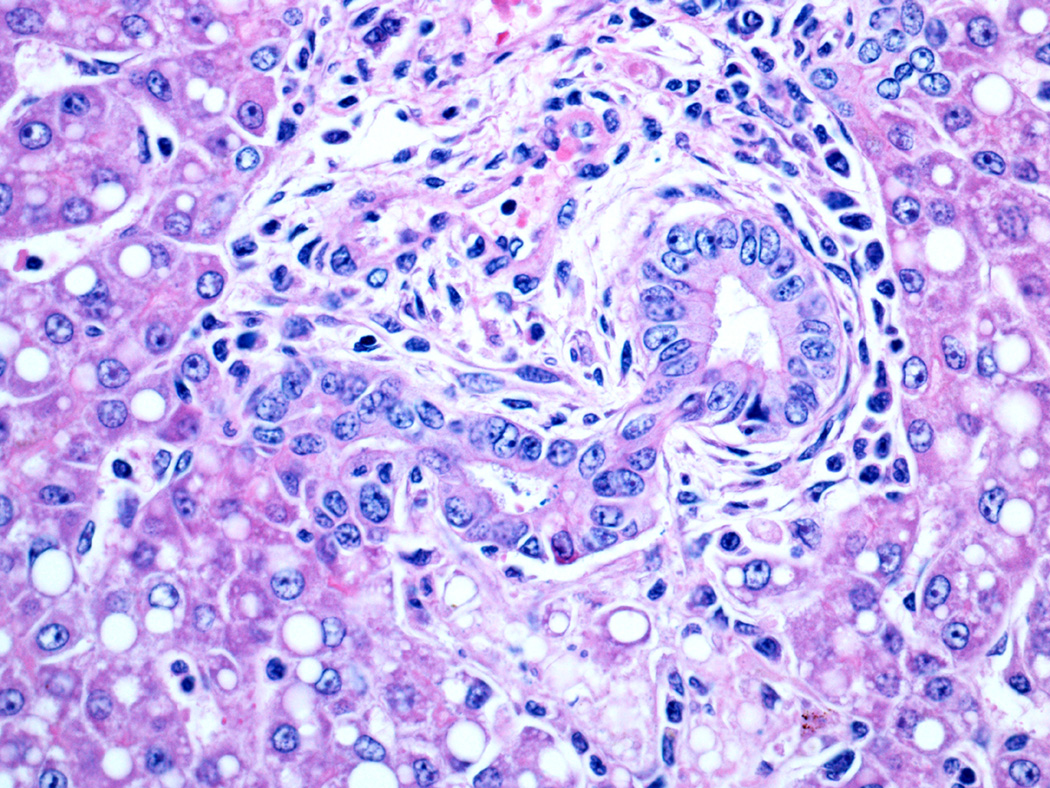
Fig 5.
Kupffer cell hypertrophy (left) and macrophagic granuloma (right) in 21 day NICU treated animals.
