Abstract
Background
On the basis of reversal of taxane resistance with AKT inhibition, we initiated a phase I trial of the AKT inhibitor perifosine with docetaxel in taxane and platinum-resistant or refractory epithelial ovarian cancer.
Methods
Patients with pathologically confirmed high-grade epithelial ovarian cancer (taxane resistant, n = 10; taxane refractory, n = 11) were enrolled. Peripheral blood samples and tumor biopsies were obtained and 18F-FDG-PET and DCE-MRI scans were performed for pharmacodynamic and imaging studies.
Results
Patients received a total of 42 treatment cycles. No dose-limiting toxicity was observed. The median progression-free survival and overall survival were 1.9 months and 4.5 months, respectively. One patient with a PTEN mutation achieved a partial remission (PR) for 7.5 months, and another patient with PIK3CA mutation had stable disease (SD) for 4 months. Two other patients without apparent PI3K pathway aberrations achieved SD. Two patients with RAS mutations demonstrated rapid progression. Decreased phosphorylated S6 correlated with 18F-FDG-PET responses.
Conclusions
Patients tolerated perifosine 150 mg PO daily plus docetaxel at 75 mg/m2 every 4 weeks. Further clinical evaluation of effects of perifosine with docetaxel on biological markers and efficacy in patients with ovarian cancer with defined PI3K pathway mutational status is warranted.
INTRODUCTION
The phosphatidylinositol-3′-kinase (PI3K)/AKT pathway regulates many biological processes including cell survival, proliferation, tumorigenesis, metastasis, and resistance to chemotherapy through downstream molecules such as mammalian target of rapamycin (mTOR), ribosomal protein S6 and p70S6 kinase1. Activation of the PI3K/AKT pathway through genomic anomalies has been identified in many types of cancer, making it a potentially exciting therapeutic target2 In one study, PIK3CA (the class IA PI3K catalytic subunit p110a) mutations and amplification occurred in 28% of ovarian cancer patients and were associated with high AKT expression and significantly lower response rates to taxane and platinum therapy3. Recently, however the TCGA (The Cancer Genome Atlas) project has demonstrated that while changes in genomic copy numbers of members of the PI3K pathway are common in high-grade serous ovarian cancer, mutations in members of the pathway are rare4. However, mutations in members of the PI3K pathway are relatively more common in non-high grade epithelial serous ovarian cancers 5,6.
Perifosine is a novel ether alkylphospholipid that inhibits tumor growth, at least in part, through inhibiting the PI3K/AKT pathway by preventing cell membrane recruitment of the N-terminal AKT pleckstrin homology (PH) domain7,8 Previous studies with perifosine demonstrated antitumor activity in multiple human trials 9–19
An AKT inhibitor may be particularly beneficial for treatment of cancers in which increased AKT signaling is associated with reduced sensitivity to cytotoxic agents. The PI3K/AKT pathway is activated in approximately 70% of ovarian cancers, as assessed by an analysis of phosphorylation of AKT and other components of the pathway, and activation of this pathway is associated with resistance to cytotoxic chemotherapy20. Inhibitors of PI3K and AKT prevent the growth of ovarian cancer xenografts and potentiate the cytotoxic effects of paclitaxel and cisplatin21,22. Preliminary studies have shown that perifosine, which blocks cell membrane recruitment of the PH domain of AKT, inhibits proliferation of ovarian cancer cells, production of neovascularizing factors, cell motility, and invasion; and sensitizes cells to paclitaxel in vitro and in vivo23. As a proof of concept study, we have conducted a phase I clinical trial to evaluate the safety of adding perifosine to a standard dose of docetaxel in platinum and taxane resistant or refractory epithelial ovarian cancer. Pharmacodynamic studies included changes in plasma levels of cytokines, and changes of intracellular signal transduction pathways, which are associated with inhibition of the PI3K/AKT pathway. Functional imaging studies with 18F-FDG-PET and DCE-MRI (dynamic contrast-enhanced MRI) as early parameters for PI3K/AKT pathway inhibition-associated mTOR inhibition-mediated changes of glucose uptake, VEGF inhibition-mediated antiangiogenesis, and antitumor activities, were among the primary endpoints of this proof of concept study combining an agent targeting the PI3K pathway with a cytotoxic chemotherapeutic agent to overcome therapeutic resistance.
MATERIALS AND METHODS
Eligibility Criteria
Patients were eligible if they had a pathologically confirmed diagnosis of high-grade epithelial cancer of the ovary, fallopian tube or peritoneum, which was considered platinum refractory (progression on or persistent disease following platinum-based therapy) or platinum resistant (progression within 6 months of completion of a platinumbased regimen)24 Similar definitions were applied to taxane refractory and resistant disease. All participants had measurable disease that had progressed prior to study entry and an ECOG performance status of 2 or better. At least two sites of lesions were required: one for biopsy and a separate site for functional imaging evaluation. Additional eligibility criteria included adequate marrow function (absolute neutrophil count > 1,500 /µl, and platelet count > 75,000 /µl), renal function (serum creatinine < 1.5 mg/dL or a calculated creatinine clearance of > 60 mL/minute), and hepatic function (serum total bilirubin < 2.0 mg/dL, and ALT > 3 times the upper limit of normal).
Study Design and Treatment Plan
This prospective, open-label phase I clinical trial of perifosine and docetaxel in patients with platinum and taxane resistant or refractory epithelial ovarian cancer was conducted at The University of Texas MD Anderson Cancer Center after approval by the Institutional Review Board (IRB). Keryx Biopharmaceuticals (New York, NY) held the Investigational New Drug Application. The primary goal of this study was to define the safety of this regimen.
Treatment was administered on an outpatient basis at MD Anderson Cancer Center. Treatment cycles were 28 days. At cycle 1, patients received 4 loading doses of oral perifosine (100 mg every 6 hours) for one day followed by a daily dose according to dose level (50, 100 or 150 mg daily) for 20 days. After 7 days of rest, patients started cycle 2 with the same schedule and dosage of perifosine plus docetaxel intravenously administered at 75 mg/m2 once on day 2. The same schedules and doses of perifosine and docetaxel administered in cycle 2 were then repeated once every 28 days as long as the patient had no evidence of tumor progression or prohibitive toxicity. All patients received antiemetics according to best medical practice.
