Abstract
The cantharidin blister model provides an in vivo assessment of the innate inflammatory response in humans. It allows researchers to profile the acute and resolving inflammatory response in healthy and diseased states and for the design of crossover trials for the testing of new treatments for acute inflammation. Below we describe the materials and methods required to prepare, induce, aspirate and analyse the forearm cantharidin blisters, in preparation for future study design.
Background
Most cardiovascular disorders are characterized by an underlying vascular inflammation that is considered to play a major causative role in pathogenesis.1 In humans, the innate inflammatory response can be assessed both in vitro and in vivo. A number of human in vivo models of acute inflammation have been developed over the past 20 years. These include the systemic inflammatory reaction following endotoxin inhalation,2 the negative pressure-induced skin blister model3 and the cantharidin-induced skin blister model.4
Of these models, the cantharidin skin blister is non-invasive, has few side-effects, is reproducible and can be maintained for several days to characterize both the induction and resolution of the innate inflammatory response. Following topical administration to the skin, cantharidin, a protein phosphatase inhibitor, induces atraumatic acantholysis and blister formation.4 This is associated with an infiltration of CD16+ neutrophils into the fluid space in the first 24 hours, and CD14+ monocyte/macrophages from 24 to 72 hours.5
We have previously described the use of the cantharidin blister to determine the induction and resolution of acute inflammation in humans, and to identify two groups of individuals, the early and the late resolvers.5 Moreover, the low within-person variability (see ref.6) in the cantharidin blister allows for the testing of immune-modifying drugs such as cyclooxygenase inhibitors,7 anti-tumour necrosis factor α (TNF-α) antibodies and corticosteroids,6 using crossover trials.
In the methodology below, we provide a brief description of participant selection, and detail the methods for blister induction, aspiration and analysis. The technique takes several weeks to become proficient at, and researchers are advised to perform repeated blisters to assess within-person variability before undertaking a crossover study.
The methodology below describes the assessment of both the acute (24 hours) and resolving (72 hours) inflammation using the cantharidin blister model.
Methodology
Study participants
Study design using the cantharidin skin blisters requires careful consideration of external factors that might influence the innate immunity. Healthy volunteers should be non-smokers, with no history of chronic or recent acute inflammatory conditions, and should remain free from aspirin for at least 7–9 days, all other anti-inflammatory medication for at least 72 hours, and alcohol for at least 24 hours prior to blister induction.