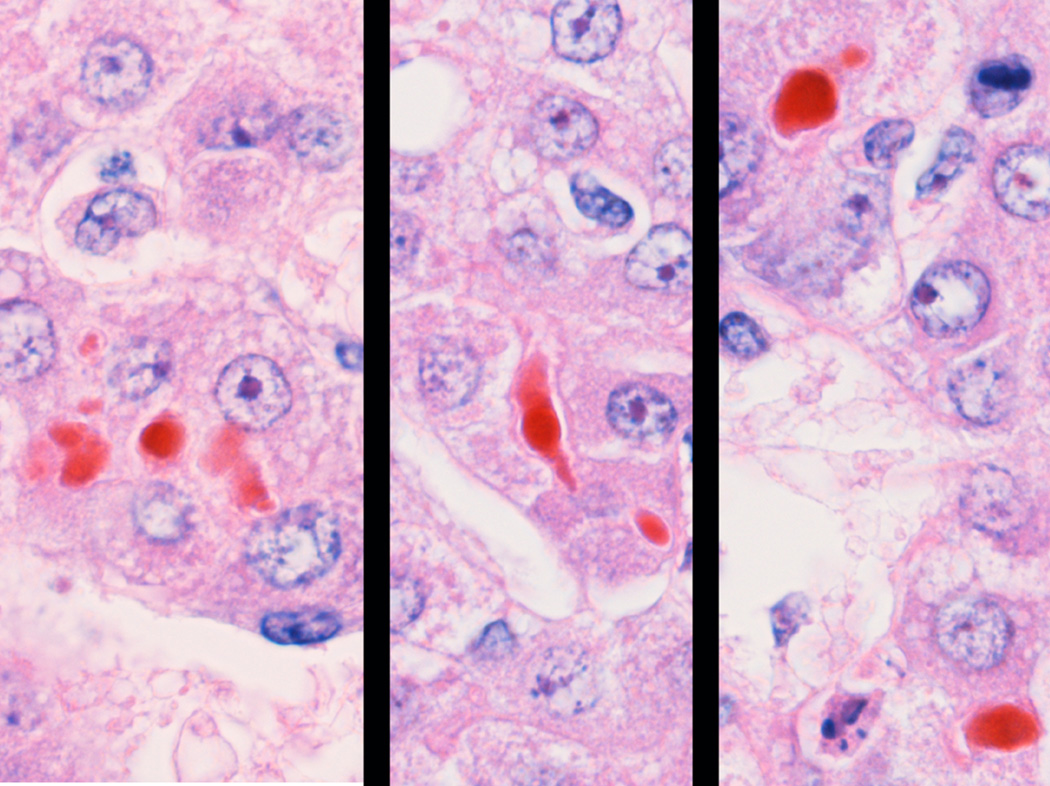
Fig 6.
Cholestasis. Canalicular and hepatocellular bile pigment in a 21 day NICU treated animal.
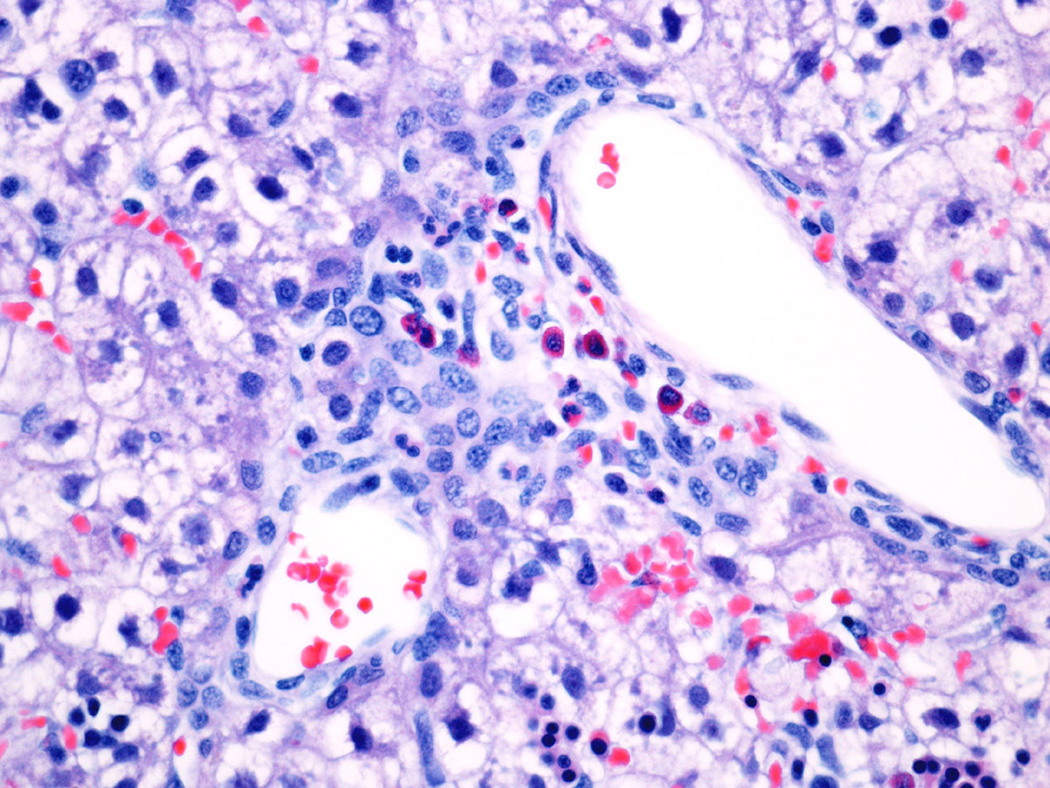
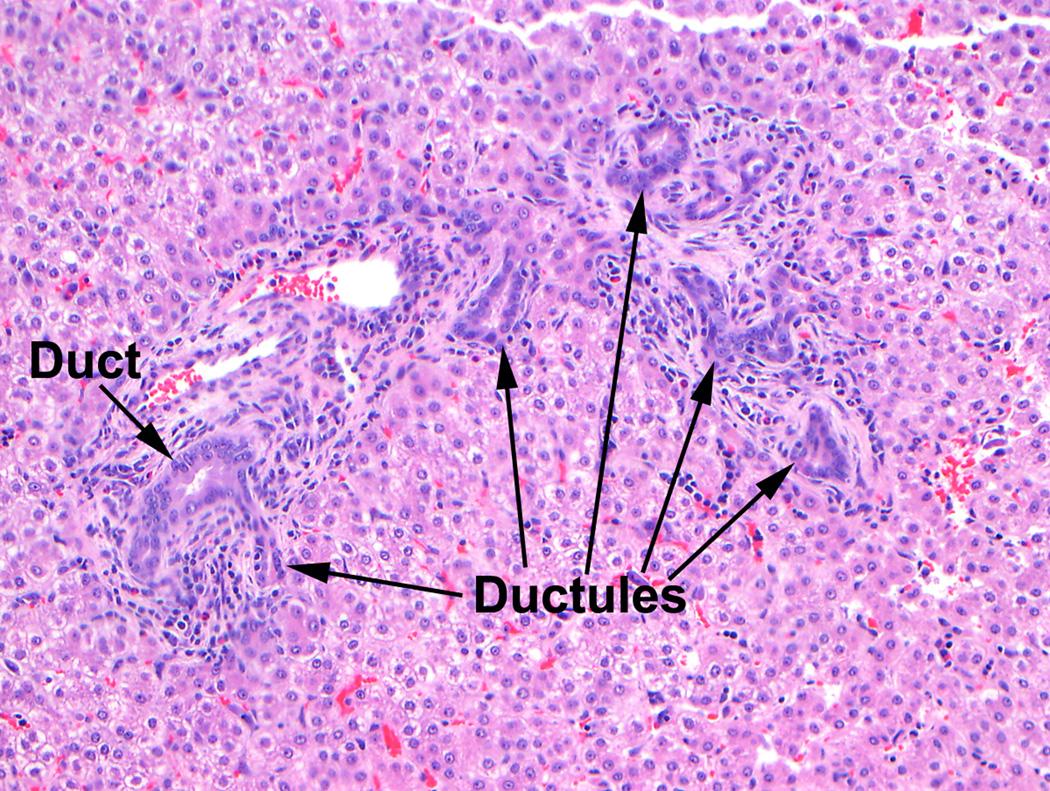
Fig 7.
Ductular proliferation in 21 day NICU treated animal. The acinar bile duct (left) runs beside the hepatic artery branch. The other duct-like structures are reactive (or proliferating) ductules.
