Abstract
Although many human melanomas express the death receptors TRAIL-R2/DR5 or TRAIL-R1/DR4 on cell surface, they often exhibit resistance to exogenous TRAIL. One of the main contributors to TRAIL-resistance of melanoma cells is upregulation of transcription factors STAT3 and NF-κB that control the expression of antiapoptotic genes, including cFLIP and Bcl-xL. On the other hand, the JNK-cJun pathway is involved in the negative regulation of cFLIP (a caspase-8 inhibitor) expression. Our observations indicated that resveratrol, a polyphenolic phytoalexin, decreased STAT3 and NF-κB activation, while activating JNK-cJun that finally suppressed expression of cFLIP and Bcl-xL proteins and increased sensitivity to exogenous TRAIL in DR5-positive melanomas. Interestingly, resveratrol did not increase surface expression of DR5 in human melanomas, while γ-irradiation or sodium arsenite treatment substantially upregulated DR5 expression. Hence, an initial increase in DR5 surface expression (either by γ-irradiation or arsenite), and subsequent downregulation of antiapoptotic cFLIP and Bcl-xL (by resveratrol), appear to constitute an efficient approach to reactivate apoptotic death pathways in TRAIL-resistant human melanomas. In spite of partial suppression of mitochondrial function and the mitochondrial death pathway, melanoma cells still retain the potential to undergo the DR5-mediated, caspase-8-dependent death pathway that could be accelerated by either an increase in DR5 surface expression or suppression of cFLIP. Taken together, these results suggest that resveratrol, in combination with TRAIL, may have a significant efficacy in the treatment of human melanomas.
Introduction
Cell death by apoptosis regulates numerous physiological and pathological processes in the human body and its deficiency is implicated in tumor development. The inability of advanced cancer cells to undergo apoptosis may be based on inactivation of proapoptotic genes, due to mutations or epigenetic regulatory mechanisms that suppress death signaling pathways [1, 2]. As a result, both the extrinsic death receptor-mediated signaling pathway (Fas- or TRAIL-R1/R2-mediated) and the intrinsic mitochondrial death pathway could be partially or completely suppressed in cancer cells [3].
Melanoma, the most aggressive form of skin cancer, is known to be highly resistant to radio- and chemotherapeutic treatment. In the USA an estimated 60,000 new cases will be diagnosed, and 8,100 deaths will occur in 2007 (ACS). Numerous observations indicate that the incidence of melanoma has significantly increased over the last ten years in the USA and worldwide. However, only limited therapies for metastatic stage of the disease are currently available. Various attempts have been made to restore high levels of apoptosis in response to treatment for this type of cancer [4-6]. One of the key contributors to radio- and chemoresistance of human melanomas is upregulation of transcription factors STAT3 and NF-κB, which control expression of numerous antiapoptotic genes in cancer cells, including cFLIP, cIAP, XIAP, Bcl-xL, Survivin, as well as suppressing proapoptotic genes [7-10]. On the other hand, via activation of cJun, JNK is involved in the negative regulation of cFLIP (an inhibitor of caspase-8) expression [11, 12] and, via activation of E3 ubiquitin-protein ligase Itch, in acceleration of proteasome-dependent cFLIP degradation [13]. Taken together, these data suggest that agents that simultaneously downregulate NF-κB and STAT3 activities, while upregulating JNK-cJun activity, might increase sensitivity to TRAIL- or Fas-mediated apoptosis. Our recent observations indicated that sodium arsenite [11] was a useful candidate for mediating these effects in melanomas.
In the present study we have used resveratrol (trans-3, 4′, 5-trihydroxystilbene) [14], as a non-toxic alternative to sodium arsenite treatment, which was previously demonstrated to be a powerful promoter of apoptosis in melanomas [15]. Resveratrol (RSV) was previously shown to suppress JAK2-STAT3, Src-STAT3 and IKK-NF-κB activation and to induce apoptosis in some cancer cell lines [16-18]. We have demonstrated that, as with sodium arsenite, RSV suppressed expression of antiapoptotic cFLIP protein in human melanomas and dramatically increased sensitivity to exogenous TRAIL in TRAIL-R2/DR5-positive melanomas. Interestingly, RSV did not increase the surface expression of DR5, whereas sodium arsenite treatment or γ-irradiation of melanoma cells substantially upregulated DR5 expression. We present evidence showing that sequential treatment of melanoma cells with γ-irradiation and then RSV, initially upregulated DR5 surface levels [19], and subsequently downregulated antiapoptotic cFLIP, Bcl-xL and survivin levels. Under conditions of pronounced deficiency of mitochondrial function in some human melanomas, RSV treatment may still activate the extrinsic TRAIL-R-mediated death pathway, thereby increasing sensitivity to TRAIL and restoring apoptotic signaling in melanomas.
Methods
Materials
Sodium arsenite, cycloheximide and resveratrol were obtained from Sigma (St. Louis, MO). Human soluble Fas Ligand (recombinant) and soluble Killer-TRAIL (recombinant) were purchased from Alexis (San Diego, CA). JNK inhibitor SP600125 and IKK-NF-κB inhibitor BAY 11-7082 were obtained from Biomol (Plymouth Meeting, PA); MEK inhibitor U0126, MAPK p38 inhibitor SB203580, caspase inhibitors zVAD-fmk, Ac-IETD-CHO (an inhibitor of caspase-8 and caspase-6) and Ac-LEHD-CHO (an inhibitor of caspase-9) were purchased from Calbiochem (La Jolla, CA).
Cell lines
Human melanoma cell lines LU1205 (also known as 1205lu), WM9, WM35, and WM793 [20], as well as OM431, FEMX, HHMSX, LOX and normal human lung fibroblasts TIG-3 were maintained in a DMEM medium supplemented with 10% fetal bovine serum (FBS), L-glutamine and antibiotics. Normal human melanocytes were maintained in TICVA medium.
Irradiation procedures
To determine sensitivity to γ-rays, the plates with melanoma cells were exposed to radiation from a Gammacell 40 137Cs irradiator (dose rate, 0.82 Gy/min) at Columbia University. Six to 24 h after irradiation, cells were analyzed by the flow cytometry or underwent additional treatments.
FACS analysis of TRAIL and TRAIL-R2/DR5 levels
Surface levels of TRAIL and TRAIL-R2/DR5 on human melanomas were determined by staining with the PE-conjugated anti-human-TRAIL or anti-human DR5 mAb (R&D System, Minneapolis, MN and eBioscience, San Diego, CA) and subsequent flow cytometry. PE-conjugated nonspecific mouse IgG1 was used as an immunoglobulin isotype control. A FACS Calibur flow cytometer (Becton Dickinson, Mountain View, CA) combined with the CellQuest program was used to perform flow cytometric analysis. All experiments were independently repeated 3-5 times.
Transfection and luciferase assay
The NF-κB luciferase reporter containing two κB binding sites, Jun2-Luc reporter and empty vector tk-Luc [21], STAT-Luc reporter containing three repeats of GAS sites from the Ly6A/E promoter were used to determine NF-κB, AP-1 and STAT transactivation, respectively. Additional reporter constructs used included: 1.5 kb TRAIL-promoter-Luc [22], 1 kb cFLIP-promoter-Luc [23, 24] and p53RE-Luc [25]. Transient transfection of different reporter constructs (1 μg) together with pCMV-βgal (0.25μg) into 5×105 melanoma cells was performed using Lipofectamine (Life Technologies-Invitrogen). Proteins were prepared for β-Gal and luciferase analysis 16 hours after transfection. Luciferase activity was determined using the Luciferase assay system (Promega, Madison, WI) and was normalized based on β-galactosidase levels. Since RSV was known to partially inhibit enzymatic activity of firefly luciferase [26], a ratio of the specific luciferase reporter activity to luciferase activity driven by the empty tk-Luc vector in the mock control culture was finally determined.
In some experiments, melanoma cells were transfected with reporter constructs together with a dominant-negative form of cJun/TAM67 in the presence of pCMV-βGal (ratio 1:3:0.25). Sixteen to 24 hours after transfection, luciferase activity was determined. Expression construct pCMV4-cFLIP was received from Dr. M. E. Peter (University of Chicago, IL) and was used for overexpression of cFLIP in melanoma lines.
cFLIP suppression by RNAi
The pSUPER retro RNA interference (RNAi) system (Oligoengine, Seattle, WA), which has been utilized for the production of small RNAi transcripts used to suppress cFLIP expression. Two variants of RNAi of 19 nucleotides each were designed to target human cFLIP were expressed using vector pSUPER.retro.puro (pSR-puro). RNAi cFLIP-92 (UGUGGUUCCACCUAAUGUC) was the most efficient in the corresponding mRNA targeting.