List of materials for one participant to receive two forearm blisters (24 and 72 hours)
Filter paper punched into 2 × 10 mm diameter discs (Whatman® grade 1; Whatman International Ltd, Maidstone, UK)
Laboratory film punched into 2 × 16 mm diameter discs (ParafilmTM; Alpha Laboratories, Eastleigh, UK)
Foam pads cut into 4 × 30 mm diameter discs (Activheal® Non-adhesive foam pad, 10 × 10 cm; Advanced Medical Solutions Ltd, Winsford, UK)
50 μL 0.1% Cantharidin solution (0.7% Cantharone®, diluted in acetone; Dormer Laboratories Inc., Toronto, ON, Canada)
1 × Adhesive dressing (IV 3000TM, 10 × 14 cm; Smith & Nephew, Hull, UK)
2 × Film dressing (Mepore®, Film & Pad 9 × 10 cm; Mölnlycke Heath Care, Gothenburg, Sweden)
1 × Ulcer dressing (Comfeel® Plus, Ulcer Dressing 10 × 10 cm; Coloplast, Peterborough, UK)
1 × Cap from 30 mL specimen tube (Sterilin, Newport, UK)
1 × Support bandage (Tubigrip®; Mölnlycke Heath Care)
Surgical tape cut into 10 × 10 cm strips (MicroporeTM; 3M Healthcare, Loughborough, UK)
3 × Alcohol wipe (UHS, Enfield, UK)
Haemocytometer (Bright-LineTM Haemocytometer; Sigma-Aldrich, Gillingham, UK)
Trypan blue stain 0.4% (Life Technologies, Ltd, Paisley, UK)
100 μL 3% Sodium citrate, dissolved in 0.9% sodium chloride (Sigma-Aldrich)
2 × 26 Gauge sterile needle (Sigma-Aldrich)
2 × 1 mL Syringe (Terumo, Egham, UK)
200 μL Roswell Park Memorial Institute (RPMI) media (Gibco®; Life Technologies)
4 × 1.5 mL Centrifuge tubes (Microcentrifuge tubes; Appleton Woods Ltd, Birmingham, UK)
P20 & P200 pipette with tips
Blister induction
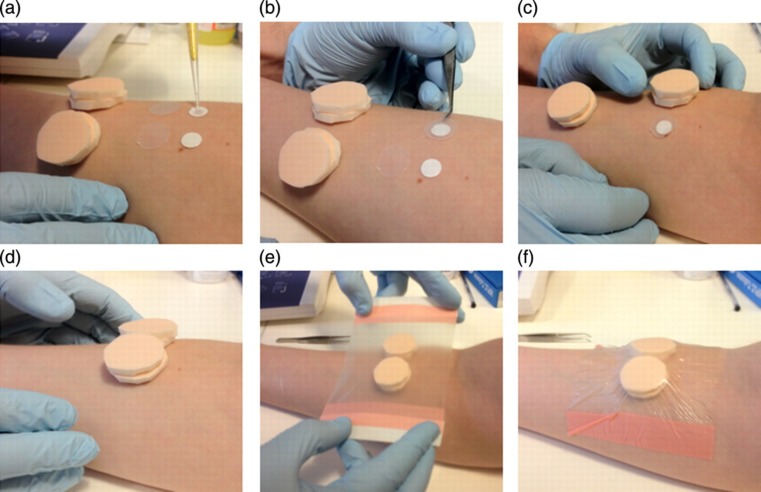
Blisters are induced in a similar manner as previously described4 (Figure 1). Two blister patches are applied to the ventral aspect of the forearm, 30–70 mm from the elbow crease, and parallel to each other, with 20–40 mm between blisters depending on forearm width. One blister is assigned to aspiration after 24 hours, and the other to 72 hours. The forearm hair is cut to allow for two 2 cm2 areas for the blisters to be formed. The skin is cleaned, the filter paper discs are positioned and the film discs and foam pads are lined up along the forearm. Cantharidin (12.5 μL of 0.1%) is then pipetted onto each paper disc using a 20-μL filter tip. Each paper disc is then covered with a 16-mm film disc and then by two foam pads. These pads are balanced on the arm and covered with an adhesive dressing, which should be applied at a constant pressure to maintain disc position and without extensive forearm skin depression. To maintain blister integrity, participants are advised to refrain from exercise, and to avoid alcohol and medication while the blisters are in position. Participants should cover the forearm while showering to keep the dressing dry. The blister is fragile during this time, so participants should be advised to take care to avoid pressure on top of the blister patch.
Figure 1.