Safety and Efficacy Evaluation
The severity of adverse events was graded according to the Common Terminology Criteria for Adverse Events v3.0 25. Dose-limiting toxicity (DLT) was defined as platelets less than 25,000 /µL, absolute neutrophil count less than 500 /µL for more than 7 days, neutropenic fever, or ≥7 days delay in initiation of the next cycle of therapy because of inadequate hematological parameters, as well as any grade 3 or greater nonhematological toxicity other than nausea, vomiting, or fatigue occurring during the initial 2 cycles of treatment. Radiographic imaging studies were repeated every 2 cycles of therapy. All patients were observed until death or December 1, 2010, to have all survived patients followed up for at least one year post-study. Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 were used to characterize tumor responses26. Serum CA125 levels were measured as a supplementary assessment of possible antitumor activity27. Efficacy endpoints included response rate, progression-free survival (PFS), and overall survival (OS), which are defined from the first dose of perifosine (cycle 1 day 1) to the defined events.
Plasma Cytokines by ELISA
Cytokines [IL-6 (interleukin 6) and IL-8] have been implicated in pharmacodynamic responses to perifosine28 Blood samples were drawn once pre-study and day 8 for ELISA using commercially available kits (R&D Systems, Inc., Quantikine, Minneapolis, MN). The lower limits of sensitivity of the assays were as follows: IL-6 (0.7 pg/mL) and IL-8 (3.5 pg/mL). A standard curve was generated using known concentrations of recombinant cytokines according to the manufacturer’s instructions and the samples were read using a plate reader (Molecular Devices, Sunnyvale, CA). Results were calculated by generating a four-variable, logistic curve fit using the SOFTmax Pro software program (version 2.6; Molecular Devices). The concentration of a particular cytokine was then determined using the standard curve.
Reverse Phase Protein Array (RPPA) and Mutation Analysis
Tumor biopsies were collected from 11 patients at baseline and between days 7–10 of cycle 1 of therapy. Ten patients had paired biopsies. Protein expression data were derived from these paired biopsies using RPPA as described previously29. Briefly, lysis buffer was used to lyse frozen biopsy samples by homogenization. Tumor lysates were normalized to 1 µg/µL concentration using a bicinchoninic acid assay and boiled with 1% SDS, and the supernatants were manually diluted in six or eight 2-fold serial dilutions with lysis buffer. A GeneTAC arrayer (Genomic Solutions, Inc., Holliston, MA) created 1,152 spot arrays on nitrocellulose-coated FAST slides (Schleicher & Schuell BioScience, Inc., Keene, NH) from the serial dilutions. Each slide was probed with one of 183 antibodies (list available upon request), and the signal was amplified using a Dako Cytomation–catalyzed system. The slides were scanned using an HP flatbed scanner, signal intensities quantified using Microvigene software (VigeneTech Inc., Carlisle, MA). To estimate the protein’s relative concentration, signal intensities within each sample’s series dilution were fitted to a logistic regression model implemented in an R package “SuperCurve” software. The protein concentrations were normalized for protein loading using the average expression levels of all measured proteins in each sample. To generate heat maps, Treeview (University of Glasgow) and X-cluster software were used.
A mass spectroscopy-based approach evaluating single nucleotide polymorphisms was used to detect mutations in PIK3CA and KRAS as described30–32, whereas PTEN mutations were identified using whole gene sequencing in the Sequencing Core at MD Anderson Cancer Center. All mutations were present in before and on-treatment biopsies.
Functional Imaging Evaluation with 18F-FDG-PET and DCE-MRI
To investigate whether perifosine could induce early changes in tumor vascularity or glucose uptake, and whether these initial changes might provide early biomarkers of antitumor efficacy of therapy, 18F-FDG PET scans ([18F] fluorodeoxyglucose positron emission tomography) and DCE-MRI (dynamic contrast-enhanced magnetic resonance imaging) were conducted within 7 days prior to, and then after at least 7 days of the treatment with perifosine (PET was conducted 2 days after the first DCE-MRI). Analyses of 18F-FDG-PET imaging data were performed by standardized uptake value (SUV) of tumor with maximal signal after visual analysis of the gross tumor and corrected for lean body mass (SUVmax). Up to five target lesions per patient were evaluated. Changes in the SUVmax during treatment (ASUVmax) were determined by the following equation: [(post-treatment SUVmax – baseline SUVmax)*100], expressed as a percentage33.
DCE-MRI images were analyzed by an independent, central image-analysis vendor (Virtual Scopics, Rochester, NY). Percentage change from baseline in volume transfer constant (Ktrans), determined by using a data-derived input function, and blood-normalized (first 90 seconds) area-under-the-enhancement curve (IAUC) were recorded for each tumor34. These parameters reflect contrast delivery (i.e., capillary blood flow) and transport across the vascular endothelium (i.e., capillary permeability-surface area product); the dominant factor depends on whether delivery is limited by flow or permeability35.
Statistical Considerations
This phase I clinical trial was conducted using a modified 5 + 5 design. Descriptive summary statistics were used to assess demographics, safety, and antitumor activity. Categorical data were summarized using frequency and percentages. Continuous data were summarized by means, median, range and coefficient of variation +/− standard deviation. Student’s t-test was used to compare two groups, and a two-sided p < 0.05 was considered statistically significant.
RESULTS
Patient Characteristics
A total of 21 patients (median age, 57 years; range, 47 to 73 years) who met the inclusion and exclusion criteria were recruited to this study. Five, five and 11 patients, respectively, were enrolled at dose level 1 (perifosine 100 mg PO q6h × 4 followed by 50 mg PO daily for 20 more days every 28 days plus docetaxel 75 mg/m2 intravenously [IV] on day 2 every 28 days after cycle 1), 2 (the same as dose level 1 except for perifosine 100 mg PO daily), and 3 (the same as dose level 1 except for perifosine 150 mg PO daily), respectively. Patient characteristics are listed in Table 1. Ten patients had platinum and taxane resistant ovarian cancer and 11 had platinum and taxane refractory ovarian cancer. Fifteen patients had received 4 or more types of systemic chemotherapy prior to enrollment onto this study.
Table 1.
Patient Characteristics (n=21)
| Number | % | |
|---|---|---|
| Age | ||
| Median, 57 years; Range, 47 – 73 years | ||
| Pathology | ||
| Serous | 10 | 48% |
| Clear cell | 4 | 19% |
| Peritoneal | 1 | 5% |
| Endometrioid | 2 | 9% |
| Transitional | 1 | 5% |
| Mixed | 3 | 14% |
| Initial Stage | ||
| I | 1 | 5% |
| II | 3 | 14% |
| III | 12 | 57% |
| IV | 5 | 24% |
| Prior Treatment | ||
| 2 regimens | 1 | 5% |
| 3 regimens | 5 | 24% |
| 4 regimens | 6 | 28% |
| 5 regimens | 4 | 19% |
| 6 regimens or more | 5 | 24% |
| Platinum and Taxane Sensitivity | ||
| Resistant | 10 | 48% |
| Refractory | 11 | 52% |
Evaluation of Safety
All 21 patients were evaluable for toxicity. No DLT or treatment-related death was observed. Grade 2 or higher toxicities for each dose level are summarized in Table 2. Frequent adverse effects were nausea, vomiting, fatigue, anorexia, diarrhea, and constipation. No greater toxicities were observed with perifosine and docetaxel than would be expected from single-agent docetaxel based on historical experiences in similar cohorts of patients36–38. No clinically significant changes in blood glucose and lipid profiles were observed in this cohort of patients.
