Abstract
Objective
To estimate the incremental delivery cost of human papillomavirus (HPV) vaccination of young adolescent girls in Peru, Uganda and Viet Nam.
Methods
Data were collected from a sample of facilities that participated in five demonstration projects for HPV vaccine delivery: school-based delivery was used in Peru, Uganda and Viet Nam; health-centre-based delivery was also used in Viet Nam; and integrated delivery, which involved existing health services, was also used in Uganda. Microcosting methods were used to guide data collection on the use of resources (i.e. staff, supplies and equipment) and data were obtained from government, demonstration project and health centre administrative records. Delivery costs were expressed in 2009 United States dollars (US$). Exclusively project-related expenses and the cost of the vaccine were excluded.
Findings
The economic delivery cost per vaccine dose ranged from US$ 1.44 for integrated outreach in Uganda to US$ 3.88 for school-based delivery in Peru. In Viet Nam, the lowest cost per dose was US$ 1.92 for health-centre-based delivery. Cost profiles revealed that, in general, the largest contributing factors were project start-up costs and recurrent personnel costs. The delivery cost of HPV vaccine was higher than published costs for traditional vaccines recommended by the Expanded Programme on Immunization (EPI).
Conclusion
The cost of delivering HPV vaccine to young adolescent girls in Peru, Uganda and Viet Nam was higher than that for vaccines currently in the EPI schedule. The cost per vaccine dose was lower when delivery was integrated into existing health services.
Résumé
Objectif
Estimer les coûts d'administration croissants du vaccin contre le papillomavirus humain (VPH) chez les jeunes adolescentes au Pérou, en Ouganda et au Viet Nam.
Méthodes
Des données ont été recueillies auprès d'un échantillon d'établissements qui ont participé à cinq projets de démonstration de l'administration du vaccin contre le VPH: un mode d'administration en milieu scolaire a été utilisé au Pérou, en Ouganda et au Viet Nam, un mode d'administration dans un centre de santé a également été utilisé au Viet Nam, et un mode d'administration intégrée, qui a impliqué les services de santé existants, a également été utilisé en Ouganda. Une approche verticale ascendante (Microcosting) a été utilisée pour guider la collecte de données sur l'utilisation des ressources (personnel, fournitures et équipement), et d'autres données ont été obtenues auprès des gouvernements, et dans les dossiers administratifs des centres de santé et des projets de démonstration. Les coûts d'administration sont exprimés en dollars des États-Unis ($) de 2009. Seules les dépenses liées au projet et les coûts du vaccin ont été exclus.
Résultats
Le coût économique d'administration par dose de vaccin variait de 1,44 $ pour une administration intégrée en Ouganda, à 3,88 $ pour une administration en milieu scolaire au Pérou. Au Viet Nam, le coût le plus bas par dose était de 1,92 $ pour une administration dans un centre de santé. Le profil des coûts a révélé qu'en général, les facteurs les plus importants étaient les coûts de démarrage du projet et les coûts récurrents du personnel. Les coûts d'administration du vaccin contre le VPH sont plus élevés que les coûts indiqués pour les vaccins traditionnels recommandés par le Programme d'immunisation élargi (PIE).
Conclusion
Les coûts d'administration du vaccin contre le VPH chez les jeunes adolescentes au Pérou, en Ouganda et au Viet Nam étaient supérieurs à ceux des vaccins actuellement indiqués dans le calendrier du PIE. Le coût par dose de vaccin était le plus bas lorsque l'administration du vaccin était réalisée dans les services de santé existants.
Resumen
Objetivo
Estimar el incremento del coste de la administración de la vacuna contra el virus del papiloma humano (VPH) a adolescentes mujeres en Perú, Uganda y Viet Nam.
Métodos
Se recabaron datos a partir de una muestra de los centros que participaron en cinco proyectos de demostración sobre la administración de la vacuna contra el VPH: en Perú, Uganda y Viet Nam, la administración se efectuó en la escuela; la prestación de asistencia sanitaria en centros de salud se realizó también en Viet Nam; y la entrega administración, que incluía los servicios de salud existentes, también se realizó en Uganda. Se emplearon métodos de microcosteo para orientar la recolección de datos sobre el uso de los recursos (es decir, el personal, los suministros y los equipos). Los datos se obtuvieron del gobierno, de los proyectos de demostración y los registros administrativos del centro de salud. Los gastos de administración se expresaron en 2009 dólares de los Estados Unidos (US$). Se excluyeron los gastos derivados exclusivamente del proyecto y del coste de las vacunas.
Resultados
El coste del envío económico por dosis de vacuna varió de US$ 1,44, en la difusión integrada en Uganda, a US$ 3,88 en la entrega en las escuelas en Perú. En Viet Nam, el coste más bajo por dosis fue de US$ 1,92 para la prestación de asistencia en centros sanitarios. Los perfiles de costes revelaron que, en general, los factores que más contribuyen son los costes de la puesta en marcha del proyecto y los del personal constante. El coste de administración de la vacuna contra el VPH fue mayor que los costes publicados de las vacunas tradicionales recomendadas por el Programa Ampliado de Inmunización (PAI).
Conclusión
El coste de la entrega de vacunas contra el VPH a las adolescentes jóvenes en Perú, Uganda y Vietnam fue mayor que el de las vacunas incluidas actualmente en el programa de EPI. El coste por dosis de vacuna fue menor cuando la entrega estaba integrada en los servicios de salud actuales.
ملخص
الغرض
تقدير تكلفة الإيتاء التكميلية للتطعيم ضد فيروس الورم الحليمي البشري للفتيات المراهقات صغيرات السن في بيرو وأوغندا وفييت نام.