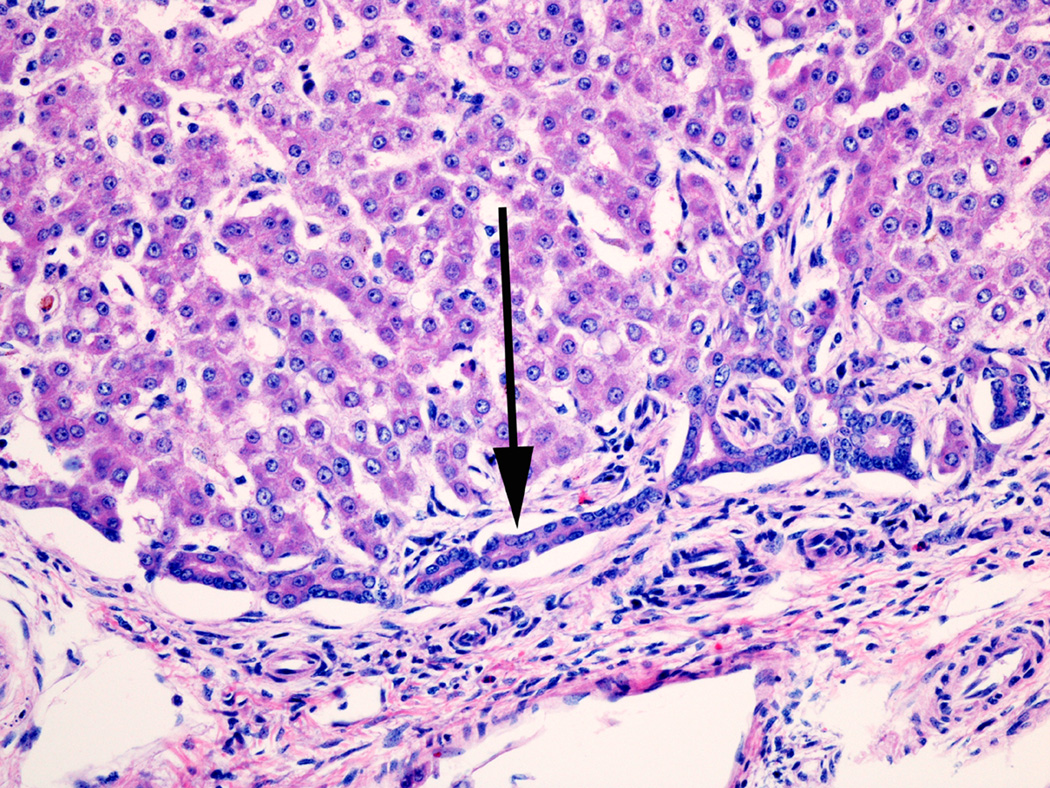
Fig 8.
Acinar bile duct forming from the ductal plate in a small portal area in a 21 day NICU treated animal.
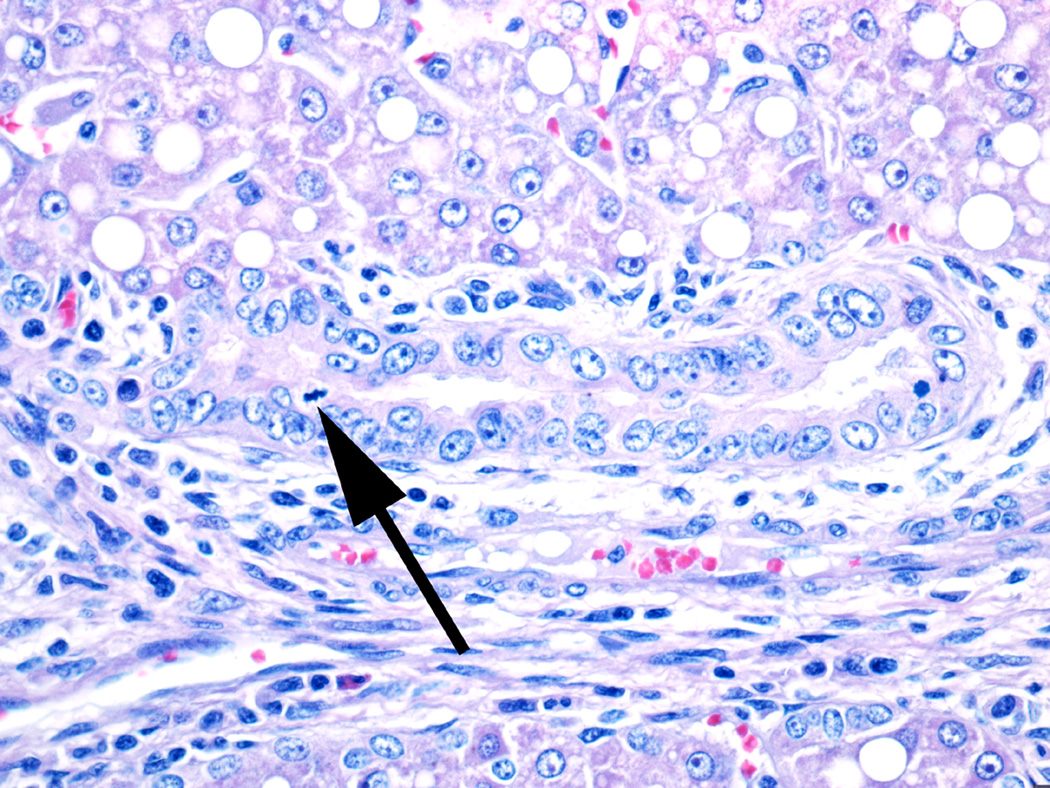
Fig 9.