Table 2.
Toxicity Profile Associated with Perifosine and Docetaxel
| Dose Level | I (n=5) | II (n=5) | III (n=11) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Toxicity Grade | II | III | IV | II | III | IV | II | III | IV |
| Nausea | 3 | 0 | 0 | 3 | 1 | 0 | 7 | 1 | 0 |
| Vomiting | 2 | 0 | 0 | 3 | 1 | 0 | 6 | 1 | 0 |
| Diarrhea | 2 | 0 | 0 | 2 | 0 | 0 | 5 | 2 | 0 |
| Constipation | 1 | 3 | 0 | 2 | 0 | 0 | 7 | 0 | 0 |
| Fatigue | 2 | 3 | 0 | 2 | 1 | 0 | 7 | 0 | 1 |
| Pain | 1 | 0 | 0 | 2 | 1 | 0 | 3 | 3 | 0 |
| Shortness of Breath | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Anorexia | 1 | 0 | 0 | 0 | 2 | 0 | 4 | 3 | 0 |
| Neutropenia | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Infection | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Intestinal Obstruction | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 |
| Dehydration | 4 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
Evaluation of Antitumor Activity
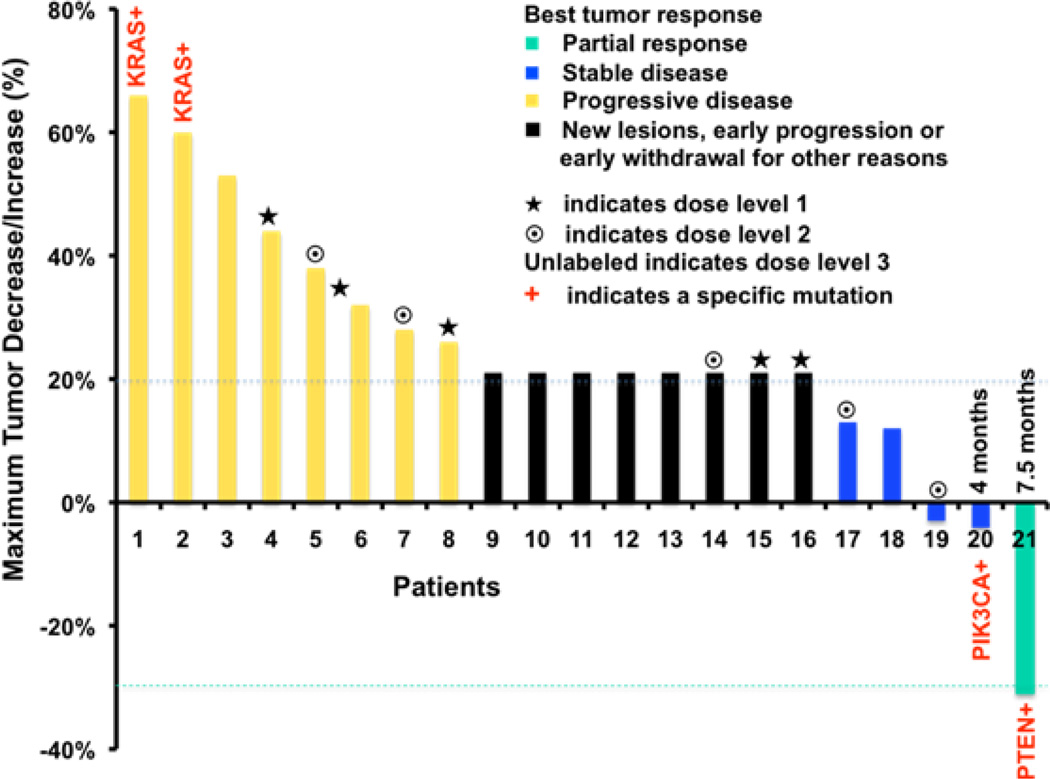
Twenty-one patients received a total of 42 cycles of therapy (eight patients received only 1 cycle of therapy without docetaxel; ten received 2 cycles; two, 4 cycles; and one, 6 cycles). The median progression-free survival was 1.9 months while the median overall survival was 4.5 months (Table 3). All five patients enrolled at dose level 1 progressed after either 1 or 2 cycles of therapy. At dose level 2, two patients achieved disease stabilization (one patient chose another treatment after 2 cycles of therapy and the other progressed after 3.5 months of therapy, whose mutation status was unknown). At dose level 3, a patient with an endometrioid cancer with a PTEN mutation achieved a partial remission by RESIST criteria for 7.5 months with the patient remaining alive for more than 24 months. CA125 levels normalized in this patient. A second patient with a clear cell tumor with a PIK3CA mutation achieved the second longest progression-free survival and was alive for more than 16 months. This patient also achieved the second largest decrease in CA125 in the study. A third patient with a clear cell tumor with no PI3K pathway mutation detected had a 2.7 month progression-free survival (Table 3 and Figure 1). As for biochemical CA125 responses, in addition to normalization of CA125 in the patient with a PR, a 91% decrease was observed in one patient at dose level 1, and four additional patients demonstrated modest CA125 level decreases with three of the four in dose level 3, as shown in Table 3. The two patients with KRAS mutations (a serous and a clear cell tumor) had rapid disease progression.
Table 3.