الطريقة
تم جمع البيانات من عينة من المرافق التي شاركت في خمسة مشاريع إيضاحية لإيتاء لقاح فيروس الورم الحليمي البشري: تم استخدام الإيتاء المستند على المدارس في بيرو وأوغندا وفييت نام؛ وتم كذلك استخدام الإيتاء المستند على المراكز الصحية في فييت نام؛ كما تم استخدام الإيتاء المتكامل، الذي اشتمل على الخدمات الصحية القائمة، في أوغندا. وتم استخدام طرق التكاليف الجزئية لتوجيه جمع البيانات بشأن استغلال الموارد (أي الموظفين والإمدادات والمعدات) وتم الحصول على البيانات من السجلات الإدارية الخاصة بالحكومة أو المشاريع الإيضاحية أو المراكز الصحية. وتم التعبير عن تكاليف الإيتاء بمبلغ 2009 دولاراً أمريكياً. وتم استبعاد النفقات المرتبطة بالمشروع على نحو حصري وتكلفة اللقاح.
النتائج
تراوحت تكلفة الإيتاء الاقتصادي لكل جرعة لقاح من 1.44 دولاراً أمريكياً بالنسبة للتواصل المتكامل في أوغندا إلى 3.88 دولاراً أمريكياً بالنسبة للإيتاء المستند على المدارس في بيرو. وفي فييت نام، كانت أقل تكلفة للجرعة الواحدة 1.92 دولاراً أمريكياً بالنسبة للإيتاء المستند على المراكز الصحية. وأظهرت مواصفات التكاليف بشكل عام أن تكاليف بدء المشاريع وتكاليف الموظفين المتكررة كانت أكثر العوامل تأثيراً. وكانت تكلفة إيتاء لقاح فيروس الورم الحليمي البشري أعلى من التكاليف المنشورة عن اللقاحات التقليدية التي أوصى بها البرنامج الموسّع للتمنيع.
الاستنتاج
كانت تكلفة إيتاء لقاح فيروس الورم الحليمي البشري للفتيات المراهقات صغيرات السن في بيرو وأوغندا وفييت نام أعلى من تكلفة اللقاحات التي يتضمنها حالياً جدول البرنامج الموسّع للتمنيع. وكانت التكلفة لكل جرعة لقاح أقل عندما تم دمج الإيتاء في الخدمات الصحية القائمة.
摘要
目的
估算秘鲁、乌干达和越南年轻少女的人类乳头状瘤病毒(HPV)疫苗的配送成本。
方法
从参与五个HPV疫苗配送示范项目的设施样本中收集数据:秘鲁、乌干达和越南使用基于学校的配送;越南还使用基于健康中心的配送;乌干达还使用包含现有卫生服务的综合配送。使用微成本计算方法指导资源(即人员、物资和设备)使用相关数据的收集并从政府、示范项目和健康中心行政记录中获取数据。以2009 年美元表示配送成本。不含项目相关的专项费用和疫苗的成本。
结果
每剂疫苗的经济配送成本为从乌干达综合推广的1.44 美元到秘鲁基于学校配送的3.88 美元不等。在越南,基于健康中心的最低每剂配送成本是1.92 美元。成本资料显示,一般来说,最大的影响因素是项目启动成本和经常性人力成本。HPV疫苗的配送成本高于由疫苗扩大免疫规划(EPI)建议的传统疫苗公开成本。
结论
秘鲁、乌干达和越南年轻少女HPV疫苗配送成本高于EPI规划当前的疫苗成本。如果将配送集成到现有卫生服务中,则每剂成本较低。
Резюме
Цель
Оценить увеличение стоимости вакцинации против вируса папилломы человека (ВПЧ) среди девушек в подростковом возрасте в Перу, Уганде и Вьетнаме.
Методы
Данные были собраны в отдельных выбранных учреждениях, которые принимали участие в пяти демонстрационных проектах по проведению вакцинации против ВПЧ. В Перу, Уганде и Вьетнаме вакцинация проводилась в школах, во Вьетнаме также проводилась вакцинация в медицинских учреждениях. В Уганде также использовалась комплексная вакцинация, с привлечением доступного медицинского обслуживания. Были использованы методы с минимальными затратами на проведение сбора данных по используемым ресурсам (т.е. сотрудникам, материалам и оборудованию), данные также были получены из административных записей государственных учреждений, демонстрационных проектов и центров здравоохранения. Стоимость вакцинации была выражена в 2009 году в долларах США. Расходы, относящиеся исключительно к организации проведения проекта и стоимость самой вакцины были исключены.
Результаты
С экономической точки зрения стоимость вакцинации в расчете на одну дозу варьировалась от 1,44 доллара США для комплексных мероприятий в Уганде до 3,88 долларов США для вакцинации в школах в Перу. Во Вьетнаме самая низкая стоимость дозы составила 1,92 доллара США для вакцинации в центрах здравоохранения. Динамика затрат указывает на то, что в целом наиболее затратными статьями были расходы на организацию проекта и циклическое привлечение персонала. Стоимость проведения вакцинации против ВПЧ была выше опубликованных расходов на традиционные вакцины, рекомендуемые Расширенной программой иммунизации (РПИ).
Вывод
Стоимость вакцинации против ВПЧ среди девушек-подростков в Перу, Уганде и Вьетнаме была выше стоимости вакцин, запланированных в настоящее время РПИ. Стоимость дозы вакцины была ниже, когда вакцинация проводилась в рамках комплексных мероприятий существующих служб здравоохранения.