Apoptosis studies
Cells were exposed to soluble TRAIL (50 ng/ml) alone or in combination with cycloheximide (2 μg/mL), TRAIL in combination with resveratrol (25-100 μM). Apoptosis was then assessed by quantifying the percentage of hypodiploid nuclei undergoing DNA fragmentation 16 h - 48 h after treatment. Flow cytometric analysis was performed on a FACS Calibur flow cytometer.
Clonogenic survival assay
Cells (500/well) were placed in triplicate on 6-well plates 16 h before treatment. For analysis of clonogenic survival of melanoma cells after treatment (24 h) with TRAIL, RSV or their combination, colonies were stained with crystal violet solution 12 days after treatment. The percentage of colony-forming efficiency (in relation to values of untreated control cells) was calculated.
Western blot analysis
Total cell lysates (50 μg protein) were resolved on 10% SDS-PAGE, and processed according to standard protocols. The antibodies (Abs) used for Western blotting included: monoclonal anti-β-Actin (Sigma), monoclonal anti-FLIP (NF6) (Axxora, San Diego, CA), monoclonal anti-XIAP (BD Biosciences, San Jose, CA), monoclonal anti-p21-WAF1 (Cell Signaling Beverly, MA); monoclonal anti-Dynamin-2 (Upstate, Lake Placid, NY); polyclonal Abs to TRAIL (human), TRAIL-R1/DR4 and TRAIL-R2/DR5 (Axxora, San Diego, CA); polyclonal Abs against: phospho-STAT3 (Tyr705) and STAT3; phospho-c-Jun (Ser73) and c-Jun; phospho-SAPK/JNK (Thr183/Tyr185) and JNK; phospho-p44/p42 MAP kinase (Thr202/Tyr204) and p44/p42 MAP kinase; phospho-p38 MAP kinase (Thr180/Tyr182) and p38 MAP kinase; phospho-FOXO3A (Ser318/321) and FOXO3A; BAX, phospho-p53 (Ser20) and p53; Bid (Cell Signaling, Beverly, MA). Optimal dilutions of primary Abs were 1:1000 to 1:5000. The secondary Abs anti-mouse or anti-rabbit) were conjugated to horseradish peroxidase (dilution 1:5000 to 1:10000); signals were detected using the ECL system (Amersham, Piscataway, NJ).
EMSA
Electrophoretic mobility shift assay (EMSA) was performed for the detection of NF-κB DNA-binding activity as previously described, using the labeled double-strand oligonucleotide AGCTTGGGGACTTTCCAGCCG. (Binding site is underlined). Ubiquitous NF-Y DNA-binding activity was used as an internal control [15].
Immunofluorescence
Immunofluorescence was performed as described previously [27]. Cells were grown on glass coverslips in growth medium, fixed (PBS, 4% paraformaldehyde for 10 min), permeabilized (PBS, 0.5% Triton X-100 for 5 min) and stained with antibodies to pyruvate dehydrogenase (Binding Site, United Kingdom). Secondary antibodies were obtained from Jackson ImmunoResearch. Nuclear staining was done with propidium iodide (PharMingen). Images were captured using a laser confocal microscope (Nikon).
Results
Sensitivity of melanoma cell lines to resveratrol
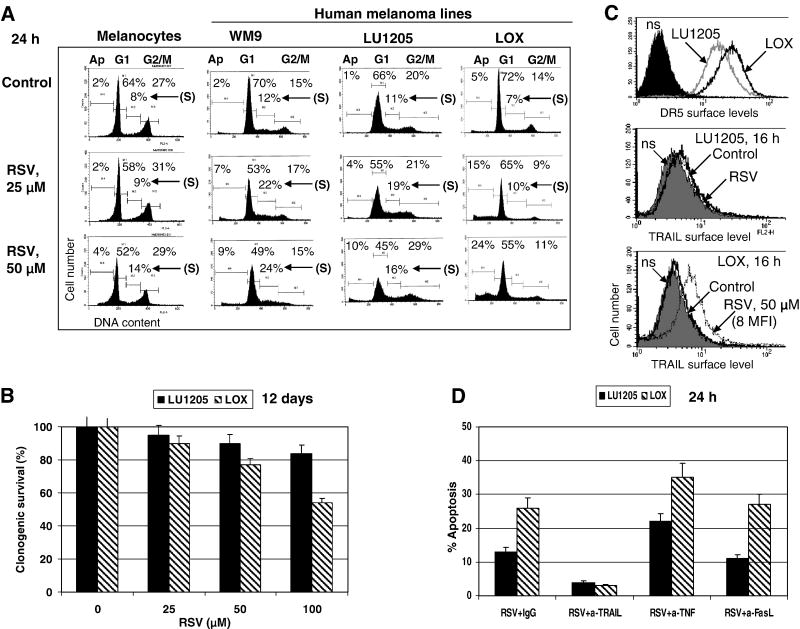
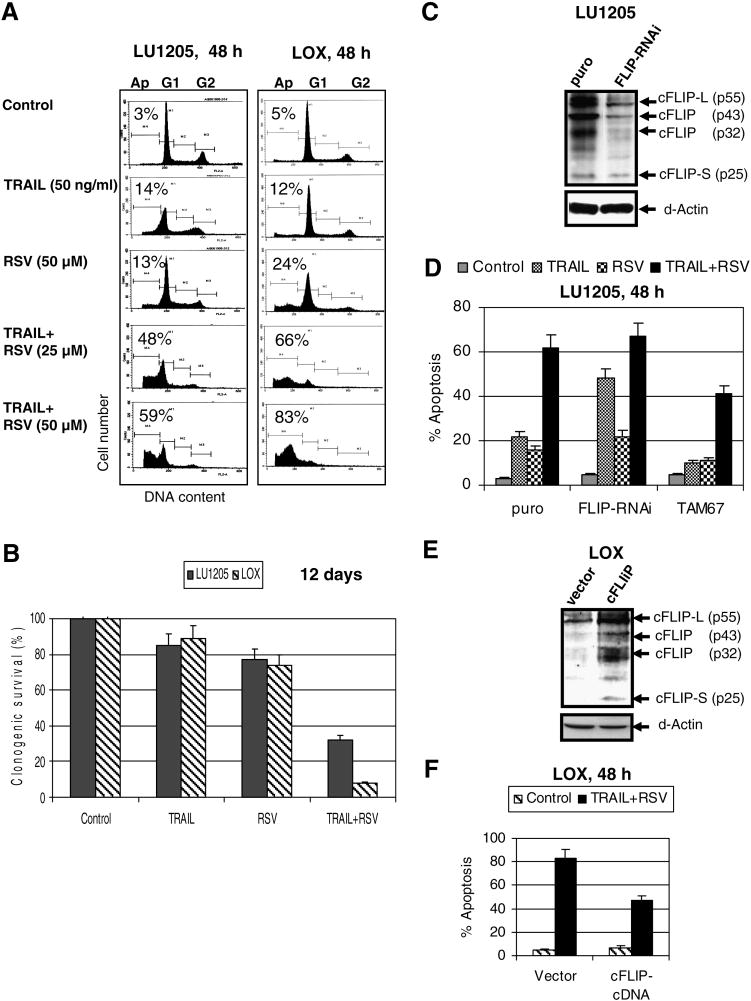
Human melanocytes, normal human fibroblasts and most metastatic human melanoma cell lines are relatively resistant to RSV-induced apoptotic stimuli (at RSV doses of 25-100 μM), demonstrating only a modest S/G2 arrest in the cell cycle (Fig. 1A and data not shown). The rare example of a moderate sensitivity to RSV was LOX human metastatic melanoma cells, which developed average levels of apoptosis 24 h after treatment (Fig. 1A). A clonogenic survival assay confirmed death in over 40% of LOX cells 12 days after treatment with 100 μM RSV. In contrast, LU1205 metastatic melanoma cells were more resistant to RSV (Fig. 1B). Both LOX and LU1205 melanoma cells express on their surface FAS, TRAIL-R2/DR5 and TNF-R1 (Fig. 1C and data not shown). RSV treatment did not change surface expression of DR5 and FAS death receptors (data not shown), but induced surface expression of endogenous TRAIL in LOX cells (Fig. 1C). RSV-induced death of LOX cells could be blocked by the addition of an inhibitory antibody to TRAIL, but not to FasL (5 μg/ml) in the culture medium (Fig. 1D). In contrast, inhibition of TNFα, which was constitutively produced by many melanoma cell lines and induced basal NF-κB activity, accelerated RSV-induced apoptosis in both LOX and LU1205 lines (Fig. 1D), linking protection against RSV/TRAIL apoptotic signaling with high basal levels of NF-κB activity. Hence, endogenously produced TRAIL after translocation to cell surface, via paracrine action, induced a receptor-mediated death signaling cascade in TRAIL-R-positive LOX cells, ultimately resulting in suicide of subpopulation of these cells (Fig. 1A).