Response Characteristics of Individual Patients Treated in the Trial
| Age | Pathology High-grade |
Platinum Resistant or Refractory |
Prior Therapy |
Best Response by RECIST1.0 |
CA125 Response |
PFS (Month) |
OS (Month) |
Mutation** | Plasma IL6 changes |
Plasma IL8 Changes |
pS6- 230s over S6 |
pS6- 240s over S6 |
PET Response |
DC-MRI Response |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 73 | Transitional | Refractory | 4 | PD (32%) | 35% | 0.9 | 3 | NA | − 46.7% | 955.9% | NA | NA | − 8.1% | NA |
| 47 | Clear cell | Resistant | 5 | PD (44%) | 137% | 1.8 | 12 | Wild-type | 136% | 783.4% | NA | NA | − 3.4% | 14% |
| 57 | Serous | Refractory | 10 | PD (*21%) | 177% | 1.8 | 3 | NA | − 55.2% | 18.1% | NA | NA | NA | NA |
| 56 | Mixed | Refractory | 7 | PD (26%) | 149% | 1.8 | 10 | Wild-type | NA | NA | NA | NA | 29.3% | NA |
| 65 | Serous | Refractory | 3 | PD (*21%) | − 91% | 1.5 | 1.5 | NA | 4.2% | 8% | NA | NA | − 10.1% | 15% |
| 50 | Serous | Resistant | 4 | PD (28%) | 398% | 1.8 | 7.5 | Wild-type | 81.6% | 35.2% | NA | NA | − 2.3% | − 59% |
| 51 | Endometrioid | Resistant | 4 | SD (13%) | 13% | 1.8 | 9 | Wild-type | − 43.9% | 3% | NA | NA | −0.4% | − 39% |
| 56 | Serous | Resistant | 6 | PD (38%) | 109% | 0.9 | 4.5 | Wild-type | 120.3% | − 75% | NA | NA | NA | NA |
| 67 | Serous | Refractory | 4 | PD (*21%) | *21% | 0.6 | 1.5 | Wild-type | 67.5% | − 40.2% | NA | NA | 13.1% | 5% |
| 65 | Serous | Refractory | 5 | SD (−3%) | − 22% | 3.5 | 21 | NA | 254.9% | 2.1% | NA | NA | − 3.4% | − 29% |
| 66 | Serous | Resistant | 4 | PD (*21%) | *21% | 0.5 | 2 | Wild-type | NA | NA | NA | NA | − 6.4% | NA |
| 65 | Serous | Refractory | 5 | PD (*21%) | − 3% | 1.8 | 4 | Wild-type | 138.1% | 1792% | 0.83 | 0.77 | 130.7% | NA |
| 48 | Mixed | Refractory | 3 | PD (*21%) | 230% | 1.8 | 3 | Wild-type | 76.6% | 403.6% | 1.11 | 1.26 | 20.3% | − 53% |
| 43 | Serous | Resistant | 3 | PD (60%) | 771% | 1.8 | 10 | KRAS* | 735.4% | 6750% | 1.02 | 0.91 | 17.3% | − 14% |
| 59 | Endometrioid | Resistant | 6 | PR (−31%) | CR | 7.5 | 24+ | PTEN | 83.1% | − 93.7% | 0.38 | 0.47 | − 3.1% | − 6% |
| 59 | Peritoneal | Refractory | 4 | PD (53%) | 110% | 0.8 | 4 | Wild-type | − 27.1% | 4705% | 0.15 | 0.30 | 5.4% | − 48% |
| 56 | Clear cell | Resistant | 3 | SD (−4%) | − 26% | 4 | 16+ | PIK3CA | − 16.7% | 19.5% | 3.22 | 1.95 | 22.7% | − 25% |
| 62 | Mixed | Refractory | 8 | PD (*21%) | 372% | 0.7 | 2 | Wild-type | 121.6% | 66.7% | 1.07 | 1.10 | NA | − 16% |
| 58 | Serous | Resistant | 3 | PD (*21%) | 22% | 0.9 | 2 | Wild-type | 183.4% | 281.2% | 0.40 | 0.48 | − 9.7% | − 28% |
| 48 | Clear cell | Resistant | 2 | SD (12%) | − 13% | 2.7 | 16+ | Wild-type | 183.8% | 1212% | 1.46 | 1.18 | NA | 31% |
| 53 | Clear cell | Refractory | 5 | PD (66%) | 98% | 1.8 | 15+ | KRAS** | 108.7% | 2931% | 1.00 | 0.98 | − 30.9% | − 41% |
Abbreviations: CR: complete remission; PR: partial remission; SD: stable disease; PD: progressive disease;
indicates new lesions, early progression or early withdrawal for other reasons; and + means that patients were alive upon the last follow up; PFS: progression-free survival; and OS: overall survival.;
includes status of PIK3CA, PTEN and KRAS mutations (PIK3CA: PIK3CA mutation E545K; PTEN: PTEN mutation R130Q/P; KRAS*: KRAS mutation G12V; KRAS**: KRAS mutation G12C; and wild-type: no mutation detected); and NA indicates not available.
Figure 1.
The waterfall plot displays best tumor responses by RECIST 1.0 criteria. All 21 patients were evaluated. Patients represented by black bars either have new lesions or early progression, or early withdrawal for other reasons. They are arbitrarily designated as having a 21% progression. Duration of response and stable disease are indicated.
Plasma Cytokines Affected by Perifosine
Plasma levels of IL-6 and IL-8 were measured at baseline and after treatment with perifosine for a week to define surrogate markers of the AKT inhibition 39–45. Compared to pre-treatment specimens, post-treatment IL-6 levels were increased at the higher dose level, as seen in Table 3. Changes of plasma IL-8 were not dose-dependent, as shown in Table 3. It was noted that three patients with tumor reduction assessed by imaging displayed a significant decrease in IL-8 by 93.7%, and minimal increases in IL-8 by 2.1% and 19.5%, respectively.