Introduction
Cervical cancer is a major public health problem: globally it is associated with over 560 000 new cases and around 275 000 deaths each year, more than 85% of which are in developing countries.1 Systematic, organized screening programmes for cervical cancer have had limited success in low-resource settings.2 However, human papillomavirus (HPV) vaccines may offer a new strategy for prevention and recent studies indicate that vaccination can greatly reduce cervical cancer incidence and mortality.3,4
As developing countries consider whether they can afford to introduce HPV vaccination, much attention has focused on the private sector price of two currently available HPV vaccines: the quadrivalent and bivalent formulations. These vaccines cost more than 100 United States dollars (US$) per dose, or more than US$ 300 for the three-dose series. Reported prices in the public sector have been declining and, in 2011, the manufacturer of the quadrivalent vaccine offered it at US$ 5 per dose to the GAVI Alliance for use in countries eligible for Alliance support. Low- and middle-income countries in Latin America can purchase HPV vaccine for US$ 10–US$ 15 per dose through the Revolving Fund of the Pan American Health Organization (PAHO). Young adolescent girls will benefit most from vaccine-based protection against cervical cancer because they are less likely than older girls to have been infected with the HPV types targeted by the vaccine. Although the price per vaccine dose will remain a key consideration when deciding whether to introduce the HPV vaccine, national governments and donors must also take into account the additional resources required for vaccine delivery.3
Between 2006 and 2010, the non-profit global health organization PATH collaborated with the governments of Peru, Uganda and Viet Nam to collect evidence that would assist government decision-making on whether and how to introduce HPV vaccination. The results of formative research5 were used to design demonstration projects of different types of vaccine delivery in partnership with each country’s ministry of health, subnational health and education sectors and other key stakeholders.6–10 Three delivery strategies were investigated: school-based outreach, health-centre-based outreach and integrated outreach, which made use of existing health services. The eligible population was selected by either school grade or age. The areas of implementation were limited geographically but large enough to cover complete administrative districts and to be broadly representative of each country’s population, thereby providing models that were suitable for scaling up in the future.
The strategies used in demonstration projects achieved high coverage among young adolescent girls and were found to be acceptable and feasible.11–17 For school-based outreach, vaccine coverage was 82.6% in Peru, 88.9% in Uganda and 87.8% in Viet Nam. In one project in Uganda, the HPV vaccination programme was integrated with Child Days Plus, a campaign that involves delivering vitamin A supplementation with one or more other child health services, and achieved 60.7% vaccine coverage. Full details of the demonstration projects’ structures and the vaccine strategies used are published elsewhere.11
The primary objective of this study was to report data on the cost of different HPV vaccination strategies for young adolescent girls, a group that is not routinely targeted by other vaccinations or health interventions18,19 The data were obtained from demonstration projects in Peru, Uganda and Viet Nam. A secondary objective was to estimate the financial cost of implementing national HPV vaccination programmes in these countries.
Methods
The feasibility and cost of HPV vaccination in young adolescent girls was assessed in a sample of the facilities that took part in demonstration projects in Peru, Uganda and Viet Nam.12,15 Facilities were selected on the basis of criteria associated with the geographical location and size of each facility, its expected workload and differences in coverage rates for diphtheria, tetanus and pertussis immunization. Details of the facilities surveyed for the cost analysis in each country are given in Table 1. In addition to the facility surveys, interviews were carried out with Expanded Programme on Immunization (EPI) managers at national, state or regional, provincial, and district or block administrative levels.
Table 1. Human papillomavirus vaccination of young adolescent girls in Peru, Uganda and Viet Nam, 2008–2010.
| Country and delivery strategy | Implementation year | Geographical area | Demonstration project |
Current study |
|||
|---|---|---|---|---|---|---|---|
| No. of eligible girlsa | No. of participating schools | No. of participating health centres | No. of facilities selectedb | ||||
| Peru | – | – | – | 264 | 161 | 12 | |
| School-based | 2008 | Piura region | 8092 | – | – | – | |
| Uganda | – | – | – | 417 | 69 | 14 | |
| School-based | 2008 | Ibanda district | 3459 | – | – | – | |
| 2009 | Ibanda district | 2835c | – | – | – | ||
| Integrated outreach | 2008–2009 | Nakasongola district | 2263d | – | – | – | |
| 2009 | Nakasongola district | 1923c | – | – | – | ||
| Viet Nam | – | – | – | 38 | 72 | 12 | |
| School-based | 2008–2009 | Quan Hoa, Nong Cong and Ninh Kieu districts | 2412 | – | – | – | |
| 2009–2010 | Quan Hoa, Nong Cong and Ninh Kieu districts | 1890c | – | – | – | ||
| Health-centre-based | 2008–2009 | Quan Hoa, Nong Cong and Binh Thuy districts | 1507 | – | – | – | |
| 2009–2010 | Quan Hoa, Nong Cong and Binh Thuy districts | 1205c | – | – | – | ||
a The number of eligible girls was determined by counting and creating a list of those eligible at the facilities participating in the human papillomavirus vaccination (HPV) demonstration projects before administration of the first vaccine dose.
b The current study investigated the feasibility and cost of HPV vaccination strategies in a selection of facilities participating in the demonstration projects.
c The decrease from the first time period occurred because of population movements, primarily emigration.
d The figure was derived from a census estimate of girls aged 10 years rather than by a head count.
