Abstract
Context
The serotonin transporter (SLC6A4) has been associated with several stress-related syndromes including posttraumatic stress disorder (PTSD). The ability to detect meaningful associations is largely dependent on reliable measures of preexisting trauma.
Objective
To study the association of genetic variants within SLC6A4 with acute and posttraumatic stress symptoms in a civilian cohort with known levels of preexisting trauma and PTSD symptoms collected prior to a shared index traumatic event.
Design
Ongoing longitudinal study.
Setting
On February 14, 2008, a lone gunman shot multiple people on the campus of Northern Illinois University in DeKalb, Illinois, killing 5 and wounding 21. As part of an ongoing longitudinal study on that campus, a cohort of female undergraduate students, interviewed prior to the shooting, completed follow-up trauma-related measures including PTSD symptom severity (follow-up survey was launched 17 days postshooting; n=691). To obtain DNA, salivary samples were collected from a subset of the original study population based on willingness to participate (n=276).
Participants
Two hundred four undergraduate women.
Main Outcome Measures
SLC6A4 polymorphisms STin2, 5-HTTLPR, and rs25531 were genotyped in 235 individuals.
Results
We found that although the STin2 variant and 5-HTTLPR alone did not associate with increased PTSD symptoms, rs25531 and the 5-HTTLPRmultimarker genotype (combined 5-HTTLPR and rs25531) were associated with significantly increased acute stress disorder symptoms at 2 to 4 weeks postshooting (n = 161; P<.05). This association remained significant when controlling for race and for level of shooting exposure (n = 123; P<.007). The association was most robust with the 5-HTTLPR multimarker genotype and avoidance symptoms (P=.003).
Conclusion
These data suggest that differential function of the serotonin transporter may mediate differential response to a severe trauma. When examined in a relatively homogenous sample with shared trauma and known prior levels of child and adult trauma, the 5-HTTLPR multimarker genotype may serve as a useful predictor of risk for PTSD-related symptoms in the weeks and months following the trauma.
In recent years, posttraumatic stress disorder (PTSD) has gained increasing recognition as a major public health issue. It is characterized in the DSM-IV by the appearance of 3 symptom clusters following an acutely traumatic event: reexperiencing (flashbacks and nightmares), avoidance of trauma-related stimuli, and hyperarousal. While PTSD has always been associated with combat veterans, it is generally accepted that any civilian trauma, including sexual or physical assault, childhood abuse, motor vehicle accident, or other type of disaster, can also lead to the disorder in at-risk individuals. The life-time prevalence of PTSD has been estimated to be 6.8% among adult Americans.1–3 Only a minority of people exposed to traumatic or life-threatening events will go on to develop PTSD. Research has shown risk factors for the development of PTSD to include severity of perceived life threat during trauma, prior trauma, family history of psychopathology, peritraumatic emotional responses, and peritraumatic dissociation.4,5
The heritability of PTSD was first described among veterans of the Vietnam War through twin studies, which suggested that genetic factors explain approximately 30% of the variance in PTSD symptoms.6 However, while the heritability of PTSD has been accepted for some time, molecular genetic association studies of PTSD have been limited. In this respect, published molecular genetics research into PTSD lags behind similar research on depression, bipolar disorder, and schizophrenia. Extant research has identified a small number of possible specific loci based on association studies, but findings to date have not been definitive.7,8 Because PTSD is a psychiatric disorder that is dependent on exposure to an environmental pathogen, genetic association studies examining genetic risk for PTSD are significantly more complex as compared with other forms of disease; this complexity has resulted in methodological inconsistencies in the current literature. For diseases involving environmental pathogens, gene × environment (G × E) studies have been a topic of increasing interest. A G × E interaction occurs when disease risk is dependent on the differential susceptibility of genotype to environmental pathogens. Gene × environment interactions are seen as a promising tool for investigating the 2-fold etiology of PTSD because the environmental pathogen (acute trauma) is known, is required for the diagnosis, and has the potential to be measured.9
Of the roughly 30 molecular genetics studies of PTSD conducted so far, 18 have focused on dopaminergic and serotonergic systems.7 Half of these studies have investigated the locus SLC6A4 coding for the serotonin transporter protein (5HTT). This protein functions to transport serotonin from the synaptic cleft back into presynaptic neurons and thus is a regulator of serotonergic neural activity.10 Of these 9 studies that investigated SLC6A4, 8 found significant associations between PTSD symptoms and polymorphisms within the locus.7
The long/short (l/s) polymorphism, 5-HTTLPR, is the most commonly described polymorphism in the psychiatric genetics literature. It is known to affect expression of the serotonin transporter protein, with the short (s) version thought to lead to decreased 5-HTTLPR expression and a higher risk for psychopathology.11,12 Additionally, studies have shown that the single-nucleotide polymorphism rs25531 may further modulate 5-HTTLPR expression, with lG alleles being equivalent to s alleles in expression.13,14 Together these 2 polymorphisms form a multimarker genotype (lA/lG/sA/sG) commonly used in research involving this locus.15 In this study, we performed analyses using this genotype, as well as the 5-HTTLPR and rs25531 polymorphisms alone. Another polymorphism, a variable number of tandem repeats in the second intron of SLC6A4 known as STin2, is less frequently described in the literature but has been shown to modulate expression in vitro along with 5-HTTLPR.16
In the present study, SLC6A4 genotype and its G × E interactions were examined to determine their effect on the severity of self-reported PTSD symptoms following a traumatic event in which we had collected prospective psychological data. Our cohort of 204 college-aged women is an ideal population in which to study the G × E interaction with PTSD because all individuals experienced a similar traumatic event: being exposed to a school shooting on the campus of Northern Illinois University.17 Importantly, the mass shooting can be considered a “fateful” event (in which the trauma exposure is outside of the victims’ control), which mitigates concerns about confounds with gene-trauma correlation.9 Self-report measures from the students allowed us to categorize their level of exposure to the incident, ranging from simply being on campus to witnessing the gunman firsthand. The examination of a population for whom we had information on prior trauma and PTSD symptoms before the trauma occurred allowed for a unique prospective G × E study. Other prospective studies, examining the interaction of acute trauma and SLC6A4 genotype on development of PTSD, have depended on retrospective data for assessment of preexisting trauma exposure.18,19 Risk factors such as child abuse, familial psychopathology, and preexisting psychopathology have significant impact on the development of PTSD.20 A challenge to all psychiatric genetic research involving PTSD is the ability to accurately capture the level of pretrauma risk, such that the genetic contribution to the development of PTSD can be more clearly assessed. By removing previous PTSD symptoms associated with pretrauma, we create a more controlled study design that allows us to focus, primarily, on the impact of the shooting event, level of exposure, and SLC6A4 genotype on the development of PTSD symptoms.