Damaged large bile duct from a 21 day NICU treated animal. The duct has irregular epithelial cells with prominent nuclei and nucleoli as well as occasional mitoses (arrow), indicating regeneration.
DISCUSSION
Liver dysfunction characterized by cholestasis is common in ELBW preterm infants who receive neonatal intensive care. It is usually associated with parenteral nutrition, but the exact pathogenesis remains unknown. Multiple etiologic factors, including immaturity of the enterohepatic circulation, absence of enteral intake, toxins, nutrient deficiency, infection and other perinatal insults may all play a role.(25) Histologic studies of tissues obtained predominantly at autopsy in a limited number of human infants, of a variety of gestational ages and postnatal ages, have described varying combinations and degrees of bile stasis, inflammation, extramedullary hematopoiesis, steatosis, bile duct injury, ductular proliferation and scarring. (11–16) One limitation of these data is that the patients in these reports had variable durations of survival; many had associated malformations and all had a variety of other complications leading to either liver biopsy or death with postmortem examination. It has not been feasible to systematically study liver histopathology in preterm human infants. Animal models have also been examined but are limited by advanced gestational age and short timespan of treatment. For example, studies in a short-term model of newborn (term) piglets treated with TPN have shown steatosis and apoptosis that can be alleviated by enteral feeding.(26–28) However, the precise sequence of events and relative contributions of pathogenetic factors, particularly in preterm infants, remains to be elucidated. The present study was undertaken to explore the possibility that the baboon model of prematurity and intensive care, initially developed to examine cardiopulmonary and neurologic conditions in preterm infants, might be suitable for study of the pathogenesis of neonatal cholestatic liver disease. We found that these animals, delivered at 125 days (67% gestation) and sacrificed at uniform intervals or 21 or 28 days, had developed histopathologic changes in the liver similar to those described in series of human premature infants. The NICU-treated premature baboon should therefore be a good model system for controlled investigations into factors that may play roles in this disease.
One major finding in our study is that the fetal baboon liver, similar to humans, is a site of extramedullary hematopoiesis. All gestational control animals had both erythropoiesis and myelopoiesis; and these tended to decrease as gestation progressed and were nearly absent at term. We found that NICU treatment, which includes transfusion of packed red blood cells as needed, is associated with decreased erythropoiesis compared to the controls. Instead, the treated animals had Kupffer cell hypertrophy with erythrophagocytosis which was often quite striking. Furthermore, there was accumulation of hemosiderin (demonstrated with the iron stain) in Kupffer cells, presumably from degradation of phagocytosed red blood cells. In the blinded review, only mild occasional erythrophagocytosis was observed in gestational controls, and Kupffer cell iron was absent. By contrast, hepatocellular iron storage was present in both controls and treated animals with a trend for increasing iron in controls with longer gestational periods and for decreasing hepatocellular iron in the NICU treated animals. Hypertrophied Kupffer cells with evidence of phagocytosis are presumed to be activated. Activated Kupffer cells produce numerous cytokines that have a myriad of effects in various pathologic conditions, and consequently their prominence in the treated animals may be a stimulus or response to the development of other pathologic findings in these animals.