Source: Adapted from LaMontagne et al.11
Cost data were collected using the ingredients-based costing methods recommended by World Health Organization (WHO) guidelines.20 After the second or third round of HPV vaccination, project staff interviewed EPI managers and health-care personnel about the resources used for the most recent vaccination round. Data were obtained by direct observation on, for example, the activities of, and time spent by, personnel during community mobilization and vaccination sessions, the cold-chain equipment used to store HPV vaccine and the types of vehicle used to distribute vaccine or transport health-care workers to vaccination sites. These data were combined with price and expenditure data to estimate start-up and recurrent costs for service delivery. The potential financial costs of scaling up HPV vaccination were estimated by extrapolating the data collected on resource use and costs. Any expenses related to project activities that would not normally have occurred during the introduction of a new vaccine, such as extensive planning, supervision, coordination and evaluation, were excluded from the analysis.
In all countries, start-up activities were carried out in accordance with WHO guidelines for the introduction of a new vaccine.21 Activities included microplanning; information, education and communication; training of health-care workers; and community mobilization and sensitization. Start-up costs were treated as fixed costs because, although start-up activities typically occur in the first or second year of a vaccine’s introduction, they influence the provision and use of services beyond the pilot phase. Our analysis included all expenses associated with training workshops, except the salaries of the health-care workers who received training. Start-up costs were estimated for each level of the health-care system, annualized over 5 years and distributed proportionately across the number of doses delivered in the three vaccination rounds.
Recurrent costs comprised the cost of: staff time required for HPV vaccination, including salaries and allowances; injection devices and supplies; waste disposal and management; and vaccine transport, storage and distribution. In all three countries, health-care workers received a per diem payment and travel allowances for HPV vaccination, regardless of the delivery strategy.
Annualized depreciation for capital goods was calculated for the vehicles and cold-chain equipment required to transport and store vaccines. The number of useful life-years of capital goods varied by country and depended on the type of vehicle or cold chain equipment used. A standard discount rate of 3% was used to annualize capital costs. Cost data were collected in each country’s national currency and converted to US$ using the exchange rate for the year of collection: US$ 1.00 equalled 3.1 Peruvian soles in 2008, 1946 Ugandan shillings in 2009 and 19 000 Vietnamese dongs in 2010. All cost estimates were adjusted to 2009 US$ using the Consumer Price Index.22
The cost analysis was performed from a government perspective and we assumed that a national HPV vaccination programme would provide vaccine without cost to beneficiaries. For each country, we calculated the economic cost, which was defined as the cost of all resources used regardless of payer, from the average cost per dose for the resources used at the health centre level and added the average cost per dose for the resources used at the national, state or regional, provincial, and district or block level, by geographical region. In estimating the total economic cost of the vaccination programme, we derived a weighted average cost per dose, which took into account the population living in different zones in each geographical region, and multiplied it by the total number of doses delivered. Subsequently, the incremental cost per fully immunized girl was calculated by dividing the total economic cost by the number of girls who received all three vaccine doses. In our study, the calculation took into account the dropout rate between doses, which was less than 3% in Peru and Viet Nam and 6% in Uganda.
We also estimated the annual incremental financial outlay needed to implement a nationwide HPV vaccination programme, where the financial outlay was defined as the actual expenditure on all goods and services. Since we assumed that currently available human resources and the capacity of the existing vaccine supply chain were sufficient for the programme, we omitted capital depreciation and salary costs shared with existing immunization or other health services. We applied the financial delivery cost per dose to a single cohort of 10-year-old girls and assumed 80% coverage, as this was the average coverage achieved in the demonstration projects.11 We also included vaccine costs: the cost per dose for Uganda and Viet Nam was US$ 0.20, which is the current country co-payment for the procurement of vaccines for poor and intermediate countries through the GAVI Alliance; the cost for Peru was US$ 14.00 per dose, which is the cost for middle-income developing countries through PAHO’s Revolving Fund.23,24 In accordance with GAVI Alliance policy,24,25 a handling fee of 4% was added to the value of the co-payment for Uganda and Viet Nam. For Peru, a 3% PAHO Revolving Fund fee and a 3% supplement for freight and insurance were added to the cost of the vaccine.26 Also added was a 5% allowance for wastage in all countries.
Table 2 presents the number of doses administered and the number of girls who were fully immunized in the demonstration projects, by country and vaccination strategy, and lists the economic and financial costs of vaccination derived using these figures. Data were processed and analysed using Excel (Microsoft, Redmond, United States of America).
Table 2. Incremental cost of delivering human papillomavirus vaccine to young adolescent girls in demonstration projects in Peru, Uganda and Viet Nam, 2008–2010.
| Country and delivery strategy | Average delivery cost per dose (2009 US$) |
No. of doses administered each year | No. of fully immunized girlsa | Annual delivery costsb (2009 US$) |
||
|---|---|---|---|---|---|---|
| Economicc | Financiald | Economicc | Financiald | |||
| Peru | ||||||
| School-based | 3.88 | 2.03 | 26 798 | 8 895 | 103 976 | 54 400 |
| Uganda | ||||||
| School-based | 3.15 | 2.10 | 9 729 | 3 038 | 30 646 | 20 431 |
| Integrated outreach | 1.44 | 1.11 | 8 624 | 2 388 | 12 419 | 9 573 |
| Viet Nam | ||||||
| School-based | 2.08 | 1.62 | 5 324 | 1 766 | 11 074 | 8 625 |
| Health-centre-based | 1.92 | 1.55 | 3 550 | 1 181 | 6 816 | 5 503 |
US$, United States dollars.
a A fully immunized girl was one who received all three vaccine doses.
b Annual delivery costs for the demonstration projects do not include the cost of the vaccine.
c The economic delivery cost was defined as the cost of all resources used, including donated or discounted goods and services, regardless of who paid.
d The financial delivery cost was defined as the actual expenditure on goods and services.