RPPA of Tumor Tissues
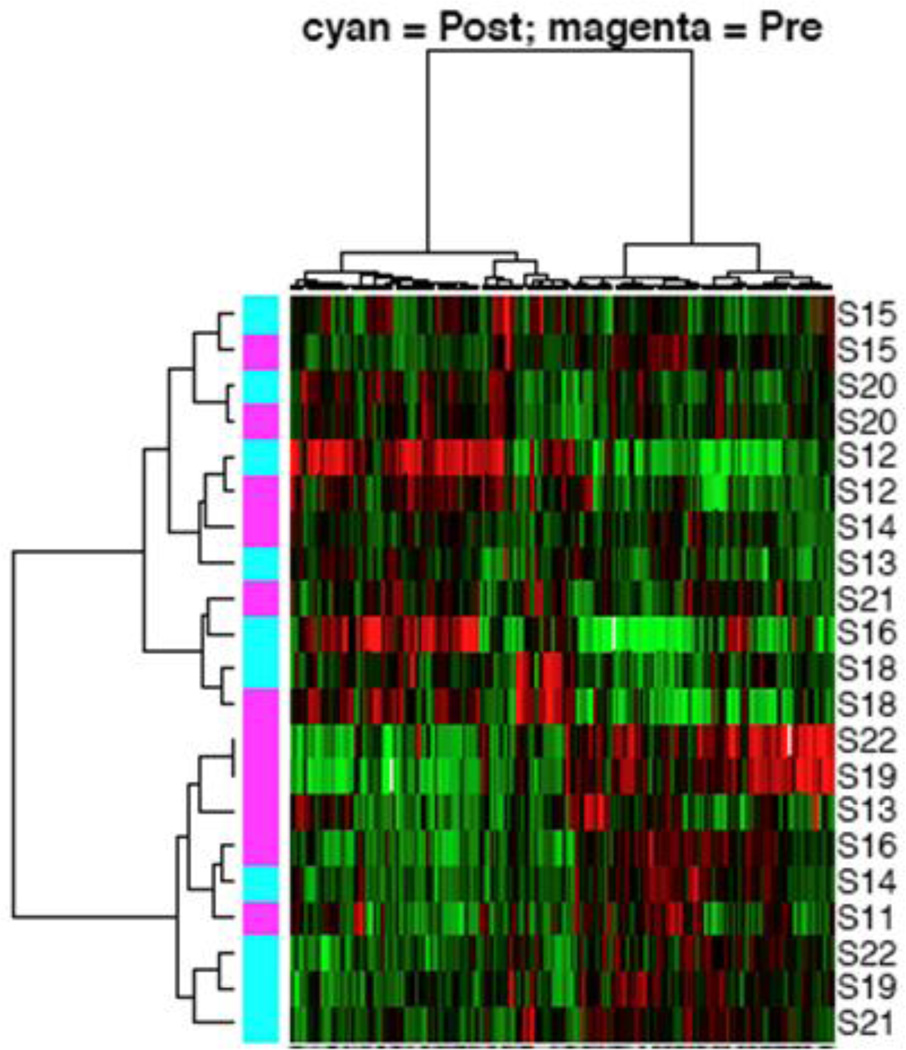
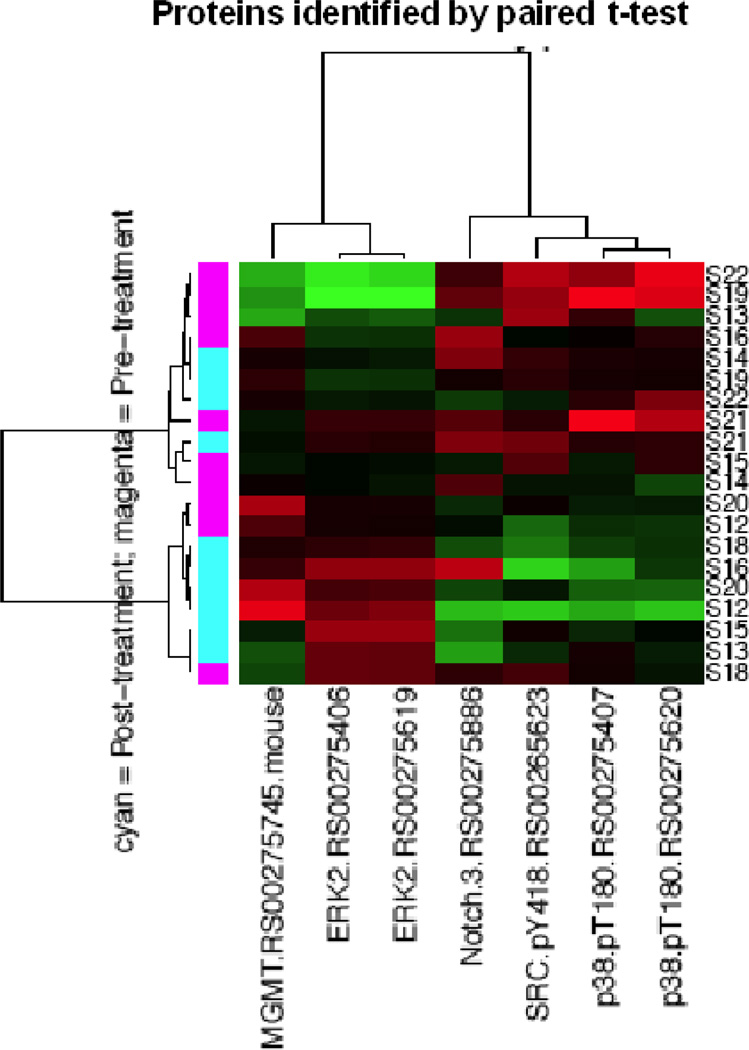
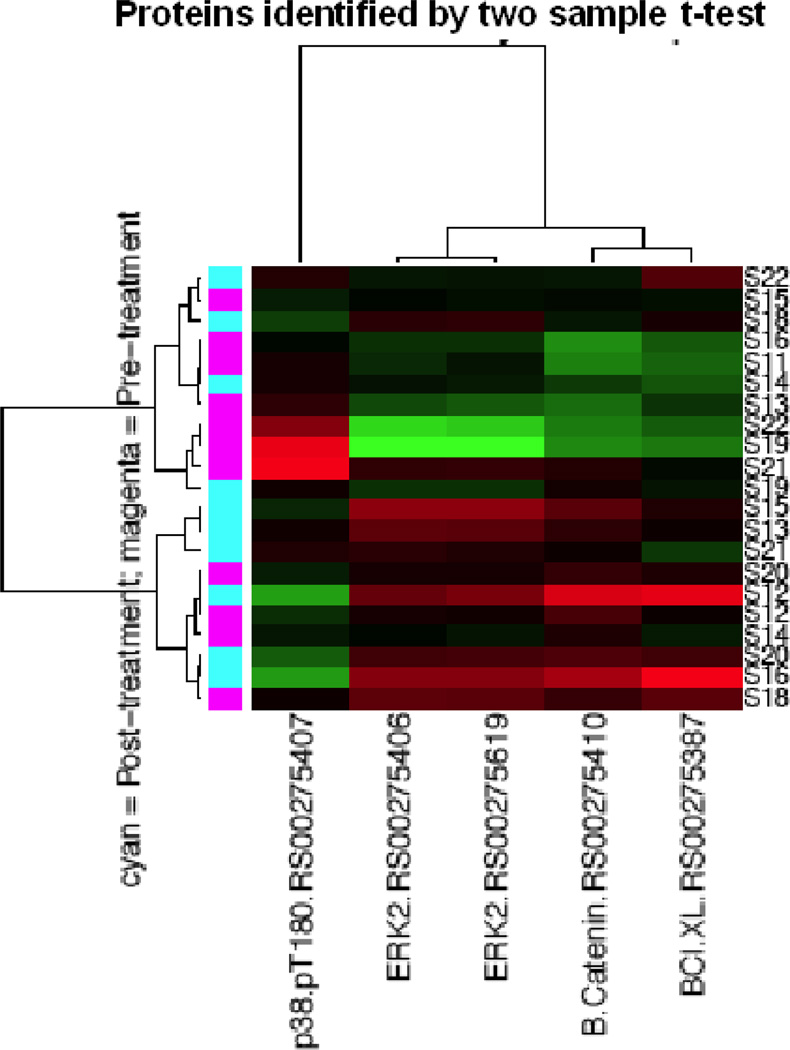
After providing informed consent, patients with platinum and taxane resistant or refractory epithelial ovarian cancer had two tumor biopsies: one was done within 7 days prior to initiation of treatment, and the other at cycle 1 day 7 of perifosine therapy. Changes in protein expression profiles in paired (pre- versus post-treatment) tumor biopsies collected from ten patients were assessed using RPPA. As shown in Figure 2A, unsupervised hierarchical clustering using all antibodies assessed showed that for many patients pre- and post-treatment biopsies clustered together, whereas for others (bottom half), there was a clear segregation by treatment. This suggested that for many patients, the overall protein expression pattern was dominant over the effects of perifosine. We thus sought differences in protein levels that would reflect treatment effects. By applying paired t-tests to determine proteins whose expression was significantly different between pre- and post-treatment tumor biopsies (Figure 2B), we found that extracellular signal-regulated kinase 2 (ERK2) and O-6-methylguanine-DNA methyltransferase (MGMT) were upregulated, and that neurogenic locus notch homolog protein 3 (Notch3), p38 phosphorylation (p38pT180), and Src phosphorylation (SRCpY418) were downregulated by perifosine (at p<0.05). By applying two-group t- tests to identify proteins whose expressions were significantly different between the pre-and post-treatment tumor biopsies (Figure 2C), we found that ERK2, β-catenin, and B-cell lymphoma extra large (BCL-xL) protein were upregulated, and that p38pT180 was downregulated by treatment with perifosine (p < 0.05).