METHODS
STUDY PARTICIPANTS
Participants were recruited from an ongoing longitudinal study following the mass shooting at Northern Illinois University on February 14, 2008.17 At the time of the shooting, 1045 undergraduate women had completed time 1 of a longitudinal study examining predictors of sexual revictimization (participants were not selected based on victimization history and needed only to be 18 years of age and fluent in English to participate). Of the original 1045, 812 agreed to be reinterviewed and were confirmed as current Northern Illinois University students at the time of the mass shooting. Seventeen days after the mass shooting, an online follow-up survey was launched and was completed by 85% of those invited (n = 691). Data collected from these surveys were designated as “time 2” measures in the current study. The time between the collection of preshooting, time 1 measures, and time 2 measures ranged from 2 to 78 weeks, averaging at 31.5 weeks between each time (σ = 22.1). The average time between the shooting and time 2 was 3.2 weeks (σ = 2.2). Data were collected again through online surveys 34 weeks, on average, after the mass shooting. This is referred to as “time 3” in the current study. At time 5 (fall 2009), participants were invited to submit a saliva sample for genetic analysis. Saliva samples were collected from 276 of the 586 women who completed time 5 (47%) using Oragene saliva collection tubes (DNA Genotek Inc, Ottawa, Ontario, Canada), and DNA was successfully extracted from 235 individuals. This sample was all female and between the ages of 18 and 45 years (time 2, mean age, 20.1 years). Race was self-reported as 77.5% white (n=158) and 13.7% black or African American (n=28). Other races accounted for 8.9% (n= 18) (Table 1).
Table 1.
Demographic of All-Female Study Populationa
| No. (%) |
||||
|---|---|---|---|---|
| All Participants |
Participants With Nominal Increase in PTSD Symptom Score, <18 |
Participants With Significant Increase in PTSD Symptom Score, ≥18 |
Test Statistics |
|
| Sample size | 204 | 138 | 66 | |
| Age,y, mean (SD)b | 20.1 (2.6) | 20.0 (2.0) | 20.3 (3.7) | P = .38 |
| Race | ||||
| White | 158 (77.5) | 105 (76.1) | 53 (80.3) | χ2 = 4.76; |
| Black | 28 (13.7) | 21 (15.2) | 7 (10.6) | P = .58 |
| Hispanic | 4 (2.0) | 2 (1.4) | 2 (3.0) | |
| Asian | 6 (2.9) | 5 (3.6) | 1 (1.5) | |
| Assyrian | 1 (0.5) | 0 | 1 (1.5) | |
| More than 1 race | 5 (2.5) | 4 (2.9) | 1 (1.5) | |
| Unknown or not reported | 2 (1.0) | 1 (0.7) | 1 (1.5) | |
| Year in collegeb | ||||
| Freshman | 101 (49.5) | 72 (52.2) | 28 (43.1) | χ2 = 4.25; |
| Sophomore | 73 (35.8) | 48 (34.8) | 25 (38.5) | P = .64 |
| Junior | 20 (9.8) | 11 (8.0) | 9 (13.8) | |
| Senior | 7 (3.4) | 4 (2.9) | 3 (4.6) | |
| Left before degree | 1 (0.5) | 1 (0.7) | 0 | |
| Graduated | 1 (0.5) | 1 (0.7) | 0 | |
| In graduate school | 1 (0.5) | 1 (0.7) | 0 | |
| No. of preshooting traumatic events, mean (SD)c | 2.3 (2.04) | 2.2 (2) | 2.6 (2.2) | P= .18 |
| Child abuse experiencedd | ||||
| Child psychological abuse | 109 (53.40) | 70 (50.7) | 39 (59.1) | χ2 = 1.26; P= .26 |
| Child physical abuse | 21 (10.30) | 15 (10.9) | 6 (9.1) | χ2 = 0.15; P= .70 |
| Child sexual abuse | 43 (21.10) | 26 (18.8) | 17 (25.8) | χ2 = 1.28; P= .26 |
Abbreviation: PTSD, posttraumatic stress disorder.
Demographic information for study population (n = 204). Study population was an all-female, predominantly white, college-aged cohort with similar trauma history.
Measure was taken at the postshooting interview.
Excludes traumatic events from child abuse before the age of 15 years.
The number represents the number of subjects ever abused; the percentage is the number of abused vs the total.
DNA EXTRACTION
Samples were mailed from Northern Illinois University to Emory University where DNA was extracted using the Agencourt DNAdvance kit (Beckman Coulter Inc, Brea, California). A DNA concentration that was less than 10 ng/µL was not used. In some cases, a second saliva sample was obtained and used for DNA extraction to replace one with a low concentration.
GENOTYPING OF SLC6A4
Genotypes were successfully obtained for 235 individuals. Individuals were genotyped using duplex polymerase chain reaction (PCR) with 2 sets of previously published primers from Wendland and colleagues14,21: 1 set for the 5-HTTLPR locus (5′-TCCTCCGCTTTGGCGCCTCTTCC-3′ and 5′-TGGGGGTTG-CAGGGGAGATCCTG-3′) and 1 set for the intron 2 variable number of tandem repeats (5′-GGGCAATGTCTGGCGCTTC-CCCTACATA-3′ and 5′-TTCTGGCCTCTCAAGAGGACCTA-CAGC-3′). All primers were obtained from Integrated DNA Technologies Inc (Coralville, Iowa). 5-HTTLPR PCR resulted in amplicons of 469 base pairs (bp) for the s allele and 512 bp for the l allele; for the intron 2 variable number of tandem repeats, PCR resulted in amplicons of 250 bp for STin2.9, 267 bp for STin.10, and 300 bp for STin2.12. Genotypes for the 5-HTTLPR and intron 2 variable number of tandem repeats loci were determined by gel electrophoresis. The PCR products were then digested using MspI (New England Biolabs Inc, Ipswich, Massachusetts), resulting in fragments of 402 + 67 bp for sG and 402 + 10 bp for lG. Samples were run by gel electrophoresis again to determine genotype at rs25531 (A/G). Unable to detect the 67- and 10-bp products, we discerned sG from lG by looking for the absence of the original PCR product for either l or s. Of all samples genotyped, 27% (n=75) were chosen at random to run in duplicate (2.6% discordance).
This multimarker genotype is reported to have an effect on 5-HTTLPR messenger RNA with the order of expression as follows: s/s, lG/lG, s/lG, lA/lG, s/lA, lA/lA.22 For our analysis, we divided these genotypes into 2 groups based on their expression profile and compared s/s, lG/lG, and s/lG against lA/lG, s/lA, and lA/lA. Since the s and lG alleles are reported to have similarly low expression, this model compares low-expression homozygotes with high-expression heterozygotes and homozygotes.