Figure 1. Effects of resveratrol (RSV) on induction of apoptosis in melanocytes and melanomas.
(A) Cells were stained by PI 16-24 h after treatment. Apoptosis (Ap) levels were determined as the percentage of cells with hypodiploid content of DNA in the pre-G0/G1 region using flow cytometry. % Cells at the distinct phases of the cell cycle is indicated. Results of typical experiments (one of three) are presented. (B) Clonogenic survival assay of LU1205 and LOX cells 12 days after indicated treatment. Error bars represent mean ± S.D. from three independent experiments. (C) Surface expression of TRAIL-R2/DR5 and TRAIL in LU1205 and LOX cells was determined by immunostainig using PE-labeled mAbs and the FACS analysis. (D) RSV induced TRAIL-mediated apoptosis in LOX melanoma cells. Introduction of anti-TRAIL (5 μg/ml), but not anti-FasL inhibitory antibodies suppressed RSV-induced apoptosis in LOX and LU1205 cells, while anti-TNF mAb (5 μg/ml) accelerated apoptosis.
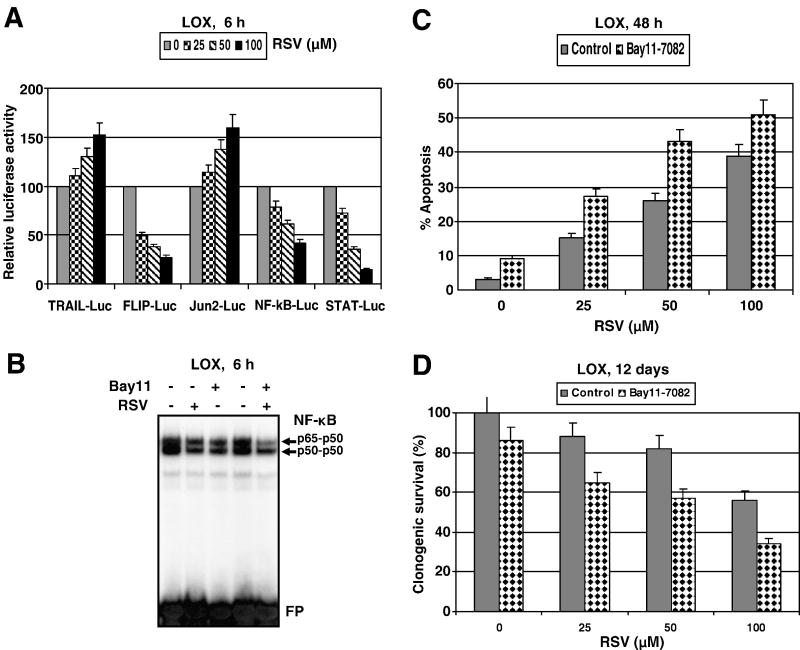
There are at least two possible mechanisms that could account for the sensitivity of LOX cells to RSV: i) down-regulation of STAT3- and NF- κB-dependent transcription, which regulate numerous survival functions in the cell (Fig. 2A); ii) upregulation of TRAIL promoter activity and increased surface expression of endogenous TRAIL (but not TRAIL-R) induced by RSV in a dose-dependent manner (Fig. 2A and 1C). In general, changes in nuclear NF-κB DNA-binding activity and NF-κB-dependent reporter activity were relatively modest after RSV (50 μM) treatment in this cell line (Fig. 2A and B). An additional suppression of NF-κB activity by pharmacological inhibitor Bay 11-7082 (5 μM) upregulated RSV-induced apoptotic levels and decreased clonogenic survival of LOX melanoma cells (Fig. 2B-D).
Figure 2. Modulation of RSV-induced apoptosis in LOX human melanoma cells.
(A) Effects of RSV (25-100 μM; 6 h) on NF-κB-, AP-1/cJun- and STAT-dependent luciferase reporter activities, TRAIL and FLIP promoter activities. The reporter constructs used were: 2×NF-κB-Luc, Jun2-Luc, 3×Ly6A/E-Luc (STAT-dependent), 1.5 kb TRAILpr-Luc, 1 kb cFLIPpr-Luc and tk-Luc, as the control vector with tk-promoter. Luciferase reporter activity was normalized based on β-gal activity; β-gal expression construct was cotransfected at a ratio of 1/4. A ratio of the specific luciferase reporter activity to luciferase activity driven by the empty tk-Luc vector in the mock control culture is indicated. Error bars represent mean ± S.D. from three independent experiments. (B) Effects of RSV (50 μM) and Bay11-7082 (5 μM) on basal nuclear NF-κB DNA binding activity in LOX cells determined by EMSA. Positions of DNA-binding complexes and free labeled probe (FP) are indicated. (C, D) Effects of pharmacological inhibitor of NF-κB (Bay 11-7082, 5 μM), on apoptosis and clonogenic survival of LOX cells after RSV treatment. Error bars represent mean ± S.D. from three independent experiments.
Signaling pathways affected by RSV in LU1205 melanoma cells
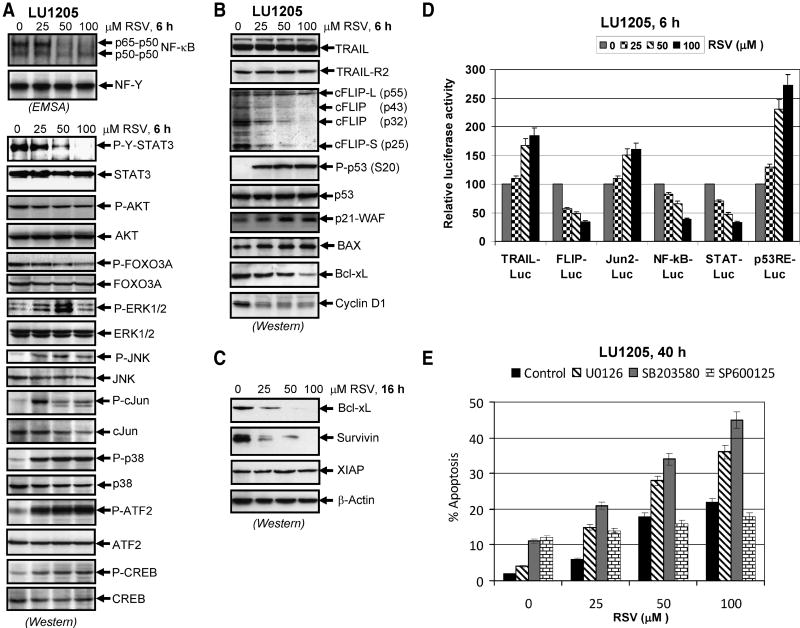
Effects of RSV on gene transcription directed by AP-1, NF-κB and STAT3 and on TRAIL-, cFLIP-promoter activities were relatively similar in both LOX and LU1205 melanoma cells (Fig. 2A and 3D). In order to determine biochemical background of RSV-induced changes in the cell cycle and RSV-induced decrease in cancer cell survival, we evaluated its effects on the main signaling pathways, the correspondent transcription factors, proapoptotic and antiapoptotic proteins in LU1205 cells (Fig. 3A-C). Pronounced downregulation of NF-κB DNA-binding activity 6 h after RSV treatment was determined by EMSA in the nuclear fraction of LU1205 cells (Fig. 3A). Levels of the active form of STAT3, phospho-STAT3 (Tyr705), were substantially decreased 6 h after treatment of LU1205 cells with RSV. Simultaneously, a decrease in phospho-FOXO3A (Ser318/321) levels was also detected (Fig. 3A). AKT-dependent phosphorylation of FOXO3A is known to induce its nuclear export and functional inactivation [28]. Partial suppression of this process by RSV should enhance the proapoptotic function of FOXO3A.
Figure 3. Effects of resveratrol (RSV) on cellular proteins controlling cell survival and apoptosis in LU1205 human melanoma cells.