Figure 2.
Protein expression profiles of tumor tissues at baseline and after treatment with perifosine for 7 days were analyzed by reverse-phase protein arrays (RPPA). Figure 2A indicates unsupervised hierarchical clustering analysis using all proteins. Figure 2B displays unsupervised hierarchical clustering analysis using proteins identified by a paired t-test analysis. Figure 2C shows unsupervised hierarchical clustering analysis using proteins identified by two-group t-test analysis.
Functional Imaging Changes Mediated by Perifosine
Further, we used 18F-FDG-PET scans to assess whether perifosine could induce early changes in glucose uptake by tumor tissues to determine whether early changes in tumor glucose uptake induced by perifosine might provide early biomarkers of antitumor efficacy in response to therapy. Percent quantitative changes in 18F-FDG-PET signals (ΔSUVmax) from baseline are summarized in Table 3. RPPA revealed that phosphorylated S6 versus total S6 protein (S6p235.236/S6, and S6p240.244/S6), as shown in Table 3, was significantly decreased (p < 0.05) in patients who had 18F-FDG-PET responses defined by <10% increases or any reduction of SUV compared to those who did not.
As shown in Table 3, percentage changes from baseline in volume transfer constant (Ktrans) obtained in a set of two DCE-MRI scans were not associated with dose levels, tumor responses, or clinical outcomes. The majority of patients showed decreased perfusion into tumor tissues after treatment with perifosine, regardless of dose or clinical response.
DISCUSSION
We tested the hypothesis that the PI3K/AKT pathway contributes to ovarian cancer progression and resistance to taxanes and should thus be evaluated as a target for novel therapeutics, alone and in combination with taxanes. Although the patient numbers are low, as would be expected in a phase I trial, the two patients with the greatest imaging response, including a PR, a normalization of CA125, the greatest PFS and who remained alive at the end of the trial had a PTEN and PIK3CA mutation in there tumors. In contrast, the two patients who had tumors with a RAS mutation demonstrated the most rapid progression. This was consistent with preclinical evidence showing that PI3K pathway inhibitors may exhibit greater activity in patients whose tumors demonstrate defined PI3K pathway aberrations and that RAS mutations may be harbingers of resistance46. Indeed, mutational status has been associated with responsiveness to a number of PI3K pathway inhibitors in the phase I setting, with PI3K pathway aberrations associated with sensitivity and RAS mutations with resistance47.
Preliminary evidence from pharmacodynamic studies of perifosine, alone and in combination with docetaxel, in patients with platinum and taxane resistant or refractory epithelial ovarian cancer demonstrates several observations that could facilitate future drug development. First, previous studies showed that IL-8 expression was decreased through inhibiting the PI3K/AKT pathway39–41, whereas IL-6 expression was markedly enhanced when the PI3K/AKT pathway was inhibited in mature monocytic blast cells42 or macrophages43 (though contradictory data do exist for the relationship between IL-6 expression and inhibition of the PI3K/AKT pathway44,45). Weekly plasma cytokine changes (e.g., in IL-6 and IL-8), as defined by ELISA were viewed as potential surrogate markers for delineating the nature of the biological activity of perifosine through inhibition of the PH domain of AKT. Second, BCL2 levels, MGMT, beta catenin and total ERK2 levels were increased and notch, phosphoSRC and phosphop38 decreased, indicating that the low clinical response rate might, in part, be caused by perifosine-induced activation of other pathways that protect cells from AKT pathway inhibition, in particular increases in levels of the anti-apoptotic mediator BCL2. Simultaneous inhibition of multiple intracellular signaling pathways might thus be required for better clinical efficacy and, in particular, a combination of a PI3K pathway inhibitor and of a BCL2 inhibitor to target cell survival could be considered. Third, in contrast to mTOR inhibitors, clinically significant increases in blood glucose and lipid profiles after treatment with perifosine were not observed in this cohort of patients, suggesting that inhibition of the PH domain of AKT by perifosine without simultaneous inhibition of mTORC248,49 has little effect on glucose metabolism or, alternatively, that the PI3K pathway was not inhibited sufficiently at doses given to alter glucose metabolism. Fourth, preclinical data have demonstrated that early 18F-FDG-PET responses correlated with tumor growth inhibition by perifosine in human ovarian cancer xenograft models23. We found that inhibition of S6 phosphorylation was associated with 18F-FDG-PET responses after about 1 week of therapy. Thus, 18F-FDG-PET responses may serve as an early indicator as a result of AKT inhibition50.
Treatment with perifosine plus docetaxel appears more effective when the PI3K/AKT pathway is mutationally activated, at least in the patient who achieved a PR. Thus, increased efficacy may be seen if patients with PIK3CA mutations and/or PTEN loss/mutations are identified before enrollment to correlate pharmacodynamic responses with clinical tumor responses.
Clearly, the turnaround time for pharmacodynamic study results must be timely to facilitate expedited drug development in the clinical setting. In addition, the laboratory and imaging procedures used for pharmacodynamic studies must be reliable and reproducible in the clinical trial setting. The role of imaging studies using 18F-FDG-PET and DCE-MRI as early clinical indicators also needs further validation.