Another major finding of the study is that in the fetal baboon liver, similar to humans, (23) acinar bile ducts are incompletely developed and appear to be actively developing in the last trimester of gestation. It is well established that bile ducts develop from the dual layer of cells called the ductal plate that is located adjacent to the portal tracts in the developing liver. As the fetus grows, the ductal plate remodels with part forming the acinar bile duct that is incorporated into the portal tract while the remainder disappears. NICU treated baboons appear to have an acceleration of bile duct development. In the gestational controls, on average, 29% of portal tracts contained a bile duct, whereas the treated animals had an average of 53% of portal tracts with ducts, similar to term newborn baboons. The reason for this is not readily apparent, but it is possible that the stress of intensive care and multiple factors associated with development of cholestatic liver disease might also stimulate bile duct development as a means to promote bile flow and alleviate cholestasis.
In addition to the changes in acinar bile ducts, ductular proliferation was noted in 33% of the treated animals but none of the gestational controls. Some publications refer to this change incorrectly as “ductal proliferation”. In hepatopathology, the acinar or interlobular bile ducts that run parallel to hepatic artery branches in the smallest portal tracts are distinguished from the ductules, which are the smallest branches of the biliary tree. The ductules, which form the connections between bile canaliculi and the bile ducts that drain the liver, are normally quite inconspicuous, but in many pathologic conditions, there is a ductular reaction characterized by the appearance of ductules that form from proliferation of hepatic progenitor cells or less often from pre-existing cholangiocytes or hepatocytes.(29) Although not specific for this situation, ductular reactions are frequently present in both acute and chronic cholestatic diseases.
Cholestasis, characterized by canalicular bile plugs, was present in 31% of treated animals but in none of the gestational controls. This is the hallmark of TPN associated liver disease in premature human infants, and it is present in most autopsy cases. The relatively lower incidence of bile stasis compared to humans probably indicates that these animals were earlier in the course of the cholestatic liver disease. None were noted to be grossly jaundiced, and since liver disease was not the focus of their protocols, systematic liver tests had not been performed to detect early disease.
In the blinded review some ducts in gestational control animals were variably surrounded by densely cellular fibrous connective tissue. It should be emphasized that in controls the fibrosis was most always mild (1+) and usually was seen around larger portal tracts, indicating that it was not a truly pathologic change. By contrast, among the treated animals 52% had moderate (2+) portal fibrosis, indicating possible progressive liver disease. No animal had bridging fibrosis or cirrhosis, but this has been described in a few human infants on long term parenteral nutrition, so it seems likely that these animals, treated for only 21 to 28 days, were in the early stages of the same process.
Our study has a number of limitations. Mainly, there are important clinical confounders in the model such as degree of respiratory illness, cardiac function, and intestinal dysmaturity/feeding tolerance that might have affected liver histopathology. In addition, our subjects represent a combined cohort drawn from several different treatment protocols. While all treatments were common to human NICU infants (eg early noninvasive ventilation), the numbers are small, and the possibility of protocol-related effects is significant. Nevertheless we feel that these preliminary histologic findings, similar to those seen in human preterm infants, suggest that this may be a useful model for hypothesis-driven studies of liver disease in premature infants.
In conclusion, the baboon model of prematurity and intensive care has been useful in studying the natural history and pathophysiology of diseases similar to those of ELBW preterm infants affecting lungs, kidneys, brain and other organs(30–34). Given the demonstrated ability to care for these infant baboons over longer time periods in a fashion similar to human neonatal intensive care, our findings indicate that the model may also be very useful in investigations into the causes and natural history of parenteral nutrition associated liver disease in this population.
Supplementary Material
Acknowledgments
Source of Funding: This work was supported in part by National Institutes of Health BPD Resource Center Grant HL526
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
Conflicts of Interest: No conflicts are declared for any author.
Disclaimer:
The views expressed in this publication are those of the authors and do not necessarily reflect the official policy or position of the Department of the Air Force, Department of Defense or the US government.