(A, B) Effects of RSV on basal nuclear NF-κB and NF-Y activities in SW1 and LU1205 cells determined by EMSA 6 h after treatment. Positions of DNA-binding complexes are indicated. Free labeled probes are not shown. Western blot analysis was performed for detection of total and phospho-protein levels of STAT3, AKT, FOXO-3A, ERK1/2, JNK1/2, cJun, p38MAPK, ATF2, CREB, TRAIL, TRAIL-R2/DR5, cFLIP, P-(Ser20) p53, total p53, p21-WAF, BAX, Bcl-xL and of Cyclin D1 6 h after treatment with indicated concentrations of RSV. (C) Western blot analysis of Bcl-xL, survivin, XIAP and β-actin levels in LU1205 cells 16 h after treatment with RSV. (D) Effects of RSV (25-100 μM; 6 h) on AP-1/cJun-, NF-κB-, STAT- and p53-dependent luciferase reporter activities, TRAIL and FLIP promoter activities. The reporter constructs used were: Jun2-Luc, 2×NF-κB-Luc, 3×Ly6A/E-Luc (STAT-dependent), p53RE-Luc, 1.5 kb TRAILpr-Luc, 1 kb cFLIPpr-Luc and tk-Luc, as the control vector with tk-promoter. Luciferase reporter activity was normalized based on β-gal activity; β-gal expression construct was cotransfected at a ratio of 1/4. A ratio of the specific luciferase reporter activity to luciferase activity driven by the empty tk-Luc vector in the mock control culture is indicated. Error bars represent mean ± S.D. from three independent experiments. (E) Effects of pharmacological inhibitors of MEK-ERK (U0126, 10 μM), p38 MAPK (SB203580, 10 μM) and JNK (SP600125, 10 μM) on RSV-induced apoptosis in LU1205 cells. Error bars represent mean ± S.D. from three independent experiments.
In contrast, RSV treatment upregulated levels of active forms of MAP kinases, phospho-ERK1/2 (Thr202/Tyr204), phospho-p38 (Thr180/Tyr182) and phospho-JNK-1/2 (Thr183/Tyr185). This was followed by an increase in phospho-cJun (Ser73) levels, with a maximum phosphorylation observed for RSV at 25 μM in LU1205 cells. There was also a strong increase in phospho-ATF2 (Thr71) levels and a modest increase in phospho-CREB (Ser133) levels after RSV treatment at 25-100 μM (Fig. 3A). Additionally, upregulation of phospho-p53 (Ser20) level, and increased expression of its target, p21-WAF, was also detected after RSV treatment of LU1205 cells (Fig. 3B) that was well correlated with a general increase of p53-dependent transcription after RSV treatment (Fig. 3D). Furthermore, p53-dependent BAX protein level [29] increased also after RSV treatment. On the other hand, pronounced downregulation of cyclin D1 level was caused by RSV (Fig. 3B). Negative regulation of cFLIP promoter activity by RSV was accompanied by a rapid decrease of cFLIP protein levels due to protein degradation, which could increase sensitivity to TRAIL-mediated apoptotic signaling. Downregulation of processed forms of cFLIP-L, FLIP-p43, FLIP-p32 and cFLIP-S (p25) was observed 6 h after treatment in LU1205 cells (Fig. 3B). Protein levels of anti-apoptotic Bcl-xL and survivin were also substantially decreased 16 h after RSV treatment (Fig. 3C). Since expression of cyclin D1, Bcl-xL and Survivin required positive transcriptional control of STAT3 [30, 31], the observed changes in these levels were expected. In contrast, levels of anti-apoptotic protein XIAP did not notably decrease after RSV treatment in LU1205.
In order to determine a role of elevated activation of the MAPK pathways (MEK-ERK, MKK6-p38-ATF2, MKK4/7-JNK-cJun) by RSV treatment in LU1205 cells, we used specific pharmacological inhibitors: U0126 (10 μM) for MEK-ERK, SB203580 (10 μM) for p38 and SP600125 (20 μM) for JNK. Inhibition of RSV-induced MEK-ERK or p38 MAPK activation substantially accelerated apoptotic response of LU1205 cells, while inhibition of JNK activity did not cause pronounced changes in apoptotic levels induced by RSV (Fig. 3E). It obviously demonstrated prosurvival function of ERK1/2 and, especially, MAPK p38 activation following RSV treatment of LU1205 melanoma cells. JNK activation by RSV in LU1205 cells appears to play dual proapoptotic and prosurvival role and suppression of JNK does not notably change the life-death balance in RSV-treated LU1205 melanoma cells.
Regulation of the main signaling pathways by RSV human LU1205 melanoma cells resulted in strong downregulation of antiapoptotic protein levels that could sensitize these cells to TRAIL-mediated apoptosis. On the other hand, direct RSV-dependent induction of cell killing additionally required an efficient surface expression of endogenous TRAIL. In contrast to LOX cells, RSV did not notably induce surface expression of endogenous TRAIL in LU1205 cells, in spite of high levels of intracellular TRAIL (Fig. 3B), demonstrating a possible deficiency in TRAIL protein translocation from the intracellular pools to cell surface. Consequently, RSV treatment did not initiate pronounced TRAIL-mediated suicide of these melanoma cells.
Cell death in human melanomas induced by either recombinant TRAIL or combination of TRAIL and RSV
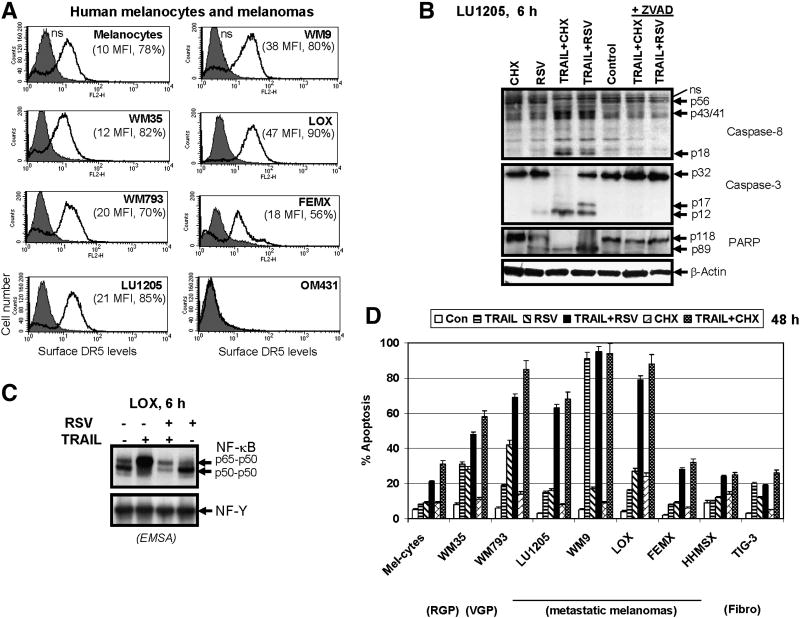
TRAIL-mediated cell death can be induced in TRAIL-R-positive cancer cells either via stimulation of expression and translocation of endogenous death ligand to cell surface with subsequent paracrine action (as we observed for RSV stimulation of LOX cells) or by exogenous TRAIL secreted by natural immunocompetent cells, or finally by pharmacological recombinant TRAIL. In all cases, surface levels of death receptors in cancer cells are critical factors regulating initiation of death signaling in cancer cells. Normal human melanocytes and many human melanomas exhibit TRAIL-Receptor-2/DR5 on their surface (Fig. 4A). TRAIL-R1/DR4 could also be detected on cell surface of some human melanomas, however, at relatively low levels [11, 32]. For early radial growth phase (RGP) WM35 melanoma, levels of surface expression of DR5 are quite similar to melanocytes. In vertical growth phase (VGP) melanoma WM793, and in some metastatic melanomas (LU1205, WM9 and LOX), surface expression of DR5 is higher than in melanocytes (Fig. 4A). In contrast, both FEMX (Fig. 4A) and HHMSX (data not shown) metastatic melanomas consist of two cell subpopulations with low (18 MFI) and negligible levels of DR5 on the cell surface. Finally, metastatic OM431 melanoma cells appear to have lost expression of DR5 on their surface almost completely (Fig. 4A).
Figure 4. Surface expression of TRAIL-R2/DR5 and TRAIL-induced apoptosis in human melanomas.
(A) Surface expression of TRAIL-R2/DR5 levels in human melanocytes and melanoma cell lines was determined using PE-labeled mAb to DR5 and FACS analysis. MFI (medium fluorescence intensity) and % positive cells are indicated; ns – nonspecific staining. (B) Western blot analysis of caspase-8, caspase-3 and PARP cleavage 8 h after apoptotic stimulation. TRAIL (50 ng/ml), RSV (50 μM), zVAD-fmk (5 μM) and their combinations were used for treatment of LU1205 human melanoma cells. (C) RSV (50 μM) suppressed TRAIL-induced up-regulation of NF-κB p65-p50 DNA-binding activity in LOX melanoma cells. RSV was added into the media 30 min before TRAIL (50 ng/ml). EMSA was performed with nuclear extracts of control and treated cells using NF-κB- and NF-Y-binding labeled probes. Positions of DNA-binding complexes are indicated. (D) Resveratrol (RSV) dramatically increased sensitivity to TRAIL in human melanomas. Effects of TRAIL (50 ng/ml), RSV (50 μM), CHX (2 μg/ml) and their combinations on apoptosis of human melanocytes, melanomas and the normal human fibroblasts TIG-3 were determined 48 h after treatment. Error bars represent mean ± S.D. from three independent experiments.