PHENOTYPE MEASURES
Demographic Information
This study uses demographic data previously described by Stephenson and colleagues.17 All individuals were interviewed on at least 3 occasions, prior to the shooting (time 1), 2 to 13 weeks postshooting (mean=3.2 weeks, time 2), and 8 to 12 months following time 2 (mean=8.4 months, time 3). To measure prior trauma, we used the Traumatic Life Events Questionnaire23 and the Distressing Event Questionnaire (DEQ)24 administered at time 1. To measure postshooting symptoms, we categorized exposure to the mass shooting and examined the DEQ scores at times 2 and 3.
Traumatic Life Events Questionnaire
Previous findings suggest that prior life trauma can strongly impact the development of psychological outcomes such as depression, anxiety, or PTSD.25 At time 1, participants reported on history of traumatic life experiences. These measures included an assessment of child abuse including physical, psychological, and sexual abuse. The number of non–child-abuse-related traumas was also recorded. To measure all previous trauma exposure, we used the Traumatic Life Events Questionnaire.23 Because childhood sexual abuse and physical abuse are separate line items in the questionnaire, we were able to separate them from other types of trauma to examine them separately.
Social Support Questionnaire
Social support data were collected using the Multidimensional Scale of Perceived Social Support to assess levels of support from family, friends, and significant other (12 questions; each question scoring 1–7 based on a 7-point rating scale ranging from very strongly disagree [1] to very strongly agree [7]).26 Assessed for internal reliability among multiple subgroups, Zimet et al27 report Cronbach coefficient a scores from .81 to .90 for the family subscale, from .90 to .94 for the friends subscale, and from .83 to .98 for the significant other subscale. In the current study, this questionnaire was given to study participants at time 2. While these questions allowed us to assess general support, they did not question the extent and satisfaction of support available specifically after the mass shooting. Two additional survey questions were included to address this. Scores for the latter 2 questions and the Multidimensional Scale of Perceived Social Support were positively and significantly correlated (r=0.432). These data did not account for support such as professional counseling.
Exposure to Mass Shooting
Participants completed a 12-item measure of exposure, modified from the Littleton et al28 Virginia Tech shooting exposure measure. An exposure variable was created by summing across yes/no items assessing participants’ personally experienced exposure to aspects of the shooting (eg, on campus, heard gunfire, saw individuals who had been wounded or killed, knew anyone wounded, in building placed on lockdown). None of the subjects in this study reported being fired on by the gunman.
PTSD Symptom Measure
The DEQ was used to assess PTSD at both times. Using a 5-point response scale, the DEQ assesses symptoms of PTSD experienced in the past 30 days as specified in the DSM-IV-TR.29 For time 2, participants were instructed to answer the DEQ with respect to the shooting event. The DEQ has demonstrated very good psychometric properties24 and contains indices for specific symptoms: hyperarousal, reexperiencing, and avoidance. The internal consistencies (Cronbach α) of these scales at time 2 in the previously reported larger sample17 from which these subjects were drawn were .92 for the total symptom subscale, .83 for the hyperarousal symptom subscale, .88 for the reexperiencing symptom subscale, and .82 for the avoidance symptom subscale.
An increase of greater than 18 points in DEQ score from time 1 to time 2 was considered significant given that a score of 18 or higher on the DEQ classifies as PTSD among women.24 Based on the DSM-IV diagnosis criteria for PTSD, an individual must have symptoms for 30 days or more.29 However, a majority of our sample population had their first postshooting interview prior to this 1-month cutoff (78%). This limited the power we had to detect factors that could significantly correlate with clinical PTSD. Hence, results presented for those individuals who were interviewed 2 to 4 weeks postshooting at time 2 will refer to acute stress disorder, while results for those individuals who were interviewed 1 month or later postshooting will refer to PTSD.
STATISTICAL ANALYSIS
SPSS version 17.0 (IBM SPSS, Chicago, Illinois) was used to perform all statistical analyses. Individuals who were interviewed 2 to 4 weeks postshooting and those who were interviewed greater than 1 month postshooting were analyzed separately. Our dependent variable for all analyses, PTSD symptoms, was measured as the difference in DEQ score between time 2 and time 1 (Δ DEQ). Using univariate analysis of variance, we tested for genetic main effects at all 3 SLC6A4 loci (STin2, 5-HT-TLPR, and rs25531) individually for the heterozygotes and homozygotes and as multimarker genotypes. In our final model, we controlled for shooting exposure by taking into account the number of high-exposure events experienced. A high-exposure event is defined as any 1 of the 5 events significantly related to the change in PTSD symptom score from baseline to postshooting (statistical significance shown in Figure 1A) in addition to the following: (1) saw police or other personnel surrounding the buildings, (2) saw individuals who had been wounded or killed, (3) know someone who was killed in the shooting, and (4) know anyone who was wounded in the shooting. We created a dichotomous variable to represent the degree of high exposure by calculating the sum of positive responses to all of the high-exposure events. Those who experienced 0 to 2 high-exposure events were classified as having a low degree of exposure and those who experienced 3 to 7 high-exposure events were classified as having a high degree of exposure. Additionally, because of allele frequency differences between races, the analyses were performed for all participants as well as for white participants (the most common self-identified race) separately. We then ran this analysis using the difference in DEQ score between time 2 and time 1 for the subcategories of PTSD (hyperarousal, reexperiencing, and avoidance) as our dependant variable. The data were considered statistically significant if the P value passed Bonferroni correction for multiple testing (6 genotype comparisons) at P<.008.