In summary, perifosine used at 150 mg PO daily showed acceptable safety and evidence of efficacy in combination with docetaxel at its standard dosage. Clinical evaluation of effects of perifosine on biological markers and efficacy in patients with ovarian cancer with defined PI3K pathway mutational status is warranted.
Hightlights.
Patients with aberrant PI3K-AKT pathway responded to combined therapy to use an AKT inhibitor perifosine plus docetaxel.
Patients with K-RAS mutations showed rapid progression.
Decreased phosphorylated S6 correlated with 18F-FDG-PET responses.
Protective pathways such as ERK were enhanced after AKT inhibition by perifosine.
Acknowledgements
The authors thank Joann Aaron in the Department of Investigational Cancer Therapeutics at MD Anderson Cancer Center for editing our manuscript, and financial support in part from The Commonwealth Foundation for Cancer Research (SF), Keryx Biopharmaceuticals (SF), MD Anderson Cancer Clinical Research Fund (SF), UT MD Anderson Ovarian Cancer SPORE philanthropic fund (RB), UT MD Anderson Ovarian Cancer SPORE (NIH P50CA83639 to RB and GBM), SUC2-AACR-DT0209 01 (GBM), an American Society of Clinical Oncology (ASCO) Cancer Foundation Career Development Award (CDA to BTH), a Science Foundation Ireland (SFI)/Health Research Board (HRB Ireland) Translational Research Award (TRA to BTH) for the clinical and correlative studies and National Cancer Institute through The University of Texas MD Anderson Cancer Center Support Grant (P30 CA016672).
Disclosure by Gordon B. Mills:
Supported research support from Keryx.
- Potential conflict of interest disclosures
-
2.1SAB/Consultant: Asuragen, Aushon, Catena, Daiichi Pharmaceutical, Foundation Medicine, Komen Foundation
-
2.2Stock/Options/Financial: Catena, PTV Ventures
-
2.3Sponsored Research: AstraZeneca, Celgene, CeMines, Exelixis, GSK, LPATH, Roche, SDI, Wyeth/Pfizer
-
2.4Discuss off label use and/or investigational use of drugs
-
2.1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors except Gordon B. Mills declare that there are no conflicts of interest.
REFERENCES
- 1.Franke TF: PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- 2.Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 3.Kolasa IK, Rembiszewska A, Felisiak A, et al. PIK3CA amplification associates with resistance to chemotherapy in ovarian cancer patients. Cancer Biol Ther. 2009;8:21–26. doi: 10.4161/cbt.8.1.7209. [DOI] [PubMed] [Google Scholar]
- 4.Integrated genomic analyses of ovarian carcinoma. Nature. 474:609–615. 2011. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willner J, Wurz K, Allison KH, et al. Alternate molecular genetic pathways in ovarian carcinomas of common histological types. Hum Pathol. 2007;38:607–613. doi: 10.1016/j.humpath.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Kuo KT, Mao TL, Jones S, et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol. 2009;174:1597–1601. doi: 10.2353/ajpath.2009.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondapaka SB, Singh SS, Dasmahapatra GP, et al. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- 8.Hideshima T, Catley L, Yasui H, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unger C, Berdel W, Hanauske AR, et al. First-time-in-man and pharmacokinetic study of weekly oral perifosine in patients with solid tumours. Eur J Cancer. 2010;46:920–925. doi: 10.1016/j.ejca.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 10.Leighl NB, Dent S, Clemons M, et al. A Phase 2 study of perifosine in advanced or metastatic breast cancer. Breast Cancer Res Treat. 2008;108:87–92. doi: 10.1007/s10549-007-9584-x. [DOI] [PubMed] [Google Scholar]
- 11.Marsh Rde W, Rocha Lima CM, Levy DE, et al. A phase II trial of perifosine in locally advanced, unresectable, or metastatic pancreatic adenocarcinoma. Am J Clin Oncol. 2007;30:26–31. doi: 10.1097/01.coc.0000251235.46149.43. [DOI] [PubMed] [Google Scholar]
- 12.Bailey HH, Mahoney MR, Ettinger DS, et al. Phase II study of daily oral perifosine in patients with advanced soft tissue sarcoma. Cancer. 2006;107:2462–2467. doi: 10.1002/cncr.22308. [DOI] [PubMed] [Google Scholar]
- 13.Argiris A, Cohen E, Karrison T, et al. A phase II trial of perifosine, an oral alkylphospholipid, in recurrent or metastatic head and neck cancer. Cancer Biol Ther. 2006;5:766–770. doi: 10.4161/cbt.5.7.2874. [DOI] [PubMed] [Google Scholar]
- 14.Knowling M, Blackstein M, Tozer R, et al. A phase II study of perifosine (D-21226) in patients with previously untreated metastatic or locally advanced soft tissue sarcoma: A National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs. 2006;24:435–439. doi: 10.1007/s10637-006-6406-7. [DOI] [PubMed] [Google Scholar]
- 15.Posadas EM, Gulley J, Arlen PM, et al. A phase II study of perifosine in androgen independent prostate cancer. Cancer Biol Ther. 2005;4:1133–1137. doi: 10.4161/cbt.4.10.2064. [DOI] [PubMed] [Google Scholar]
- 16.Ernst DS, Eisenhauer E, Wainman N, et al. Phase II study of perifosine in previously untreated patients with metastatic melanoma. Invest New Drugs. 2005;23:569–575. doi: 10.1007/s10637-005-1157-4. [DOI] [PubMed] [Google Scholar]
- 17.Van Ummersen L, Binger K, Volkman J, et al. A phase I trial of perifosine (NSC 639966) on a loading dose/maintenance dose schedule in patients with advanced cancer. Clin Cancer Res. 2004;10:7450–7456. doi: 10.1158/1078-0432.CCR-03-0406. [DOI] [PubMed] [Google Scholar]
- 18.Crul M, Rosing H, de Klerk GJ, et al. Phase I and pharmacological study of daily oral administration of perifosine (D-21266) in patients with advanced solid tumours. Eur J Cancer. 2002;38:1615–1621. doi: 10.1016/s0959-8049(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 19.Pal SK, Reckamp K, Yu H, et al. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs. 2010;19:1355–1366. doi: 10.1517/13543784.2010.520701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bast RC, Jr., Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le XF, Hittelman WN, Liu J, et al. Paclitaxel induces inactivation of p70 S6 kinase and phosphorylation of Thr421 and Ser424 via multiple signaling pathways in mitosis. Oncogene. 2003;22:484–497. doi: 10.1038/sj.onc.1206175. [DOI] [PubMed] [Google Scholar]