In general, most human melanomas are relatively resistant to apoptosis induced by TRAIL alone. Furthermore, there was no simple linear correlation between surface levels of DR5 and apoptotic response to recombinant TRAIL (50 ng/ml) for the melanoma lines tested, including LOX and LU1205 cells (Fig. 4A, D). Evidently, additional components may be involved in regulating the death signaling pathway. Indeed, TRAIL in the presence of an inhibitor of protein synthesis, cycloheximide (CHX, 2 μg/ml), induced caspase-8/caspase-3 activation, leading to PARP cleavage (Fig. 4B), and dramatically accelerated TRAIL-mediated apoptotic signaling that in this case was proportional to death receptor surface expression levels (Fig. 4A and D). Caspase activation and death signaling was suppressed by the universal inhibitor of caspases, zVAD-fmk. Enhancing effects of CHX on TRAIL-induced death signaling were probably due to translational suppression of short-lived protein inhibitors of apoptosis, including cFLIP [33, 34].
Apart from protein synthesis inhibitors, RSV was previously implicated in sensitization to TRAIL-mediated apoptosis in different cancer cell lines [16, 17, 35]. Based on its effects on signaling pathways and expression of anti-apoptotic proteins (Fig. 3), RSV dramatically increased sensitivity of DR5-positive metastatic melanomas, including LU1205 and LOX, to exogenous recombinant TRAIL that was revealed by an enhanced level of activation of the death signaling cascade after combined treatment (Fig. 4B). We observed that the death signaling pathway was highly active after either TRAIL+CHX or TRAIL+RSV stimulation of the DR5 receptor. As early as 6-8 h after treatment, protein processing of caspase-8, caspase-3 and cleavage of PARP, the classical target of caspase-3, were easily detectable. RSV (50 μM) by itself demonstrated low to moderate caspase activation in LU1205 cells (Fig. 4B). Determination of nuclear NF-κB DNA-binding activity by EMSA revealed again modest negative effects of RSV on the basal levels of nuclear NF-κB in LU1205 and LOX melanoma cells, while a negative effect of RSV on TRAIL-induced nuclear NF-κB p65-p50 activities was more pronounced (Fig. 4C and data not shown). Importantly, RSV suppressed initial survival signals induced by TRAIL via NF-κB activation and shifted the life-death balance to favor TRAIL-R2-mediated death signaling.
The levels of TRAIL+RSV induced apoptotic death in human melanomas were determined 48 h after treatment. Combined treatment of DR5-positive metastatic melanomas, LU1205 and LOX, with exogenous TRAIL (50 ng/ml) and RSV (25-50 μM) resulted in an effective induction of apoptosis, which was more pronounced in LOX cells with higher levels of DR5 surface expression (Fig. 4D). WM9 cells displayed elevated sensitivity to TRAIL alone and did not require any additional stimulation. On the other hand, OM431 melanoma cells, which were deficient in surface DR5 expression (Fig. 4A) but possessed modest DR4 surface levels [11], exhibited TRAIL+RSV mediated apoptosis at low levels (data not shown). FEMX and HHMSX metastatic melanoma cells (Fig. 4D) also weakly responded to combined treatment of TRAIL+RSV. Although TIG-3 (DR5-positive normal human lung fibroblasts) modestly responded to TRAIL alone, neither CHX nor RSV increased TRAIL-induced apoptotic levels in these cells. Melanocytes also developed only low levels of apoptosis after combined treatment (Fig. 4D). This remarkable feature of normal human cells allowed select TRAIL+RSV combination for specific targeting of TRAIL-R-positive cancer cells.
As we observed in the present study, RSV treatment notably induced endogenous TRAIL surface expression in LOX, but not in LU1205 cells, and consequently initiated moderate TRAIL-mediated apoptosis in LOX cells (Fig. 1). However, a combination of exogenous recombinant TRAIL and RSV induced pronounced apoptosis in both cell lines, which still was notably higher for LOX cells, probably due to higher DR5 surface expression in these cells (Fig. 5A). A clonogenic survival assay also demonstrated very low survival of LOX cells when compared to LU1205 cells 12 days after combined treatment of TRAIL and RSV (Fig. 5B).
Figure 5. Synergistic interaction of TRAIL and RSV for induction of apoptosis in human metastatic melanomas LU1205 and LOX. A role of cFLIP in resistance to TRAIL.
(A) Effects of TRAIL (50 ng/ml), RSV (25 μM and 50 μM) and their combinations on apoptosis of LU1205 and LOX human melanoma cells. Cells were stained by PI 48 h after treatment. Apoptosis levels were determined as percentage of cells with hypodiploid content of DNA in the pre-G0/G1 region using flow cytometry. Results of typical experiments (one of four) are presented. (B) Clonogenic survival assay of LU1205 and LOX cells 12 days after indicated treatment. Error bars represent mean ± S.D. from three independent experiments. (C) Suppression of cFLIP expression by specific RNAi. Protein levels of cFLIP and β-actin were determined by Western blot analysis of LU1205 cells stably transfected by the empty vector (puro) and FLIP-RNAi expression construct. (D) Apoptosis levels 48 h after treatment of control (puro), TAM67-transfected LU1205 cells and cFLIP-RNAi transfected LU1205 cells with indicated stimili: TRAIL (50 ng/ml), RSV (50 μM) or their combination. Apoptosis levels were determined as the percentage of cells with hypodiploid content of DNA in the pre-G0/G1 region using flow cytometry. Error bars represent mean + S.D. from three independent experiments. (E, F) LOX melanoma cells were transfected by the empty vector or cFLIP expression construct in the presence of pEF-GFP. Apoptotic levels were determined in GFP-positive cells 48 h after indicated treatment using PI staining and the flow cytometry.
Effects of modulation of cFLIP protein levels on TRAIL-induced apoptosis
Activation of the JNK-cJun pathway is involved in downregulation of cFLIP expression [11, 13], which has been described as one of the main negative regulators of the extrinsic apoptotic pathway. Since inhibition of JNK activity modulates numerous cell functions besides cFLIP regulation, we used direct cFLIP suppression by specific RNAi to determine a role of cFLIP in TRAIL+RSV induced apoptosis. Suppression of cFLIP by specific RNAi (Fig. 5C) increased the levels of TRAIL-induced apoptosis, but did not additionally affect TRAIL+RSV induced apoptosis, suggesting that RSV and FLIP-RNAi affected the same target (Fig. 5D). In contrast, TAM67, a dominant negative cJun expression construct, substantially upregulated cFLIP expression and protein levels [11]. LU1205 melanoma cells with increased levels of cFLIP after transfecion with TAM67 were also more resistant to TRAIL+RSV induced apoptosis (Fig. 5D). Mass cultures of both LU1205 and LOX cells transfected with the cFLIP expression construct demonstrated upregulation of cFLIP expression and decreased levels of TRAIL+RSV induced apoptosis (Fig. 6E, F and data not shown). Taken together, these data further demonstrated a role for cJun-dependent negative regulation of cFLIP expression, including RSV-induced downregulation of cFLIP, for the sensitization and upregulation of TRAIL-induced cell death in human melanomas.
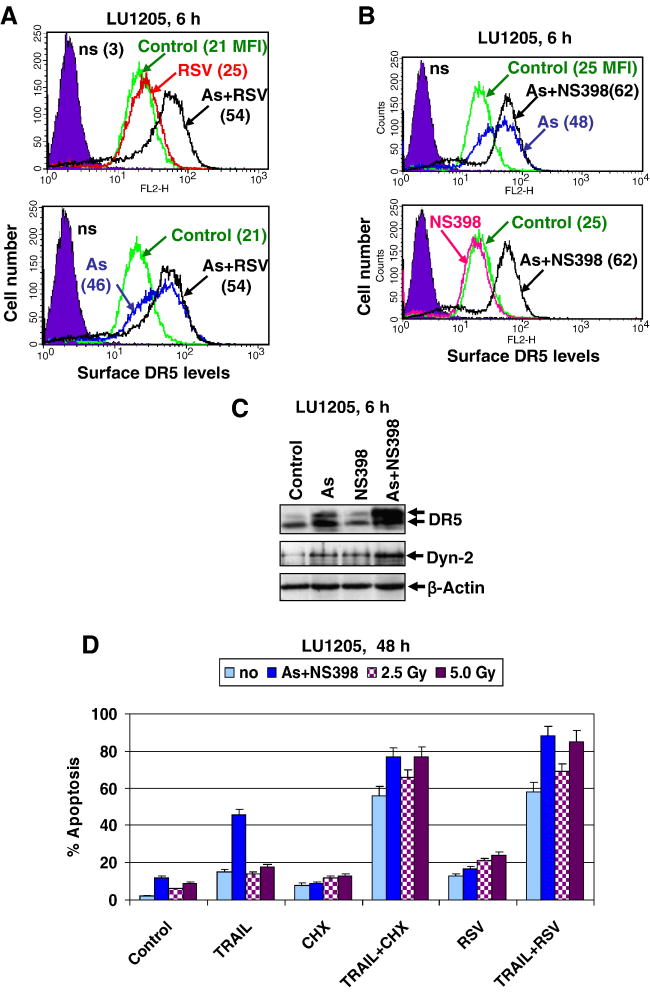
Figure 6. Upregulation of TRAIL and RSV induced apoptosis in LU1205 melanoma cells.