Results
The average economic delivery cost per HPV vaccine dose ranged from US$ 1.44 for integrated outreach in Uganda to US$ 3.88 for school-based outreach in Peru (Table 2). In general, vaccination programmes delivered in schools had a higher economic cost than those delivered in health centres or via integrated outreach. However, in Viet Nam there was only a small difference in economic cost between school-based outreach and health-centre-based outreach: US$ 2.08 versus US$ 1.92 per dose, respectively. A larger difference in cost between delivery strategies was observed in Uganda: the economic cost of school-based outreach was US$ 3.15 per dose, compared with US$ 1.44 per dose for integrated outreach. The average incremental economic cost per fully immunized girl was highest with school-based outreach in Peru and Uganda, at US$ 11.69 and US$ 10.09, respectively; it was lowest with integrated outreach in Uganda, at US$ 5.20.
The average financial delivery cost per dose was both highest and lowest in Uganda: US$ 1.11 with integrated outreach and US$ 2.10 with school-based outreach. Correspondingly, the average financial cost per fully immunized girl in the country ranged from US$ 4.01 with integrated outreach to US$ 6.73 for school-based outreach.
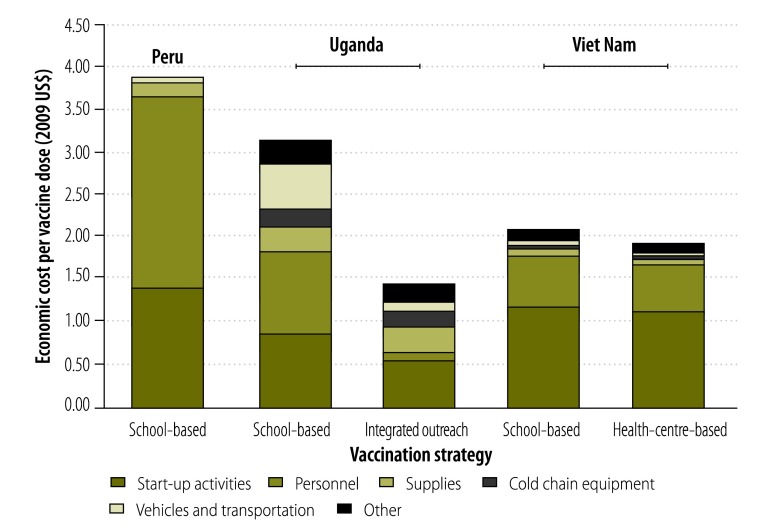
The profiles of the economic cost of an HPV vaccine dose for different delivery strategies in Peru, Uganda and Viet Nam are shown in Fig. 1. Start-up costs contributed the largest share – approximately 60% – of the cost for the two delivery strategies used in Viet Nam but only 30 to 40% of the cost in Peru and Uganda. Information, education and communication activities and community mobilization and sensitization accounted for approximately 40% of start-up costs, whereas staff training and microplanning accounted for 25 to 30%. All other costs were classified as recurrent delivery costs. Personnel costs formed the largest component of these costs, except for integrated outreach in Uganda, where salary costs were low because they were shared with the Child Days Plus campaign. Other important recurrent costs were associated with monitoring the programme and facilitating adverse event reporting.
Fig. 1.
Profile of the economic cost of human papillomavirus vaccination strategies for young adolescent girls, Peru, Uganda and Viet Nam, 2008–2010
US$, United States dollars.
More detailed profiles of both economic and financial costs are provided in Appendices A, B, C, D and E (available at: http://www.rho.org/files/PATH-WHO-Bulletin-HPV-vac-delivery-costs-appendices-2012.pdf). The profiles of financial and economic costs were similar. The largest component of the financial costs was the start-up costs, followed by personnel allowances and the cost of supplies.
Outlay for national programmes
The incremental annual financial outlays needed in Peru, Uganda and Viet Nam to implement nationwide HPV vaccination programmes that would achieve 80% coverage are shown in Table 3. The highest financial outlay was for school-based outreach in Peru: US$ 14 438 519, which comprised 13% of the country’s total estimated budget for immunization in 2009. The lowest outlay was for integrated outreach in Uganda: US$ 1 400 179 or 4% of the planned immunization budget for 2009. School-based outreach in Uganda would cost US$ 2 443 243 (i.e. 7% of the immunization budget). In Viet Nam, school-based outreach would cost slightly more than health-centre-based outreach and both strategies would have accounted for 10 to 11% of the country’s immunization budget in 2009.
Table 3. Incremental annual financial cost of nationwide human papillomavirus vaccination for young adolescent girls, Peru, Uganda and Viet Nam, 2008–2010.
| Country and delivery strategy | No. of girls targeted for vaccinationa | Cost (2009 US$) |
National immunization budget in 2009b |
|||||
|---|---|---|---|---|---|---|---|---|
| Vaccinec | Vaccine delivery | Total | Delivery cost as a fraction of total cost (%) | Total (2009 US$) | Percentage allocated to vaccination strategy | |||
| Peru | 228 480 | – | – | – | – | 113 963 713 | – | |
| School-based | – | 13 047 076 | 1 391 443 | 14 438 519 | 10 | NA | 13 | |
| Uganda | 351 200 | – | – | – | – | 35 672 010 | – | |
| School-based | – | 230 683 | 2 212 560 | 2 443 243 | 91 | – | 7 | |
| Integrated outreach | – | 230 683 | 1 169 496 | 1 400 179 | 84 | – | 4 | |
| Viet Nam | 534 720 | – | – | – | – | 28 083 812 | – | |
| School-based | – | 351 227 | 2 598 739 | 2 949 966 | 88 | – | 11 | |
| Health-centre-based | – | 351 227 | 2 486 448 | 2 837 675 | 88 | – | 10 | |
NA, not available; US$, United States dollars.