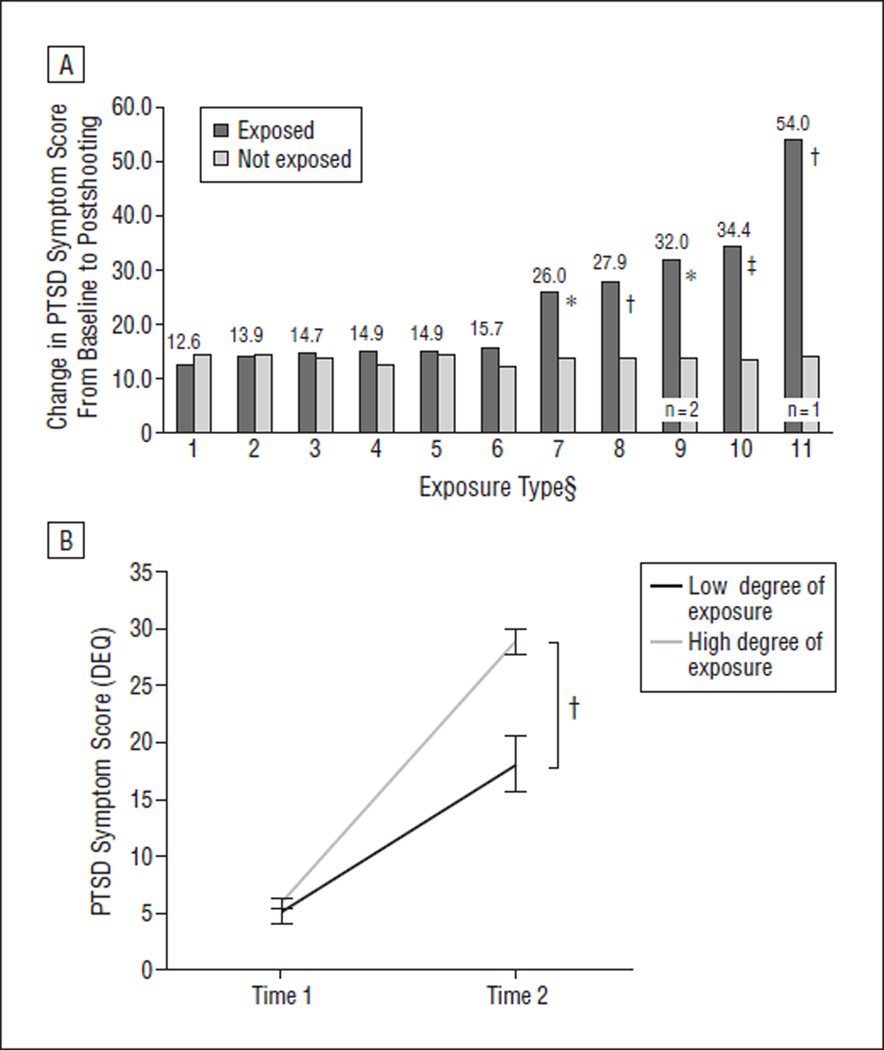
Figure 1.
Effects of mass shooting on posttraumatic stress disorder (PTSD) symptoms in a prospectively assessed sample. A, Comparison of PTSD symptom severity 2 to 4 weeks after shooting by type of exposure to shooting. Each number on the x-axis represents a specific yes or no question about each individual’s exposure to the shooting: 1 =knew someone killed in shooting, 2 = knew someone wounded in shooting, 3=were in a building placed on lockdown, 4=on campus during shooting, 5=saw individuals wounded or killed, 6=saw police or other personnel surrounding the building, 7=in Cole Hall during shooting, 8=heard sound of gunfire, 9=saw gunman fire on someone, 10=saw gunman during shooting, 11=were hurt in shooting. Part A includes all individuals who had the second interview 2 to 4 weeks postshooting. B, Severity of PTSD symptoms 2 to 4 weeks after shooting by degree of exposure. This graph shows the mean PTSD symptom score (Distressing Event Questionnaire [DEQ]) at time 1 and time 2 (± standard error) A sum ranging from 0 to 2 for positive responses to high-exposure events is classified as a low degree of exposure. A sum ranging from 3 to 7 for positive responses to high-exposure events is classified as a high degree of exposure. *P< .05. †P< .005. ‡P< .001 §Sample sizes less than 5 are noted.
To confirm that the analytical P values were not dependent on distributional assumptions, we computed empirical P values via permutation tests. In each of 10 000 permutations of the data set, we reperformed the data analysis after randomly reassigning values of the dependent variable. The empirical P value was then computed as the proportion of permutations for which we observed a genetic association of greater or equal strength to that originally observed.
RESULTS
DEMOGRAPHICS
The primary study sample (n = 691) that was initially interviewed at time 2 was described in detail by Stephenson and colleagues.17 Of the total study sample, 41% (n = 280) had a total score of 18 or more on the DEQ at time 2, indicating that a large portion of the sample had clinical symptoms of PTSD.24 Complete DNA extractions were obtained for 276 participants. We were able to obtain useable DNA for 269 of these participants (based on a qualifying DNA concentration > 10 ng/µL). Only participants with confident genotype calls for all 3 loci were included. We further limited the study set to 235 participants who had complete phenotype measures. Participants who presented with PTSD symptoms at time 1, as defined by a DEQ score greater than or equal to 18, were also excluded (n=31), to only include subjects who did not have clinically significant PTSD prior to the shooting. This left us with a final cohort of 204 individuals who were used for current analyses (Table 1). All participants were female with 77.5% (n=158) self-identifying race as white and 13.7% (n = 28) self-identifying as black. The mean age for this cohort was 20.1 years. About half of the students were freshman at time 2 (49.5%), while the other half were predominantly sophomores and juniors (35.8% and 9.8%, respectively). Comparisons of the included participants (n=204), as compared with the other participants (n=487) on key variables, indicated that included participants reported slightly higher PTSD symptoms at time 2 and were more likely to be white but did not differ in age or the shooting exposure variable. Notably, for our study sample, 20% of those who presented with acute stress disorder at 2 to 4 weeks went on to develop PTSD at time 3.
INCIDENCE OF PRESHOOTING TRAUMA
Of the 204 individuals included in the study, 74.5% (n=152) reported no child abuse before the age of 18 years. A little more than 1% of the individuals reported experiencing 2 or more abuse types that involved fear, helplessness, or horror, while 10% experienced sexual abuse involving fear, helplessness, or horror before the age of 15 years, including abuse by a peer. Less than 5% had experienced physical abuse more than once a year. Comparing the number of individuals who had ever experienced child abuse in the form of sexual abuse (before the age of 18 years), physical abuse (14 years or younger), or psychological abuse (14 years or younger), there was no statistically significant difference between the group that developed PTSD symptoms following the shooting and the group that did not (Table 1) (P>.10). The number of preshooting traumatic events as measured by the Traumatic Life Events Questionnaire was not significantly associated with more severe PTSD symptoms postshooting, with the low-symptom group experiencing an average of 2.2 traumatic events (σ = 2.0) and the high-symptom group experiencing an average of 2.6 traumatic events (σ = 2.0).
SOCIAL SUPPORT
Social support measures (Multidimensional Scale of Perceived Social Support and additional items examining the specific availability and perception of support related to the shooting) were performed to examine if support predicted PTSD symptoms postshooting. Analysis of our data revealed that the majority of individuals who did not present with acute stress disorder (55.7%) or PTSD (56.2%) following the shooting claimed to be extremely satisfied with the extent of support available to them. The majority of those who did have acute stress disorder (54.4%) or PTSD (52.6%) felt the same. Across different analyses of social support relative to PTSD, we found no significant relationship.