- 22.Hu L, Hofmann J, Lu Y, et al. Inhibition of phosphatidylinositol 3'-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002;62:1087–1092. [PubMed] [Google Scholar]
- 23.Hennessy BT, Lu Y, Poradosu E, et al. Pharmacodynamic markers of perifosine efficacy. Clin Cancer Res. 2007;13:7421–7431. doi: 10.1158/1078-0432.CCR-07-0760. [DOI] [PubMed] [Google Scholar]
- 24.Fu S, Hu W, Iyer R, et al. Phase 1b-2a study to reverse platinum resistance through use of a hypomethylating agent, azacitidine, in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Cancer. 2011;117:1661–1669. doi: 10.1002/cncr.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 26.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 27.Rustin GJ, Nelstrop AE, Crawford M, et al. Phase II trial of oral altretamine for relapsed ovarian carcinoma: evaluation of defining response by serum CA125. J Clin Oncol. 1997;15:172–176. doi: 10.1200/JCO.1997.15.1.172. [DOI] [PubMed] [Google Scholar]
- 28.Huston A, Leleu X, Jia X, et al. Targeting Akt and heat shock protein 90 produces synergistic multiple myeloma cell cytotoxicity in the bone marrow microenvironment. Clin Cancer Res. 2008;14:865–874. doi: 10.1158/1078-0432.CCR-07-1299. [DOI] [PubMed] [Google Scholar]
- 29.Carey MS, Agarwal R, Gilks B, et al. Functional proteomic analysis of advanced serous ovarian cancer using reverse phase protein array: TGF-beta pathway signaling indicates response to primary chemotherapy. Clin Cancer Res. 16:2852–2860. doi: 10.1158/1078-0432.CCR-09-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas RK, Baker AC, Debiasi RM, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39:347–351. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 32.Jurinke C, van den Boom D, Cantor CR, et al. The use of MassARRAY technology for high throughput genotyping. Adv Biochem Eng Biotechnol. 2002;77:57–74. doi: 10.1007/3-540-45713-5_4. [DOI] [PubMed] [Google Scholar]
- 33.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 34.Liu G, Rugo HS, Wilding G, et al. Dynamic contrast-enhanced magnetic resonance imaging as a pharmacodynamic measure of response after acute dosing of AG-013736, an oral angiogenesis inhibitor, in patients with advanced solid tumors: results from a phase I study. J Clin Oncol. 2005;23:5464–5473. doi: 10.1200/JCO.2005.04.143. [DOI] [PubMed] [Google Scholar]
- 35.Evelhoch JL. Key factors in the acquisition of contrast kinetic data for oncology. J Magn Reson Imaging. 1999;10:254–259. doi: 10.1002/(sici)1522-2586(199909)10:3<254::aid-jmri5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Verschraegen CF, Sittisomwong T, Kudelka AP, et al. Docetaxel for patients with paclitaxel-resistant Mullerian carcinoma. J Clin Oncol. 2000;18:2733–2739. doi: 10.1200/JCO.2000.18.14.2733. [DOI] [PubMed] [Google Scholar]
- 37.Kavanagh JJ, Kudelka AP, de Leon CG, et al. Phase II study of docetaxel in patients with epithelial ovarian carcinoma refractory to platinum. Clin Cancer Res. 1996;2:837–842. [PubMed] [Google Scholar]
- 38.Markman M, Zanotti K, Webster K, et al. Phase 2 trial of single agent docetaxel in platinum and paclitaxel-refractory ovarian cancer, fallopian tube cancer, and primary carcinoma of the peritoneum. Gynecol Oncol. 2003;91:573–576. doi: 10.1016/j.ygyno.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Dong G, Chen Z, Li ZY, et al. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res. 2001;61:5911–5918. [PubMed] [Google Scholar]
- 40.Xu L, Pathak PS, Fukumura D. Hypoxia-induced activation of p38 mitogen-activated protein kinase and phosphatidylinositol 3'-kinase signaling pathways contributes to expression of interleukin 8 in human ovarian carcinoma cells. Clin Cancer Res. 2004;10:701–707. doi: 10.1158/1078-0432.ccr-0953-03. [DOI] [PubMed] [Google Scholar]
- 41.Wen XF, Yang G, Mao W, et al. HER2 signaling modulates the equilibrium between pro-and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25:6986–6896. doi: 10.1038/sj.onc.1209685. [DOI] [PubMed] [Google Scholar]
- 42.Birkenkamp KU, Esselink MT, Kruijer W, et al. An inhibitor of PI3-K differentially affects proliferation and IL-6 protein secretion in normal and leukemic myeloid cells depending on the stage of differentiation. Exp Hematol. 2000;28:1239–1249. doi: 10.1016/s0301-472x(00)00529-4. [DOI] [PubMed] [Google Scholar]
- 43.Medina EA, Morris IR, Berton MT. Phosphatidylinositol 3-Kinase Activation Attenuates the TLR2-Mediated Macrophage Proinflammatory Cytokine Response to Francisella tularensis Live Vaccine Strain. J Immunol. 2010 doi: 10.4049/jimmunol.0903790. [DOI] [PubMed] [Google Scholar]
- 44.Rabot M, El Costa H, Polgar B, et al. CD160-activating NK cell effector functions depend on the phosphatidylinositol 3-kinase recruitment. Int Immunol. 2007;19:401–409. doi: 10.1093/intimm/dxm005. [DOI] [PubMed] [Google Scholar]
- 45.Takai S, Tokuda H, Hanai Y, et al. Phosphatidylinositol 3-kinase/Akt plays a part in tumor necrosis factor-alpha-induced interleukin-6 synthesis in osteoblasts. Horm Metab Res. 2006;38:563–569. doi: 10.1055/s-2006-950502. [DOI] [PubMed] [Google Scholar]
- 46.Mohseni M, Park BH. PIK3CA and KRAS mutations predict for response to everolimus therapy: now that's RAD001. J Clin Invest. 2010;120:2655–2658. doi: 10.1172/JCI44026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janku F, Tsimberidou AM, Garrido-Laguna I, et al. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther. 2011;10:558–565. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar A, Lawrence JC, Jr., Jung DY, et al. Fat cell-specific ablation of rictor in mice impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism. Diabetes. 2010;59:1397–1406. doi: 10.2337/db09-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar A, Harris TE, Keller SR, et al. Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances Basal glycogen synthase activity. Mol Cell Biol. 2008;28:61–70. doi: 10.1128/MCB.01405-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma WW, Jacene H, Song D, et al. [18F]fluorodeoxyglucose positron emission tomography correlates with Akt pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy. J Clin Oncol. 2009;27:2697–2704. doi: 10.1200/JCO.2008.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]