References
- 1.Beale EF, Nelson RM, Bucciarelli RL, Donnelly WH, Eitzman DV. Intrahepatic cholestasis associated with parenteral nutrition in premature infants. Pediatrics. 1979;64:342–347. [PubMed] [Google Scholar]
- 2.Stormon MO, Dorney SF, Kamath KR, O'Loughlin EV, Gaskin KJ. The changing pattern of diagnosis of infantile cholestasis. JPaediatrChild Health. 2001;37:47–50. doi: 10.1046/j.1440-1754.2001.00613.x. [DOI] [PubMed] [Google Scholar]
- 3.Christensen RD, Henry E, Wiedmeier SE, Burnett J, Lambert DK. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. JPerinatol. 2007;27:284–290. doi: 10.1038/sj.jp.7211686. [DOI] [PubMed] [Google Scholar]
- 4.Robinson DT, Ehrenkranz RA. Parenteral nutrition-associated cholestasis in small for gestational age infants. JPediatr. 2008;152:59–62. doi: 10.1016/j.jpeds.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Steinbach M, Clark RH, Kelleher AS, et al. Demographic and nutritional factors associated with prolonged cholestatic jaundice in the premature infant. JPerinatol. 2008;28:129–135. doi: 10.1038/sj.jp.7211889. [DOI] [PubMed] [Google Scholar]
- 6.Venigalla S, Gourley GR. Neonatal cholestasis. SeminPerinatol. 2004;28:348–355. doi: 10.1053/j.semperi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Buchman A. Total parenteral nutrition-associated liver disease. JPEN JParenterEnteral Nutr. 2002;26:S43–S48. doi: 10.1177/014860710202600512. [DOI] [PubMed] [Google Scholar]
- 8.Guglielmi FW, Regano N, Mazzuoli S, et al. Cholestasis induced by total parenteral nutrition. ClinLiver Dis. 2008;12:97–110. doi: 10.1016/j.cld.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Kubota A, Yonekura T, Hoki M, et al. Total parenteral nutrition-associated intrahepatic cholestasis in infants: 25 years' experience. JPediatrSurg. 2000;35:1049–1051. doi: 10.1053/jpsu.2000.7769. [DOI] [PubMed] [Google Scholar]
- 10.Stewart SM, Uauy R, Kennard BD, Waller DA, Benser M, Andrews WS. Mental development and growth in children with chronic liver disease of early and late onset. Pediatrics. 1988;82:167–172. [PubMed] [Google Scholar]
- 11.Cohen C, Olsen MM. Pediatric total parenteral nutrition. Liver histopathology. ArchPatholLab Med. 1981;105:152–156. [PubMed] [Google Scholar]
- 12.Dahms BB, Halpin TC., Jr Serial liver biopsies in parenteral nutrition-associated cholestasis of early infancy. Gastroenterology. 1981;81:136–144. [PubMed] [Google Scholar]
- 13.Mullick FG, Moran CA, Ishak KG. Total parenteral nutrition: a histopathologic analysis of the liver changes in 20 children. ModPathol. 1994;7:190–194. [PubMed] [Google Scholar]
- 14.Naini BV, Lassman CR. Total parenteral nutrition therapy and liver injury: a histopathologic study with clinical correlation. Human pathology. 2012;43:826–833. doi: 10.1016/j.humpath.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Postuma R, Trevenen CL. Liver disease in infants receiving total parenteral nutrition. Pediatrics. 1979;63:110–115. [PubMed] [Google Scholar]
- 16.Zambrano E, El-Hennawy M, Ehrenkranz RA, Zelterman D, Reyes-Mugica M. Total parenteral nutrition induced liver pathology: an autopsy series of 24 newborn cases. PediatrDevPathol. 2004;7:425–432. doi: 10.1007/s10024-001-0154-7. [DOI] [PubMed] [Google Scholar]
- 17.Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med. 1999;160:1333–1346. doi: 10.1164/ajrccm.160.4.9810071. [DOI] [PubMed] [Google Scholar]
- 18.McCurnin DC, Yoder BA, Coalson J, et al. Effect of ductus ligation on cardiopulmonary function in premature baboons. Am J Respir Crit Care Med. 2005;172:1569–1574. doi: 10.1164/rccm.200502-230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoder BA, Siler-Khodr T, Winter VT, Coalson JJ. High-frequency oscillatory ventilation: effects on lung function, mechanics, and airway cytokines in the immature baboon model for neonatal chronic lung disease. Am J Respir Crit Care Med. 2000;162:1867–1876. doi: 10.1164/ajrccm.162.5.9912145. [DOI] [PubMed] [Google Scholar]