THE EFFECT OF SHOOTING EXPOSURE ON PTSD SYMPTOMS
Of the 12 questions the students were asked regarding the shooting, we found that positive responses to the following 5 were significantly associated with outcome for PTSD symptom severity at 2 to 4 weeks after the shooting: Were you in Cole Hall (where the mass shooting took place) during the shooting? (P<.05), Did you hear the sound of gunfire? (P<.005), Did you see the gunman fire on someone? (P <.05), Did you see the gunman during the shooting? (P<.001), and Were you hurt in the shooting? (P<.005) (Figure 1A). We found that the degree of exposure, calculated as the sum of high-exposure events experienced, had a positive additive effect on change in DEQ score from time 1 to time 2 (Figure 1B) (P<.005). This same relationship extended to time 3 (P<.005) (data not shown).
MAIN EFFECTS OF SLC6A4 GENOTYPES ON SHOOTING-RELATED PTSD SYMPTOMS
Genotypes for all 3 loci examined were in Hardy-Weinberg equilibrium (for white participants, P>.05) (Table 2). 5-HTTLPR allele frequencies matched previously reported findings for European or European Americans.30–32 The STin2 frequencies closely matched those reported in Europeans.14,18,26 The 5-HTTLPR multimarker genotype frequencies matched well with the frequencies reported by Wendland and colleagues14 for a similar sample size of white Americans (our frequencies: sA=42.4%; sG=0.60%; lA=49.7%; lG=7.3%; Wendland et al: sA=43.2%; sG = 0.25%; lA=50.0%; lG = 6.5%).
Table 2.
Genotypes for SLC6A4a
| No. (%) |
||
|---|---|---|
| All Races | White Participants Only |
|
| Sample size | 204 | 158 |
| STin2 genotype | ||
| 12/12 | 75.0 (36.8) | 51.0 (32.3) |
| 12/10 | 98.0 (48.0) | 78.0 (49.4) |
| 12/9 | 5.0 (2.5) | 5.0 (3.2) |
| 10/10 | 26.0 (12.7) | 24.0 (15.2) |
| Test statistics | HWE: χ2 = 1.07; P= .30 | |
| 5-HTTLPR genotype | ||
| l / l | 65.0 (31.9) | 48.0 (30.4) |
| l /s | 106.0 (52.0) | 84.0 (53.2) |
| s/s | 33.0 (16.2) | 26.0 (16.5) |
| Test statistics | HWE: χ2 = 1.12; P= .29 | |
| rs25531 Genotype | ||
| AA | 168.0 (82.4) | 132.0 (83.5) |
| AG | 31.0 (15.2) | 23.0 (12.8) |
| GG | 3.0 (1.5) | 1.0 (0.6) |
| Test statistics | HWE: χ2 = 3.03 × 10−6; P < .99 | |
| 5-HTTLPR multimarker genotype | ||
| s/s | 33.0 (16.2) | 26.0 (16.5) |
| lG/lG | 2.0 (1.0) | 0.0 |
| s/lG | 17.0 (8.3) | 13.0 (8.2) |
| lA/lG | 14.0 (6.9) | 10.0 (6.3) |
| s/lA | 89.0 (43.6) | 71.0 (44.9) |
| lA/lA | 49.0 (24.0) | 38.0 (24.1) |
Abbreviation: HWE, Hardy-Weinberg equilibrium.
Genotype frequencies for study population at 3 SLC6A4loci. 5-HTTLPR multimarker genotype refers to the genotype composed of 5-HTTLPR and rs25531. The χ2 test of HWE and associated P values are shown for each individual locus for white participants only
We next examined the effect of serotonin transporter genotypes on the main effect of postshooting PTSD symptoms in this group that did not exhibit PTSD symptoms prior to the shooting. Genotypes were examined for all races together and for self-reported white participants only to control for possible population stratification. We did not observe main effects for STin2 or 5-HTTLPR alone on PTSD symptom score (Δ DEQ) (Table 3). However, both rs25531 and the 5-HTTLPR multimarker genotypes were significantly associated for all races combined (P = .01 and .03, respectively) (Table 3). rs25531 was significant for the postshooting PTSD symptoms when examined in both an additive allelic (P = .01) and in a dominant/recessive fashion (P=.003). When self-identifying white participants were examined alone, the association remained significant with a P value that survived Bonferroni correction for multiple testing for both rs25531 and the 5-HTTLPR multimarker genotype (P = .003 and P=.006, respectively).
Table 3.
Association Between Genotype and PTSD Symptom Scorea
| Postshooting P Value |
||||
|---|---|---|---|---|
| 2–4 wk |
>1 mo |
|||
| All Races | White Participants Only |
All Races | White Participants Only |
|
| Sample size | 161 | 123 | 43 | 35 |
| Stin2 genotype | ||||
| 12/12 vs 12/10 and 12/9 vs 10/10 | .81 | .90 | .80 | .55 |
| 5-HTTLPR genotype | ||||
| l/l vs l/s vs s/s | .31 | .24 | .99 | .83 |
| l/l and l/s vs s/s | .13 | .09 | .97 | .81 |
| rs25531 Genotype | ||||
| A/A vs A/G vs G/G | .01b | .004c | .65 | .499 |
| A/Avs A/G and G/G | .003c | .003c | .84 | .499 |
| 5-HTTLPR multimarker genotype | ||||
| s/s, lG/lG, and s/lG vs lA/lG, s/lA, and lA/lA | .03b | .006d | .61 | .40 |
Abbreviation: PTSD, posttraumatic stress disorder.
Main effect of genotypes on difference in Distressing Event Questionnaire score between times 1 and 2. The sample population was divided into 2 groups those who were interviewed 2 to 4 weeks postshooting and those who were interviewed more than 1 month postshooting. Because of potential population stratification, all races and white participants are analyzed separately.
P< .05.