(A) Combined effects of sodium arsenite (As, 5 μM) and resveratrol (RSV, 50 μM) on DR5 surface expression in LU1205 cells. RSV was added into cell media 1 h after sodium arsenite. Cells were stained 6 h after treatment with PE-labeled anti-human DR5 mAb and analyzed by the flow cytometry; MFI was indicated (B) Combined effects of sodium arsenite (As, 5 μM) and NS398 (50 μM) on DR5 surface expression. (C) Western blot analysis of total protein levels of DR5 and dynamin-2 six h after indicated treatment of LU1205 cells. (D) Acceleration of TRAIL-mediated apoptosis in LU1205 cells after pretreatment with sodium arsenite (5 μM) + NS398 (50 μM) for 16 h, or after γ-irradiation (2.5-5 Gy). TRAIL (50 ng/ml), RSV (50 μM) and CHX (2 μg/ml) were added 16 h after an initial treatment and remained in the media for the next 32 h. Error bars represent mean + S.D. from three independent experiments.
Increasing DR5 surface expression in metastatic melanoma cells enhances TRAIL and RSV induced apoptosis
We attempted to further increase DR5 surface expression in LU1205 cells in order to achieve effective killing of these metastatic melanoma cells. We have recently described several approaches to increase surface expression of DR5 in LU1205 human melanoma, including the use of sodium arsenite treatment and γ-irradiation [11, 19]. Treatment of LU1205 melanoma cells by RSV (50 μM) alone had only minor effects on surface DR5 levels, while sodium arsenite in combination with RSV demonstrated some additive effect, compared to sodium arsenite alone (Fig. 6A). However, a combination of sodium arsenite and NS398 (50 μM), a COX-2 inhibitor, had the most pronounced effects on up-regulating DR5 surface levels in LU1205 melanoma cells (Fig. 6B). The effect of sodium arsenite+NS398 on DR5 surface expression was dependent on the upregulation of intracellular protein levels of both DR5 and dynamin-2, an adaptor protein critically involved in death receptor translocation from the Golgi to the cell surface [19, 36] (Fig. 6C).
Finally, an initial treatment of LU1205 cells with arsenite+NS398 or with γ-irradiation (2.5--5 Gy) and subsequent treatment with TRAIL+RSV caused strong upregulation of LU1205 cell death (Fig. 6D). Taken together, these results confirmed a role of elevated DR5 surface expression to further accelerate TRAIL+RSV induced apoptosis in LU1205 melanoma cells.
Upregulation of TRAIL-mediated apoptosis in highly resistant HHMSX metastatic melanoma cells using the extrinsic receptor-mediated pathway
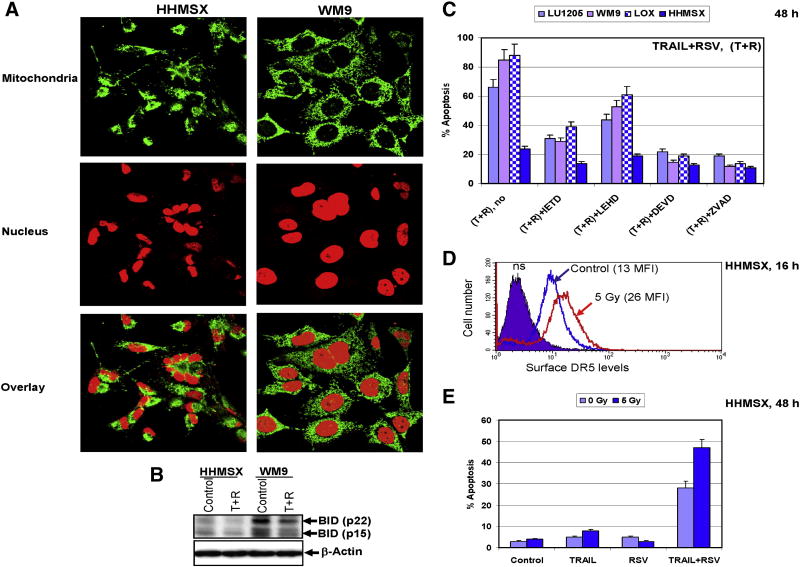
HHMSX metastatic melanoma cells were highly resistant to the death-receptor-mediated apoptosis. Mitochondrial phenotype of these cells was evaluated using immunostaining. In normal human fibroblasts and melanoma WM9 cells the mitochondrial staining consistently displayed a characteristic elongated morphology was mainly perinuclear (Fig. 7A and data not shown). In contrast, the mitochondrial staining of HHMSX melanoma cells differed significantly from cell to cell and had an inconsistent and variable morphology, indicating probably an alteration of mitochondrial function in these cells (Fig. 7A). Intracellular levels of BID-p22 and its cleaved form BID-p15 proteins, which were critically important for the transmission of caspase-8-mediated signaling to mitochondria, were substantially decreased in HHMSX cells, further demonstrating a low efficiency of the mitochondrial pathway (Fig. 7B).
Figure 7. HHMSX melanoma cells have dysfunctional mitochondrial apoptotic pathway, but undergo TRAIL-mediated apoptosis.
(A) Mitochondrial morphology of melanoma cell lines. WM9 and HHMSX metastatic melanomas were stained with anti-Pyruvate dehydrogenase (PDH - green) to visualize mitochondria, and propidium iodide (PI - red) to observe the nucleus. The mitochondrial and nuclear staining of HHMSX cells was aberrant, indicative of a highly transformed cell line. (B) Western blot analysis of BID (p22 and p15) levels in control and TRAIL+RSV treated melanoma cells. (C) Induction of apoptosis in melanoma lines 48 h after indicated treatment. TRAIL (50 ng/ml) and RSV (50 μM) were used. Caspase inhibitors: IETD, LEHD, DEVD and zVAD (20 μM) have been added to the media 30 min before TRAIL+RSV. Cells were stained by PI 48 h after treatment. Apoptosis levels were determined as the percentage of cells with hypodiploid content of DNA in the pre-G0/G1 region using flow cytometry. Error bars represent mean ± S.D. from three independent experiments. (D) Upregulation of surface DR5 levels in HHMSX cells following γ-irradiation (16 h) or sequential treatment with irradiation and RSV (16 h after γ-irradiation for the next 16 h) and was determined by immunostaining with PE-labeled anti-human DR mAb using the flow cytometry; MFI is indicated. (E) Acceleration of apoptosis in HHMSX cells with increased levels of DR5. Cell were not treated or γ-irradiated at 5 Gy; 16 h after irradiation TRAIL (50 ng/ml), RSV (50 μM) or TRAIL+RSV were added and remained in the media for an additional 32 h. Cell cycle-apoptosis analysis was performed using PI staining DNA and the FACS analysis. Results of a typical experiment are indicated.
Specific inhibitors of caspases also indicated that HHMSX melanoma cells have a dysfunctional mitochondrial death pathway. Indeed, TRAIL+RSV induced apoptosis in several human melanomas was suppressed by Ac-DEVD-CHO, a caspase-3 inhibitor, and zVAD-fmk, a universal inhibitor of caspases. Ac-IETD-CHO (5 μM), a caspase-8 inhibitor, and Ac-LEHD-CHO (5 μM), a caspase-9 inhibitor, provided some protection against TRAIL-mediated apoptosis in WM9 and LU1205 cells. In contrast, Ac-LEHD-CHO was not effective in HHMSX cells, revealing a deficiency in their mitochondrial pathway (Fig. 7C). These data correlated well with the involvement of both caspase-8 and caspase-9 mediated apoptotic pathways in response to TRAIL in WM9 and LU1205 melanoma cell lines. However, it also indicated that apoptotic death of melanoma cells, such as HHMSX, with their suppressed mitochondrial pathway, could be independent of caspase-9 activity.