a The number of 10-year-old girls in each country was estimated using United Nations Development Programme population data. The number targeted was 80% of that number because it was assumed that only 80% would be fully vaccinated (i.e. would receive three vaccine doses).
b For Uganda and Viet Nam, the figure was derived from government multi-year plans; for Peru, information obtained from the National Expanded Program for Immunization was used.
c Each vaccine dose was assumed to cost US$ 14.00 for Peru and US$ 0.20 for Uganda and Viet Nam (US$ 0.20 is the current country co-payment for the procurement of vaccines for poor and intermediate countries through the GAVI Alliance). For Peru, a 3% Pan American Health Organization Revolving Fund fee and 3% supplement for freight and insurance was added. For Uganda and Viet Nam, a 4% handling fee was added. A 5% allowance was added for wastage in all countries.
Discussion
This analysis provides new information on the delivery cost of HPV vaccination in three low-resource countries that used school-based, health-centre-based and integrated outreach for vaccine delivery. The average economic delivery cost per dose of fully vaccinating around 80% of eligible girls ranged from US$ 1.44 for integrated outreach in Uganda to US$ 3.88 for school-based outreach in Peru. These figures are higher than the published cost of delivering vaccines in traditional EPI schedules, which ranges from US$ 0.75 to US$ 1.40 per dose, depending on vaccine, country and the year of the study.27–32
In Uganda, school-based outreach cost more than integrated outreach primarily because of personnel (e.g. payment for travelling time and allowances) and transportation costs. However, coverage was higher with school-based outreach than integrated outreach: 88.9% versus 60.9%, respectively. In Viet Nam, the difference in the cost of school- and health-centre-based strategies was not as great because each required substantial personnel time and other resources to raise awareness among teachers, parents and communities about the benefits of HPV vaccine.
Our findings are consistent with those reported in the literature, which show that the cost of vaccination per fully immunized child varies according to the mix of delivery strategies used, the cost of key inputs (e.g. personnel and transportation) and the scale of the programme.32 In addition, the variation in cost reflects several key contextual factors, such as national income level, which affects public health service costs and personnel salaries, and health system policies and programmes, which influence country-specific implementation plans and lead to variations in resource use.6–10,12 For example, countries used a variety of approaches and materials for microplanning, community sensitization, raising awareness and staff training. In Peru, Uganda and Viet Nam, national immunization programmes scheduled separate microplanning and training activities for HPV vaccination at multiple tiers of the health system rather than integrating them with scheduled meetings and used an established training-of-trainers strategy. In addition, in the demonstration projects, even workers based at health centres received per diem payments for the days on which they administered HPV vaccine to girls.
Start-up costs were high for the demonstration projects in all countries, with the bulk of these costs being due to activities for raising awareness and community mobilization. Investment in communications increased the community’s acceptance of vaccination, which translated into a high vaccine uptake.33 The cost of introducing new vaccines was similarly high in other settings. For example, the start-up cost for the pentavalent vaccine in Ethiopia was an additional US$ 4.7 million, or US$ 1.50 per fully vaccinated child.34 In Cambodia, the introduction of Japanese encephalitis vaccine cost approximately US$ 1.50 per child, or 60% of the total cost per child vaccinated.35
The economies of scale that occur in national HPV vaccination programmes mean that the unit cost of the development and production of materials for information, education and communication, and for training and the unit cost of community mobilization meetings at the national or subnational levels, are likely to be lower than in a demonstration project. Since the costs of these activities tends to be fixed, they will be spread over a higher number of delivered doses when a country’s vaccination programme is scaled up, resulting in a lower unit cost per dose. In addition, total start-up costs should also decline, depending on how quickly a country decides to introduce and scale up HPV vaccination.
Evidence from the HPV vaccine demonstration projects indicates that the cost per dose was lower when vaccine delivery was integrated into existing health services. For example, in Uganda, personnel and transportation costs were lower with integrated outreach than school-based outreach because HPV vaccination took place alongside an existing programme delivering other health services. Integrating the distribution of HPV vaccine and injection devices with the distribution of other EPI vaccines and immunization supplies is likely to reduce the delivery cost per dose. However, any reduction will depend on existing vaccine storage and transport capacity in the country and on whether the HPV vaccine is introduced in conjunction with other new vaccines, such as rotavirus or pneumococcal vaccine.
In the immediate future, considerable government support will be needed to pay for the delivery of HPV vaccine as well as for its purchase through either the GAVI Alliance or another public sector provider. Although immunization budgets have been increasing recently in countries that took part in the demonstration projects, national decision-makers have noted that co-financing a national HPV vaccination programme is challenging.32 Our analysis indicates that introducing HPV vaccination for young adolescent girls could increase national immunization budgets by 5 to 13%. Consequently, countries will need to allocate public health resources and seek greater support from external donors to cover these costs. Peru increased its national immunization budget by 500% between 2006 and 201036 and, in 2011, the country successfully funded and launched a nationwide HPV vaccination programme.37 In 2012, the GAVI Alliance announced support for introducing the HPV vaccine in low-income countries and Uganda has applied for continued support.38,39
Our cost estimates for countries that took part in the demonstration projects have several limitations. First, in some instances it was not possible to identify all the costs that were integral to the projects but that may not make up the same proportion of the costs for a national immunization campaign. Second, although the same methods were used for all countries and costs were calculated in the same way, some costs may have been unique to a particular country and may have affected both the absolute cost per dose and the cost profile. Third, we omitted labour costs for volunteers who participated in community mobilization and for the time health-care workers spent in training or microplanning sessions. These costs are likely to vary across countries and their inclusion may change relative costs and cost profiles. Finally, the estimated financial outlays for introducing national HPV vaccination programmes are merely indicative, since scaling up a programme to the national level may involve additional costs related to the development of a comprehensive cervical cancer prevention programme and to investment in human and capital resources that are not captured in this analysis.