P<.005
P < .01
G X E INTERACTION BETWEEN SLC6A4 GENOTYPE AND SHOOTING EXPOSURE
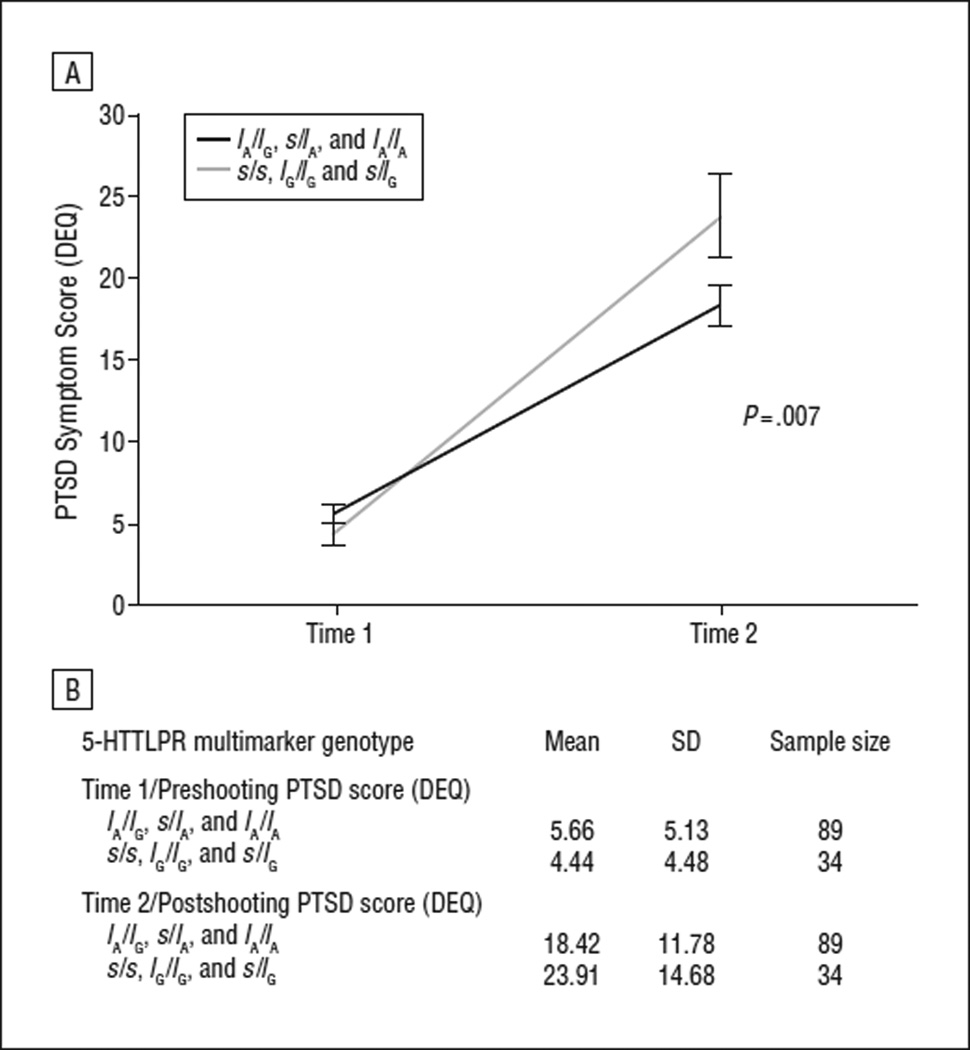
As described earlier, we observed a main effect for shooting exposure on DEQ score (Figure 1B). We next examined the relationship between the 5-HTTLPR multimarker genotype and PTSD symptom score while accounting for exposure to the shooting to ensure that our effect was not driven primarily by differences in exposure across genotypes. In this analysis, the relationship remained strong (P = .007) (Table 4), despite covariation for degree of shooting exposure. Subjects carrying the low-expressing s/s, lG/lG, and s/lG genotypes had significantly higher PTSD symptom scores postshooting (mean DEQ score = 23.91) compared with those with the higher-expressing lA/lG,s/lA, and lA/lA genotypes postshooting (mean DEQ score = 18.42) (Figure 2). Permutation tests confirmed that this result was not dependent on distributional assumptions (permutation P=.005). There was no correlation between genotype and shooting exposure (r=0.04; P=.68).
Table 4.
Association Between Genotype and PTSD Symptom Score Controlling for Shooting Exposurea
| Postshooting P Value White Participants Only |
||
|---|---|---|
| 2–4 wk | >1 mo | |
| Sample size | 123 | 35 |
| STin2 genotype | ||
| 12/12 vs 12/10 and 12/9 vs 10/10 | .93 | .36 |
| 5-HTTLPR genotype | ||
| l/l vs l/s vs s/s | .25 | .95 |
| l/land l/s vs s/s | .10 | .97 |
| rs25531 Genotype | ||
| A/A vs A/G vs G/G | .004b | .32 |
| A/A vs A/G and G/G | .004b | .32 |
| 5-HTTLPR multimarker genotype | ||
| s/s, lG/lG, and s/lG vs lA/lG, s/lA, and lA/lA | .007c | .55 |
Abbreviation: PTSD, posttraumatic stress disorder.
Results for univariate analysis of variance comparing the effect of all genotypes (for white participants only) on PTSD symptom score after controlling for the degree of shooting exposure. The sample population was divided into 2 groups: those who were interviewed 2 to 4 weeks postshooting and those who were interviewed more than 1 month postshooting
P< .005
P< .01
Figure 2.
The 5-HTTLPR multimarker genotype predicts posttraumatic stress disorder (PTSD) symptom severity 2 to 4 weeks posttrauma. The graph shows the mean differences (± standard error) in PTSD symptom severity before and after the shooting relative to genotype. The graph includes white participants only who had their second interview within 2 to 4 weeks after the shooting. The postshooting PTSD symptoms × genotype association (P=.007) accounts for degree of exposure to the shooting. Actual mean values for the Distressing Event Questionnaire (DEQ) scores at each time are given in the Table below the graph.
As mentioned earlier, we were also able to obtain measurements for the hyperarousal, reexperiencing, and avoidance subscales of PTSD symptoms. These differential symptom clusters of PTSD were analyzed individually to determine which types of symptoms were predominant in our genetic risk group. Reexperiencing symptoms were not significantly different. Although hyperarousal symptoms were statistically significant (P<.05), the greatest effect was seen for avoidance symptoms (P = .003) (Table 5).
Table 5.
Association Between Genotype and PTSD Symptom Subscale Score Controlling for Shooting Exposurea
| Postshooting P Value White Participants Only |
||
|---|---|---|
| 2–4 wk | >1 mo | |
| Sample size | 123 | 35 |
| 5-HTTLPR multimarker genotype | ||
| Hyperarousal | .03b | .27 |
| Reexperiencing | .10 | .16 |
| Avoidance | .003c | .21 |
Abbreviation: PTSD, posttraumatic stress disorder.
Results for univariate analysis of variance comparing the effect of the 5-HTTLPR multimarker genotype (for white participants only) on PTSD subscale symptom score after controlling for the degree of shooting exposure. The sample population was divided into 2 groups: those who were nterviewed 2 to 4 weeks postshooting and those who were interviewed more than 1 month postshooting.
P< .05.