- 20.Thomson MA, Yoder BA, Winter VT, Giavedoni L, Chang LY, Coalson JJ. Delayed extubation to nasal continuous positive airway pressure in the immature baboon model of bronchopulmonary dysplasia: lung clinical and pathological findings. Pediatrics. 2006;118:2038–2050. doi: 10.1542/peds.2006-0622. [DOI] [PubMed] [Google Scholar]
- 21.Thomson MA, Yoder BA, Winter VT, et al. Treatment of immature baboons for 28 days with early nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2004;169:1054–1062. doi: 10.1164/rccm.200309-1276OC. [DOI] [PubMed] [Google Scholar]
- 22.Miranda RN, Omurtag K, Castellani WJ, De las Casas LE, Quintanilla NM, Kaabipour E. Myelopoiesis in the liver of stillborns with evidence of intrauterine infection. Arch Pathol Lab Med. 2006;130:1786–1791. doi: 10.5858/2006-130-1786-MITLOS. [DOI] [PubMed] [Google Scholar]
- 23.Kahn E, Markowitz J, Aiges H, Daum F. Human ontogeny of the bile duct to portal space ratio. Hepatology. 1989;10:21–23. doi: 10.1002/hep.1840100105. [DOI] [PubMed] [Google Scholar]
- 24.Van Eyken P, Sciot R, Callea F, Van der SK, Moerman P, Desmet VJ. The development of the intrahepatic bile ducts in man: a keratin-immunohistochemical study. Hepatology. 1988;8:1586–1595. doi: 10.1002/hep.1840080619. [DOI] [PubMed] [Google Scholar]
- 25.Kelly DA. Preventing parenteral nutrition liver disease. Early HumDev. 2010;86:683–687. doi: 10.1016/j.earlhumdev.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Jain AK, Stoll B, Burrin DG, Holst JJ, Moore DD. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs. American journal of physiology Gastrointestinal and liver physiology. 2012;302:G218–G224. doi: 10.1152/ajpgi.00280.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoll B, Horst DA, Cui L, et al. Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. The Journal of nutrition. 2010;140:2193–2200. doi: 10.3945/jn.110.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Khaoustov VI, Krishnan B, et al. Total parenteral nutrition induces liver steatosis and apoptosis in neonatal piglets. The Journal of nutrition. 2006;136:2547–2552. doi: 10.1093/jn/136.10.2547. [DOI] [PubMed] [Google Scholar]
- 29.Roskams TA, Theise ND, Balabaud C, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 30.Blanco CL, Liang H, Joya-Galeana J, DeFronzo RA, McCurnin D, Musi N. The ontogeny of insulin signaling in the preterm baboon model. Endocrinology. 2010;151:1990–1997. doi: 10.1210/en.2009-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffith JL, Shimony JS, Cousins SA, et al. MR imaging correlates of white-matter pathology in a preterm baboon model. Pediatr Res. 2012;71:185–191. doi: 10.1038/pr.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gubhaju L, Sutherland MR, Yoder BA, Zulli A, Bertram JF, Black MJ. Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. American journal of physiology Renal physiology. 2009;297:F1668–F1677. doi: 10.1152/ajprenal.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quinn AR, Blanco CL, Perego C, et al. The ontogeny of the endocrine pancreas in the fetal/newborn baboon. The Journal of endocrinology. 2012;214:289–299. doi: 10.1530/JOE-12-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waleh N, McCurnin DC, Yoder BA, Shaul PW, Clyman RI. Patent ductus arteriosus ligation alters pulmonary gene expression in preterm baboons. Pediatr Res. 2011;69:212–216. doi: 10.1203/PDR.0b013e3182084f8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.