In conclusion, the cost of delivering HPV vaccine to young adolescent girls is likely to be higher than the cost of delivering vaccines currently included in the EPI schedule but may decline as delivery becomes integrated into immunization and school-based health services. Our findings can assist donors and national governments estimate budgetary requirements and can provide information on the resources needed to introduce and eventually scale up HPV vaccination.
Funding:
This project was funded in whole by a grant from the Bill & Melinda Gates Foundation.
Competing interests:
None declared.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization [Internet]. Immunization, vaccines and biologicals. Human papillomavirus (HPV). Geneva: World Health Organization. Available from: http://www.who.int/immunization/topics/hpv/en/ [accessed 16 June 2013].
- 3.Goldie SJ, O’Shea M, Campos NG, Diaz M, Sweet S, Kim SY. Health and economic outcomes of HPV 16,18 vaccination in 72 GAVI-eligible countries. Vaccine. 2008;26:4080–93. doi: 10.1016/j.vaccine.2008.04.053. [DOI] [PubMed] [Google Scholar]
- 4.Marra F, Cloutier K, Oteng B, Marra C, Ogilvie G. Effectiveness and cost effectiveness of human papillomavirus vaccine: a systematic review. Pharmacoeconomics. 2009;27:127–47. doi: 10.2165/00019053-200927020-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bingham A, Janmohamed A, Bartolini R, Creed-Kanashiro H, Katahoire A, Khan I, et al. An approach to formative research in HPV vaccine introduction planning in low-resource settings. Open Vaccine J. 2009;2:1–16. doi: 10.2174/1875035400902010001. [DOI] [Google Scholar]
- 6.Katahoire RA, Jitta J, Kivumbi G, Murokora D, Arube WJ, Siu G, et al. An assessment of the readiness for introduction of the HPV vaccine in Uganda. Afr J Reprod Health. 2008;12:159–72. [PubMed] [Google Scholar]
- 7.Bartolini RM, Drake JK, Creed-Kanashiro HM, Díaz-Otoya MM, Mosqueira-Lovón NR, Penny ME, et al. Investigación formativa para diseñar estrategias para la introducción de la vacuna contra el VPH en el Perú. [Formative research to shape HPV vaccine introduction strategies in Peru]. Salud Publica Mex 201052226–33.Spanish 10.1590/S0036-36342010000300007 [DOI] [PubMed] [Google Scholar]
- 8.Nghi NQ, Lamontagne DS, Bingham A, Rafiq M, Mai TP, Lien N, et al. Human papillomavirus vaccine introduction in Vietnam: formative research findings. Sex Health. 2010;7:262–70. doi: 10.1071/SH09123. [DOI] [PubMed] [Google Scholar]
- 9.Jacob M, Mawar N, Menezes L, Kaipilyawar S, Gandhi S, Khan I, et al. Assessing the environment for introduction of human papillomavirus vaccine in India. Open Vaccine J. 2010;3:96–107. doi: 10.2174/1875035401003010096. [DOI] [Google Scholar]
- 10.Biellik R, Levin C, Mugisha E, LaMontagne DS, Bingham A, Kaipilyawar S, et al. Health systems and immunization financing for human papillomavirus vaccine introduction in low-resource settings. Vaccine. 2009;27:6203–9. doi: 10.1016/j.vaccine.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 11.LaMontagne DS, Barge S, Le NT, Mugisha E, Penny ME, Gandhi S, et al. Human papillomavirus vaccine delivery strategies that achieved high coverage in low- and middle-income countries. Bull World Health Organ 201189821–830B. .[REMOVED HYPERLINK FIELD] 10.2471/BLT.11.08986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penny M, Bartolini R, Mosqueira NR, LaMontagne DS, Mendoza MA, Ramos I, et al. Strategies to vaccinate against cancer of the cervix: feasibility of a school-based HPV vaccination program in Peru. Vaccine. 2011;29:5022–30. doi: 10.1016/j.vaccine.2011.04.078. [DOI] [PubMed] [Google Scholar]
- 13.Bartolini RM, Winkler JL, Penny ME, LaMontagne DS. Parental acceptance of HPV vaccine in Peru: a decision framework. PLoS One. 2012;7:e48017. doi: 10.1371/journal.pone.0048017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cover JK, Nghi NQ, LaMontagne DS, Huyen DT, Hien NT, Nga T. Acceptance patterns and decision-making for human papillomavirus vaccination among parents in Vietnam: an in-depth qualitative study post-vaccination. BMC Public Health. 2012;12:629. doi: 10.1186/1471-2458-12-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PATH, National Institute of Hygiene and Epidemiology & National Center for Health Education and Communication. HPV vaccination in Southeast Asia: lessons learned from a pilot program in Vietnam. Seattle: PATH; 2012. Available from: http://www.rho.org/files/rb4/HPV_lessons_learned_Vietnam_PATH_2012.pdf [accessed 16 June 2013].
- 16.Katahoire AR, Murokora D, Arube-Wani J, Mugisha E, LaMontagne DS.Acceptability of HPV vaccine among young adolescent girls in Uganda: young people’s perspectives count. Intl J Child Adolesc Health 20136In press [Google Scholar]
- 17.PATH, Child Health and Development Centre & Uganda National Expanded Program on Immunization. HPV vaccination in Africa: lessons learned from a pilot program in Uganda. Seattle: PATH; 2011. Available from: http://www.rho.org/files/rb2/HPV_lessons_learned_Uganda_PATH_2011.pdf [accessed 16 June 2013].