P< .005
COMMENT
One of the critical questions surrounding PTSD is why some individuals are at risk for developing the disorder following an index trauma while others appear to be relatively resilient. It is known that genetic heritability is one component of the differential risk for PTSD, but the mechanisms remain relatively unknown. It has been difficult to demonstrate how genetic factors interact with trauma to cause PTSD symptoms for several reasons. It is difficult to normalize the level of trauma exposure across a sample set, self-reported symptoms and personal histories can be unreliable, and baseline levels of PTSD symptoms prior to the index trauma are often impossible to establish. We believe that the unique nature of this cohort, while perhaps not entirely eliminating these problems, may substantially decrease the influence of these confounding factors.
In this study, we have looked at college students who had been interviewed prior to, and shortly after, a mass shooting on the Northern Illinois University campus. We used these prospective psychological data to explicitly examine the association between polymorphisms within the serotonin transporter gene promoter region and PTSD/ acute stress disorder symptoms that developed in the aftermath of exposure to the shooting. We found that (1) proximity to the shooting was highly associated with PTSD symptom severity (DEQ score difference) between times 1 and 2 for individuals who were interviewed 2 to 4 weeks postshooting and for individuals who were interviewed more than 1 month postshooting; (2) the STin2 and 5-HTTLPR genotypes had no main effect on DEQ score difference between times 1 and 2; (3) the rs25531 and 5-HTTLPR–rs25531 combined multimarker genotypes were strongly associated with differential risk for PTSD postshooting; and (4) this association between the 5-HTTLPR multimarker genotype and DEQ score difference survived adjustment for multiple testing (Bonferroni) and survived adjustment for shooting proximity variables.
Genetics have been clearly shown to play an important role in the differential risk for development of PTSD. The SLC6A4 polymorphisms are of particular interest in relation to PTSD and a host of other psychiatric illnesses. The majority of findings implicate the 5-HTTLPR s allele as a risk allele for developing PTSD. This seems biologically plausible given the following evidence: (1) the presence of 1 and 2 s alleles reduces the expression of 5HTT by 27% and 30%, respectively,33 and (2) the s allele associates with greater amygdala activity in response to emotional stimuli.34 Conflicting evidence still exists, however.19,35 rs25531 (A/G) has also been examined for its role in mental health.36,37 It has been hypothesized that the G allele creates an AP2 binding site, which, in turn, can decrease transcription.20 Additionally, rs25531 has been shown to have an effect on amygdala activity in response to angry, happy, or sad emotions.38 Compared with the other 2 loci, there is less literature concerning STin2, but this polymorphism has been associated with harm avoidance,39 suicidality,40 psychosis,41 and migraine headaches.42 Thus, despite evidence that this polymorphism is likely functional,12 to our knowledge, there is no evidence associating this variant with risk for PTSD.
Sample size is an important limitation in this study. Although we were unable to obtain saliva samples for the majority of those in the primary study population, we continue to make efforts to genotype more members of this study sample. Most prospective, shared trauma disasters will likely share similar sample size limitations. Additionally, the majority of our subjects (78%) were interviewed within 2 to 4 weeks after the shooting. This limited our ability to make conclusions regarding PTSD rather than acute stress disorder. Later time measures (time 3) were collected but too few in the study sample (n = 21) had a likely diagnosis of PTSD, diminishing the power to obtain meaningful results regarding genetic association. Another limitation is the DEQ measure of PTSD symptoms. Although this is a validated measure of symptoms, it is not a clinical measure of PTSD diagnosis, which was not practical given the unique circumstances of this study design and subject population.
Despite its limitations, this study population was uniquely predisposed toward the analysis of PTSD G × E interactions. The strength of the current study is the availability of the same validated survey measure to assess PTSD symptoms prior to and after a shared acute traumatic event. By accounting for this measure in our calculation of PTSD symptoms after the shooting, we are essentially controlling for other factors that might be contributing to PTSD other than SLC6A4 genotype and exposure to the shooting. Definitive clarification of the role of genetic heritability in the prediction of risk for psychopathology will not be possible without such prospective and retrospective approaches.
In summary, the examination of a population for which we had information on lifetime trauma and pretrauma PTSD symptoms allowed for a unique prospective G × E study. These data suggest that differential function of the serotonin transporter may mediate differential response to a severe trauma. When examined in a relatively homogenous sample with shared trauma and known prior levels of child and adult trauma, the 5-HTTLPR multimarker genotype may serve as a useful predictor of risk for PTSD-related symptoms in the weeks and months following the trauma.
Acknowledgments
Funding/Support: This research was funded by grants from the Joyce Foundation (Dr Orcutt) and the Burroughs Wellcome Fund (Dr Ressler), grant HD049907 from the National Institute of Child Health and Human Development (Dr Orcutt), and grants MH085436 (Dr Orcutt) and MH071537 (Dr Ressler) from the National Institute of Mental Health.
Footnotes
Author Contributions: Dr Ressler had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
Additional Contributions: We express our gratitude to the research participants for their contributions to this work.