We attempted to increase the levels of TRAIL-mediated apoptosis in highly resistant HHMSX melanoma cells via the extrinsic TRAIL-R2-mediated pathway. Pretreatment with γ-irradiation significantly upregulated DR5 surface levels in HHMSX cells (Fig. 7D). The sequential treatment of HHMSX cells by γ-irradiation, and 16 h after irradiation with TRAIL (50 ng/ml) + RSV (50 μM) caused a substantial increase in levels of TRAIL-mediated apoptosis (Fig. 7E). Clonogenic survival assay further demonstrated that combined treatment with TRAIL and RSV of HHMSX melanoma cells was especially efficient following γ-irradiation, while normal human fibroblasts TIG-3 were affected substantially less under these conditions (data not shown).
Hence, we observed an efficient TRAIL/DR5/caspase-8/caspase-3-mediated death signaling in the presence of RSV, in melanomas harboring defects in mitochondrial death pathway, demonstrating a possibility for the upregulation of apoptotic signaling in these cancer cells via the extrinsic pathway.
Discussion
Numerous investigations over the last two decades have attempted to restore apoptotic signaling pathways in cancer cells. Understanding the role of NF-κB, STAT3 and AP1 in general regulation of cell survival, via transcriptional control of genes with pro-survival and pro-apoptotic functions, was an important goal of these investigations [7, 37, 38]. The discovery of TRAIL and detailed analysis of TRAIL-R1/R2-mediated death signaling in normal and cancer cells led to the theory that TRAIL-R-mediated pathway might be effective for the induction of apoptosis [39, 40]. One important advantage of TRAIL, compared to TNFα and FasL, is its relatively low toxicity in vivo [41]. The safety and efficacy of soluble TRAIL and anti-TRAIL-R1/R2 agonistic mAbs combined with chemotherapy are currently undergoing evaluation in several clinical trials [41]. Unfortunately, sensitivity to TRAIL is not a universal feature of cancer cells. Most melanomas do not respond to apoptotic stimulation induced by TRAIL alone and require cotreatment with an additional sensitizing agent [42].
The internal strategy of genetic and epigenetic regulation during progression of cancer, based on Darwinian selection, is to suppress death signaling at all levels, including down-regulation of transcription of death receptor genes, inhibition of receptor's modification, suppression of translocation of death receptors to the cell surface, elevated mutagenesis in the death domains, the dramatic STAT3/NF-κB-dependent upregulation of expression of apoptotic inhibitors and upregulation of surface expression of decoy receptors, DcR1 and DcR2 [9, 34, 43-45]. Investigation of natural and pharmacological inhibitors of STAT3 and NF-κB represents a significant and novel tactics in the struggle against cancer. In this respect, correlation of anticancer activity of RSV and its capacity to suppress the activation of STAT3 and NF-κB has provided insights into the regulation of apoptosis in cancer cells [16, 46]. Jang et al. [47] have demonstrated the abilities of RSV to inhibit carcinogenesis at multiple stages, and the systemic administration of RSV was shown to inhibit tumor progression in different mouse cancer models [14].
The results of the present study highlighted a significance of RSV-induced p53-p21WAF activation and RSV-induced downregulation of cyclin D1 levels for RSV-induced cell cycle arrest in LU1205 (Fig. 1 and 3). However, RSV has many additional targets within the cell, including general modulators of transcription Sirtuins (class III histon deacetylases) [48, 49], transcription factors NF-κB and STAT3 [16, 17] and many enzymes of general cell metabolism [46]. RSV treatment of several melanoma lines induced a relatively modest decrease in the high basal NF-κB activity and NF-κB-dependent transcription in these cells, while downregulation of STAT3 levels and STAT3-dependent transcription was more pronounced (Fig. 2 and 3). The well known control of Bcl-xL, Survivin, TRAIL and Fas genes by STAT3 certainly demonstrated its integral role as a regulator of apoptosis in melanomas [9]. An additional important target of RSV in melanoma cells was the activation of the JNK-cJun pathway, which was involved in regulating the expression and turnover of cFLIP [11, 13] and the upregulation of sensitivity to TRAIL. Interestingly, that strong activation of MAPK p38-ATF2 by RSV in melanoma cells was involved in the regulation of cell survival that was strongly decreased after specific inhibition of this pathway (Fig. 3).
The results of our study demonstrated that RSV rarely initiates notable upregulation of endogenous TRAIL expression on the cell surface or TRAIL-mediated cell suicide in human melanomas, as most melanomas tested were relatively resistant to either RSV or recombinant TRAIL alone. Importantly, the combined treatment with RSV and TRAIL had a profound synergistic effect on the induction of apoptosis in certain metastatic melanomas. In addition, pretreatment of melanoma cells by either γ-irradiation or sodium arsenite (especially in combination with COX-2 inhibitor) that upregulates DR5 surface levels [19] and subsequent treatment with TRAIL+RSV has promising therapeutic potential, as evidenced by the induction of apoptosis in the most resistant melanoma lines. A role of COX-2 inhibitors in transcriptional upregulation of dynamin-2 expression, which was important for death receptor translocation, was described previously [50].
We should highlight that an alternative approach, which used γ-irradiation and RSV treatment was effective only for killing early stage human melanomas (such as WM35 and WM793) and had only modest improvement in metastatic melanomas (such as LOX). In contrast, this approach was very effective for mouse SW1 metastatic melanoma cells, where TRAIL was actively translocated to cell surface after RSV treatment (unpublished obsevations). Mechanisms that control TRAIL translocation from the intracellular pools to the cell surface [51] are currently unknown and are the subject of continuing investigation. Monoubiquitination was shown to play an important role in the protein translocation of Fas Ligand [52]. However, protein modifications of TRAIL linked with its intracellular trafficking are still incompletely investigated.
Many cancer cells, including melanoma cells, have mitochondrial respiration defects and suppressed mitochondrial function [53]. We observed a similar situation in some metastatic melanomas investigated in our study. Despite inherent mitochondrial deficiencies, we demonstrated in the present study that the extrinsic DR5-mediated death pathway could still be upregulated in metastatic melanoma cells, using pretreatment with γ-irradiation that was followed by combined treatment of TRAIL and RSV. This finding provides a potentially important rational approach to more efficacious melanoma treatment.
Acknowledgments
We would like to thank Drs. A. Chan and S. Y. Fuchs for discussion, Drs. M. Herlyn and Z. Ronai for melanoma cell lines, Drs. R. Davis, W. S. El-Deiry, G. J. Gores, J. Hiscott, M. Karin, J. J. Manfredi, M. E. Perer, S.-Y. Sun, A. N. Shajahan and H. Wajant and for plasmid constructs. This work was supported by NIH Grants CA 49062, ES 11804, Superfund Grant P42 ES 10349, and Environmental Center Grant P30 ES 09089.