- 18.HPV vaccine adoption in developing countries: cost and financing issues New York & Seattle: International AIDS Vaccine Initiative & PATH; 2007. [Google Scholar]
- 19.Temin M, Levin R. Start with a girl: a new agenda for global health. Washington: Center for Global Development; 2009. Available from: www.cgdev.org/files/1422899_file_Start_with_a_Girl_FINAL.pdf [accessed 7 June 2013].
- 20.Guidelines for estimating costs of introducing new vaccines into the national immunization system Geneva: World Health Organization; 2002 (WHO/V&B/02.11).
- 21.Preparing for the introduction of HPV vaccines: policy and programme guidance for countries Geneva: World Health Organization; 2006. [Google Scholar]
- 22.The World Bank [Internet]. Consumer price index (2005 = 100). Washington: The World Bank; 2012. Available from: http://data.worldbank.org/indicator/FP.CPI.TOTL [accessed 7 June 2013].
- 23.Pan American Health Organization PAHO Revolving Fund: vaccine and syringe prices, 2011. Immunization Newsletter. 2011;XXXIII:5. [Google Scholar]
- 24.Co-financing new vaccines and sustainability: meeting report of the Africa Region workshop New York: United Nations Children’s Fund; 2009. [Google Scholar]
- 25.GAVI Alliance [Internet]. Co-financing policy. Geneva: GAVI Alliance; 2010. Available from: www.gavialliance.org/about/governance/programme-policies/co-financing [accessed 7 June 2013].
- 26.Operating procedures of the PAHO Revolving Fund for the purchase of vaccines, syringes and other related supplies Washington: Pan American Health Organization; 2008. [Google Scholar]
- 27.Acharya A, Diaz-Ortega JL, Tambini G, de Quadros C, Arita I. Cost-effectiveness of measles elimination in Latin America and the Caribbean: a prospective analysis. Vaccine. 2002;20:3332–41. doi: 10.1016/S0264-410X(02)00296-7. [DOI] [PubMed] [Google Scholar]
- 28.Walker D, Mosqueira NR, Penny ME, Lanata CF, Clark AD, Sanderson CF, et al. Variation in the costs of delivering routine immunization services in Peru. Bull World Health Organ. 2004;82:676–82. [PMC free article] [PubMed] [Google Scholar]
- 29.Griffiths UK, Wolfson LJ, Quddus A, Younus M, Hafiz RA. Incremental cost-effectiveness of supplementary immunization activities to prevent neonatal tetanus in Pakistan. Bull World Health Organ. 2004;82:643–51. [PMC free article] [PubMed] [Google Scholar]
- 30.Dayan GH, Cairns L, Sangrujee N, Mtonga A, Nguyen V, Strebel P. Cost-effectiveness of three different vaccination strategies against measles in Zambian children. Vaccine. 2004;22:475–84. doi: 10.1016/j.vaccine.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Hoang MV, Nguyen TB, Kim BG, Dao LH, Nguyen TH, Wright P. Cost of providing the expanded programme on immunization: findings from a facility-based study in Viet Nam, 2005. Bull World Health Organ. 2008;86:429–34. doi: 10.2471/BLT.07.045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenzel L, Wolfson LJ, Fox-Rusby J, Miller M, Halsey NA. Vaccine preventable diseases. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al., editors. Disease control priorities in developing countries. 2nd ed. Washington: The World Bank; 2006. [Google Scholar]
- 33.Galagan SR, Paul P, Menezes L, LaMontagne DS.Influences on parental acceptance of HPV vaccination in demonstration projects in Uganda and Vietnam. Vaccine 2013. Epub May 15 [DOI] [PubMed] [Google Scholar]
- 34.Griffiths UK, Korczak VS, Ayalew D, Yigzaw A. Incremental system costs of introducing combined DTwP-hepatitis B-Hib vaccine into national immunization services in Ethiopia. Vaccine. 2009;27:1426–32. doi: 10.1016/j.vaccine.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 35.Touch S, Suraratdecha C, Samnang C, Heng S, Gazley L, Huch C, et al. A cost-effectiveness analysis of Japanese encephalitis vaccine in Cambodia. Vaccine. 2010;28:4593–9. doi: 10.1016/j.vaccine.2010.04.086. [DOI] [PubMed] [Google Scholar]
- 36.Perú, Ministerio de Economía y Finanzas [Internet]. Presupuesto público. Lima: MEF; 2013. Available from: www.mef.gob.pe [accessed 7 June 2013].
- 37.Ministerio de Salud [Internet]. Llega al Perú primer lote de vacunas contra el cáncer de cuello uterino. [The first batch of cervical cancer vaccines arrives in Peru]. Lima: Ministerio de Salud; 2011. Spanish. Available from: http://www.minsa.gob.pe/portada/prensa/notas_auxiliar.asp?nota=9896http://[accessed 7 June 2013].
- 38.GAVI Alliance [Internet]. Human papillomavirus vaccine support. Geneva: GAVI; 2013. Available from: http://www.gavialliance.org/support/nvs/human-papillomavirus-vaccine-support/ [accessed 16 June 2013]
- 39.Okino P. First Lady launches cervical cancer fight. Kampala: New Vision 7 September 2012. Available from: http://www.newvision.co.ug/news/635003-first-lady-launches-cervical-cancer-vaccination-drive.html [accessed 7 June 2013]