REFERENCES
- 1.Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48(3):216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(suppl 5):4–12. discussion 13–14. [PubMed] [Google Scholar]
- 3.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication [published correction appears in Arch Gen Psychiatry. 2005;62(7):768] Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 4.Marmar CR, Weiss DS, Schlenger WE, Fairbank JA, Jordan BK, Kulka RA, Hough RL. Peritraumatic dissociation and posttraumatic stress in male Vietnam theater veterans. Am J Psychiatry. 1994;151(6):902–907. doi: 10.1176/ajp.151.6.902. [DOI] [PubMed] [Google Scholar]
- 5.Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129(1):52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 6.True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50(4):257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 7.Cornelis MC, Nugent NR, Amstadter AB, Koenen KC. Genetics of post-traumatic stress disorder: review and recommendations for genome-wide association studies. Curr Psychiatry Rep. 2010;12(4):313–326. doi: 10.1007/s11920-010-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norrholm SD, Ressler KJ. Genetics of anxiety and trauma-related disorders. Neuroscience. 2009;164(1):272–287. doi: 10.1016/j.neuroscience.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koenen KC, Amstadter AB, Nugent NR. Gene-environment interaction in post-traumatic stress disorder: an update. J Trauma Stress. 2009;22(5):416–426. doi: 10.1002/jts.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed BA, Bukhari IA, Jeffus BC, Harney JT, Thyparambil S, Ziu E, Fraer M, Rusch NJ, Zimniak P, Lupashin V, Tang D, Kilic F. The cellular distribution of serotonin transporter is impeded on serotonin-altered vimentin network. PLoS One. 2009;4(3):e4730. doi: 10.1371/journal.pone.0004730. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 12.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- 14.Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11(3):224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 15.Parsey RV, Hastings RS, Oquendo MA, Hu X, Goldman D, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry. 2006;163(1):48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- 16.Ali FR, Vasiliou SA, Haddley K, Paredes UM, Roberts JC, Miyajima F, Klenova E, Bubb VJ, Quinn JP. Combinatorial interaction between two human serotonin transporter gene variable number tandem repeats and their regulation by CTCF. J Neurochem. 2010;112(1):296–306. doi: 10.1111/j.1471-4159.2009.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephenson KL, Valentiner DP, Kumpula MJ, Orcutt HK. Anxiety sensitivity and posttrauma stress symptoms in female undergraduates following a campus shooting. J Trauma Stress. 2009;22(6):489–496. doi: 10.1002/jts.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayin A, Kucukyildirim S, Akar T, Bakkaloglu Z, Demircan A, Kurtoglu G, Demirel B, Candansayar S, Mergen H. A prospective study of serotonin transporter gene promoter (5-HTT gene linked polymorphic region) and intron 2 (variable number of tandem repeats) polymorphisms as predictors of trauma response to mild physical injury. DNA Cell Biol. 2010;29(2):71–77. doi: 10.1089/dna.2009.0936. [DOI] [PubMed] [Google Scholar]
- 19.Thakur GA, Joober R, Brunet A. Development and persistence of posttraumatic stress disorder and the 5-HTTLPR polymorphism. J Trauma Stress. 2009;22(3):240–243. doi: 10.1002/jts.20405. [DOI] [PubMed] [Google Scholar]
- 20.Koenen KC. Developmental epidemiology of PTSD: self-regulation as a central mechanism. Ann N Y Acad Sci. 2006;1071:255–266. doi: 10.1196/annals.1364.020. [DOI] [PubMed] [Google Scholar]
- 21.Wendland JR, Kruse MR, Murphy DL. Functional SLITRK1 var321, varCDfs and SLC6A4 G56A variants and susceptibility to obsessive-compulsive disorder. Mol Psychiatry. 2006;11(9):802–804. doi: 10.1038/sj.mp.4001848. [DOI] [PubMed] [Google Scholar]
- 22.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychol Assess. 2000;12(2):210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- 24.Kubany ES, Leisen MB, Kaplan AS, Kelly MP. Validation of a brief measure of posttraumatic stress disorder: the Distressing Event Questionnaire (DEQ) Psy-chol Assess. 2000;12(2):197–209. doi: 10.1037//1040-3590.12.2.197. [DOI] [PubMed] [Google Scholar]
- 25.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support. J Pers Assess. 1988;52(1):30–41. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 27.Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J Pers Assess. 1990;55(3–4):610–617. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 28.Littleton H, Grills-Taquechel A, Axsom D. Resource loss as a predictor of post-trauma symptoms among college women following the mass shooting at Virginia Tech. Violence Vict. 2009;24(5):669–686. doi: 10.1891/0886-6708.24.5.669. [DOI] [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. text rev. [Google Scholar]
- 30.Gelernter J, Cubells JF, Kidd JR, Pakstis AJ, Kidd KK. Population studies of polymorphisms of the serotonin transporter protein gene. Am J Med Genet. 1999;88(1):61–66. [PubMed] [Google Scholar]
- 31.Koenen KC, Aiello AE, Bakshis E, Amstadter AB, Ruggiero KJ, Acierno R, Kilpatrick DG, Gelernter J, Galea S. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. Am J Epidemiol. 2009;169(6):704–711. doi: 10.1093/aje/kwn397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 2000;5(1):32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- 33.Hranilovic D, Stefulj J, Schwab S, Borrmann-Hassenbach M, Albus M, Jernej B, Wildenauer D. Serotonin transporter promoter and intron 2 polymorphisms: relationship between allelic variants and gene expression. Biol Psychiatry. 2004;55(11):1090–1094. doi: 10.1016/j.biopsych.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Gru¨sser SM, Flor H, Schumann G, Mann K, Bu¨chel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8(1):20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- 35.Grabe HJ, Spitzer C, Schwahn C, Marcinek A, Frahnow A, Barnow S, Lucht M, Freyberger HJ, John U, Wallaschofski H, Vo¨lzke H, Rosskopf D. Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to post-traumatic stress disorder in the general population. Am J Psychiatry. 2009;166(8):926–933. doi: 10.1176/appi.ajp.2009.08101542. [DOI] [PubMed] [Google Scholar]
- 36.Bryant RA, Felmingham KL, Falconer EM, Pe Benito L, Dobson-Stone C, Pierce KD, Schofield PR. Preliminary evidence of the short allele of the serotonin transporter gene predicting poor response to cognitive behavior therapy in posttraumatic stress disorder. Biol Psychiatry. 2010;67(12):1217–1219. doi: 10.1016/j.biopsych.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Mellman TA, Alim T, Brown DD, Gorodetsky E, Buzas B, Lawson WB, Goldman D, Charney DS. Serotonin polymorphisms and posttraumatic stress disorder in a trauma exposed African American population. Depress Anxiety. 2009;26(11):993–997. doi: 10.1002/da.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dannlowski U, Ohrmann P, Bauer J, Kugel H, Baune BT, Hohoff C, Kersting A, Arolt V, Heindel W, Deckert J, Suslow T. Serotonergic genes modulate amygdala activity in major depression. Genes Brain Behav. 2007;6(7):672–676. doi: 10.1111/j.1601-183X.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- 39.Saiz PA, Garcia-Portilla MP, Herrero R, Arango C, Corcoran P, Morales B, Bascara´n MT, Alvarez V, Coto E, Paredes B, Ferna´ndez JM, Bobes J. Interactions between functional serotonergic polymorphisms and demographic factors influence personality traits in healthy Spanish Caucasians. Psychiatr Genet. 2010;20(4):171–178. doi: 10.1097/YPG.0b013e32833a20b9. [DOI] [PubMed] [Google Scholar]
- 40.Lopez de Lara C, Dumais A, Rouleau G, Lesage A, Dumont M, Chawky N, Alda M, Benkelfat C, Turecki G. STin2 variant and family history of suicide as significant predictors of suicide completion in major depression. Biol Psychiatry. 2006;59(2):114–120. doi: 10.1016/j.biopsych.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Proitsi P, Lupton MK, Reeves SJ, Hamilton G, Archer N, Martin BM, Iyegbe C, Hollingworth P, Lawlor B, Gill M, Brayne C, Rubinsztein DC, Owen MJ, Williams J, Lovestone S, Powell JF. Association of serotonin and dopamine gene pathways with behavioral subphenotypes in dementia [published online August 2, 2010] Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Schürks M, Rist PM, Kurth T. STin2 VNTR polymorphism in the serotonin transporter gene and migraine: pooled and meta-analyses. J Headache Pain. 2010;11(4):317–326. doi: 10.1007/s10194-010-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]