Abbreviations
- Ac-IETD-CHO
N-acetyl-Ile-Glu-Thr-Asp-CHO (aldehyde)
- Ac-LEHD-CHO
N-acetyl-Leu-Glu-His-Asp-CHO (aldehyde)
- AP-1
activator protein-1
- DR4
death receptor-4
- DR5
death receptor-5
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-regulated kinase
- FACS
fluorescence-activated cell sorter
- FasL
Fas ligand
- GFP
green fluorescent protein
- JNK
Jun N-terminal kinase
- IκB
inhibitor of NF-κB
- IKK
inhibitor nuclear factor kappa B kinase
- MAPK
mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- MFI
medium fluorescence intensity
- NF-κB
nuclear factor kappa B
- PI
propidium iodide
- ROS
reactive oxygen species
- RNAi
RNA interference
- RSV
resveratrol
- STAT
signal transducers and activators of transcription
- TNFα
tumor necrosis factor alpha
- TRAIL
TNF-related apoptosis inducing ligand
- TRAIL-R
TRAIL-Receptor
- XIAP
X-linked inhibitor of apoptosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krammer PH, Kaminski M, Kiessling M, Gulow K. No Life Without Death. Adv Cancer Res. 2007;97C:111–138. doi: 10.1016/S0065-230X(06)97005-5. [DOI] [PubMed] [Google Scholar]
- 2.Reed JC. Drug insight: cancer therapy strategies based on restoration of endogenous cell death mechanisms. Nature clinical practice. 2006;3:388–398. doi: 10.1038/ncponc0538. [DOI] [PubMed] [Google Scholar]
- 3.Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950–2966. doi: 10.1038/sj.onc.1207558. [DOI] [PubMed] [Google Scholar]
- 4.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 5.Perlis C, Herlyn M. Recent advances in melanoma biology. Oncologist. 2004;9:182–187. doi: 10.1634/theoncologist.9-2-182. [DOI] [PubMed] [Google Scholar]
- 6.Hersey P, Zhuang L, Zhang XD. Current strategies in overcoming resistance of cancer cells to apoptosis melanoma as a model. International review of cytology. 2006;251:131–158. doi: 10.1016/S0074-7696(06)51004-6. [DOI] [PubMed] [Google Scholar]
- 7.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 8.Darnell JE., Jr Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 9.Ivanov VN, Bhoumik A, Ronai Z. Death receptors and melanoma resistance to apoptosis. Oncogene. 2003;22:3152–3161. doi: 10.1038/sj.onc.1206456. [DOI] [PubMed] [Google Scholar]
- 10.Ueda Y, Richmond A. NF-kappaB activation in melanoma. Pigment cell research/sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 2006;19:112–124. doi: 10.1111/j.1600-0749.2006.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivanov VN, Hei TK. Sodium arsenite accelerates TRAIL-mediated apoptosis in melanoma cells through upregulation of TRAIL-R1/R2 surface levels and downregulation of cFLIP expression. Exp Cell Res. 2006;312:4120–4138. doi: 10.1016/j.yexcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Zhang X, Olumi AF. MG-132 sensitizes TRAIL-resistant prostate cancer cells by activating c-Fos/c-Jun heterodimers and repressing c-FLIP(L) Cancer Res. 2007;67:2247–2255. doi: 10.1158/0008-5472.CAN-06-3793. [DOI] [PubMed] [Google Scholar]
- 13.Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov VN, Hei TK. Arsenite sensitizes human melanomas to apoptosis via tumor necrosis factor alpha-mediated pathway. J Biol Chem. 2004;279:22747–22758. doi: 10.1074/jbc.M314131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer research. 2004;24:2783–2840. [PubMed] [Google Scholar]
- 17.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, Nair AS, Shishodia S, Aggarwal BB. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 18.Kotha A, Sekharam M, Cilenti L, Siddiquee K, Khaled A, Zervos AS, Carter B, Turkson J, Jove R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther. 2006;5:621–629. doi: 10.1158/1535-7163.MCT-05-0268. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov VN, Zhou H, Hei TK. Sequential Treatment by Ionizing Radiation and Sodium Arsenite Dramatically Accelerates TRAIL-Mediated Apoptosis of Human Melanoma Cells. Cancer Res. 2007;67:5397–5407. doi: 10.1158/0008-5472.CAN-07-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satyamoorthy K, DeJesus E, Linnenbach AJ, Kraj B, Kornreich DL, Rendle S, Elder DE, Herlyn M. Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res. 1997;7(2):S35–42. [PubMed] [Google Scholar]
- 21.van Dam H, Huguier S, Kooistra K, Baguet J, Vial E, van der Eb AJ, Herrlich P, Angel P, Castellazzi M. Autocrine growth and anchorage independence: two complementing Jun-controlled genetic programs of cellular transformation. Genes Dev. 1998;12:1227–1239. doi: 10.1101/gad.12.8.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baetu TM, Kwon H, Sharma S, Grandvaux N, Hiscott J. Disruption of NF-kappaB signaling reveals a novel role for NF-kappaB in the regulation of TNF-related apoptosis-inducing ligand expression. J Immunol. 2001;167:3164–3173. doi: 10.4049/jimmunol.167.6.3164. [DOI] [PubMed] [Google Scholar]
- 23.Bartke T, Siegmund D, Peters N, Reichwein M, Henkler F, Scheurich P, Wajant H. p53 upregulates cFLIP, inhibits transcription of NF-kappaB-regulated genes and induces caspase-8-independent cell death in DLD-1 cells. Oncogene. 2001;20:571–580. doi: 10.1038/sj.onc.1204124. [DOI] [PubMed] [Google Scholar]
- 24.Ricci MS, Jin Z, Dews M, Yu D, Thomas-Tikhonenko A, Dicker DT, El-Deiry WS. Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol Cell Biol. 2004;24:8541–8555. doi: 10.1128/MCB.24.19.8541-8555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Resnick-Silverman L, St Clair S, Maurer M, Zhao K, Manfredi JJ. Identification of a novel class of genomic DNA-binding sites suggests a mechanism for selectivity in target gene activation by the tumor suppressor protein p53. Genes Dev. 1998;12:2102–2107. doi: 10.1101/gad.12.14.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakhtiarova A, Taslimi P, Elliman SJ, Kosinski PA, Hubbard B, Kavana M, Kemp DM. Resveratrol inhibits firefly luciferase. Biochem Biophys Res Commun. 2006;351:481–484. doi: 10.1016/j.bbrc.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 27.Partridge MA, Huang SX, Hernandez-Rosa E, Davidson MM, Hei TK. Arsenic induced mitochondrial DNA damage and altered mitochondrial oxidative function: implications for genotoxic mechanisms in mammalian cells. Cancer Res. 2007;67:5239–5247. doi: 10.1158/0008-5472.CAN-07-0074. [DOI] [PubMed] [Google Scholar]
- 28.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 29.Selvakumaran M, Lin HK, Miyashita T, Wang HG, Krajewski S, Reed JC, Hoffman B, Liebermann D. Immediate early up-regulation of bax expression by p53 but not TGF beta 1: a paradigm for distinct apoptotic pathways. Oncogene. 1994;9:1791–1798. [PubMed] [Google Scholar]
- 30.Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, Yoder S, Enkemann S, Eschrich S, Lee JH, Beam CA, Cheng J, Minton S, Muro-Cacho CA, Jove R. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 31.Sinibaldi D, Wharton W, Turkson J, Bowman T, Pledger WJ, Jove R. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene. 2000;19:5419–5427. doi: 10.1038/sj.onc.1203947. [DOI] [PubMed] [Google Scholar]
- 32.Xiao C, Yang BF, Song JH, Schulman H, Li L, Hao C. Inhibition of CaMKII-mediated c-FLIP expression sensitizes malignant melanoma cells to TRAIL-induced apoptosis. Exp Cell Res. 2005;304:244–255. doi: 10.1016/j.yexcr.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 35.Fulda S, Debatin KM. Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 2004;64:337–346. doi: 10.1158/0008-5472.can-03-1656. [DOI] [PubMed] [Google Scholar]
- 36.Ivanov VN, Ronai Z, Hei TK. Opposite roles of FAP-1 and dynamin in the regulation of Fas (CD95) translocation to the cell surface and susceptibility to Fas ligand-mediated apoptosis. J Biol Chem. 2006;281:1840–1852. doi: 10.1074/jbc.M509866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 38.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 39.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 40.Takimoto R, El-Deiry WS. Wild-type p53 transactivates the KILLER/DR5 gene through an intronic sequence-specific DNA-binding site. Oncogene. 2000;19:1735–1743. doi: 10.1038/sj.onc.1203489. [DOI] [PubMed] [Google Scholar]
- 41.Schaefer U, Voloshanenko O, Willen D, Walczak H. TRAIL: a multifunctional cytokine. Front Biosci. 2007;12:3813–3824. doi: 10.2741/2354. [DOI] [PubMed] [Google Scholar]
- 42.Hersey P, Zhang XD. How melanoma cells evade trail-induced apoptosis. Nat Rev Cancer. 2001;1:142–150. doi: 10.1038/35101078. [DOI] [PubMed] [Google Scholar]
- 43.Wajant H. CD95L/FasL and TRAIL in tumour surveillance and cancer therapy. Cancer Treat Res. 2006;130:141–165. doi: 10.1007/0-387-26283-0_7. [DOI] [PubMed] [Google Scholar]
- 44.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K, Huw L, Katta V, Cavet G, Hymowitz SG, Amler L, Ashkenazi A. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 45.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 46.Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007;12:4839–4854. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- 47.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 48.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, DuBois RN. Detection of differentially expressed genes in human colon carcinoma cells treated with a selective COX-2 inhibitor. Oncogene. 2001;20:4450–4456. doi: 10.1038/sj.onc.1204588. [DOI] [PubMed] [Google Scholar]
- 51.Cassatella MA, Huber V, Calzetti F, Margotto D, Tamassia N, Peri G, Mantovani A, Rivoltini L, Tecchio C. Interferon-activated neutrophils store a TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) intracellular pool that is readily mobilizable following exposure to proinflammatory mediators. J Leukoc Biol. 2006;79:123–132. doi: 10.1189/jlb.0805431. [DOI] [PubMed] [Google Scholar]
- 52.Zuccato E, Blott EJ, Holt O, Sigismund S, Shaw M, Bossi G, Griffiths GM. Sorting of Fas ligand to secretory lysosomes is regulated by mono-ubiquitylation and phosphorylation. J Cell Sci. 2007;120:191–199. doi: 10.1242/jcs.03315. [DOI] [PubMed] [Google Scholar]
- 53.Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, Hu Y, Ramdas L, Hu L, Keating MJ, Zhang W, Plunkett W, Huang P. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol. 2006;175:913–923. doi: 10.1083/jcb.200512100. [DOI] [PMC free article] [PubMed] [Google Scholar]