Induction of cantharidin blisters. Cantharidin (0.1%) is pipetted onto each filter paper disc (a). Each filter paper disc is then covered by a film disc (b) and two foam pads (c,d) before being secured by an adhesive dressing (e,f)
Blister aspiration after 24 hours
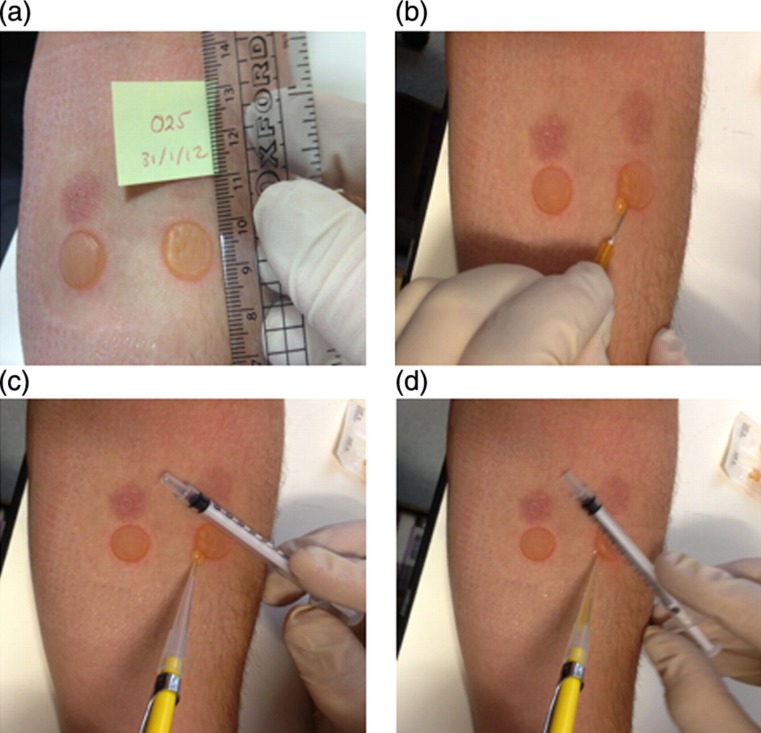
After 24 hours, participants return for the aspiration of the first blister (Figure 2). The forearm is rested on a flat surface, and the dressing, foam pads, parafilm and filter paper are carefully removed. The blisters are measured for size (typically 11–16 mm diameter), and checked for evidence of fluid loss onto the skin or foam pads. The fluid is extracted by piercing the epidermis at the lateral border using a 26-gauge sterile needle. The oedema is allowed to drain, and a 1 mL syringe is rolled across the blister to push the fluid onto the skin. The fluid is carefully aspirated during this using a sterile tip, before being immediately transferred to a sterile centrifuge tube containing 50 μL sodium citrate. All fluid is collected where possible. The pierced blister is then wiped clean, and dressed using a film dressing.
Figure 2.
Fluid aspiration from cantharidin blisters after 24 hours. Once the dressing has been removed, blisters are measured (a), before being pierced (b) and the fluid aspirated from the adjacent skin (c, d)
Securing the 72-hour blister
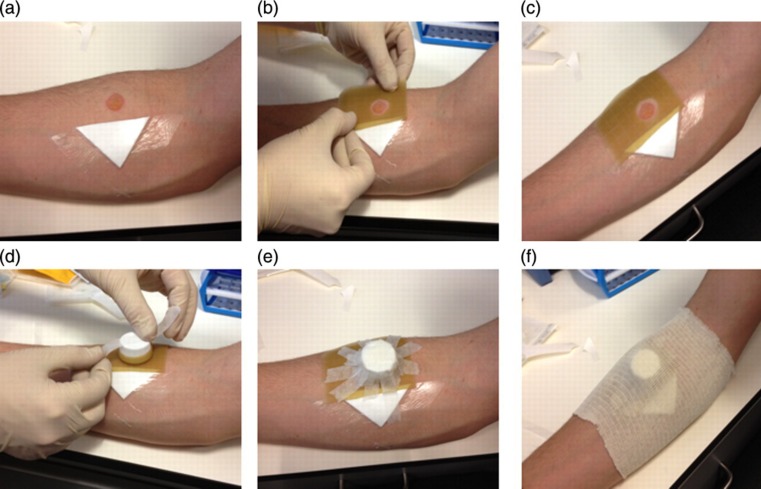
The remaining intact blister is covered for two additional days (Figure 3). First, a cap is removed from a specimen tube, and a strip of the ulcer dressing is cut to cover the rim. The remaining ulcer dressing is cut to fit around the blister, and the cap is placed over the blister, which is then secured using medical tape. The cap and ulcer dressing are covered by support bandage.
Figure 3.
Securing the 72-hour blister. The pierced blister is dressed (a) and an ulcer dressing is cut and fixed around the intact blister (b & c). The cap is then attached to the ulcer dressing with surgical tape (d & e) before being covered with a support bandage (f)
Following an additional 48 hours, the protective cap is removed and the blister aspirated as at 24 hours. Once aspirated, the blisters sites are cleaned and covered by a film dressing. For a crossover trial, at least one week should be left between blister induction, and alternate arms used for repeated pairs of blisters.
Fluid analysis
Once stored in citrate, tubes are weighed and immediately centrifuged (2000 g, 5 minutes, 10°C). The supernatant is removed, aliquoted and stored at −80°C for future assessment of inflammatory mediators, such as cytokines, chemokines and lipoxins (e.g. TNF-α, interleukin-6 [IL-6], prostaglandin E2, 15-epi-LxA4). The fluid volume of the blister oedema is often below 150 μL, and the low sample volume makes multiple enzyme-linked immunosorbent assays challenging. This can be overcome by making 1:10 and 1:100 dilutions of the fluid, and by using assays that provide multiple results from a single sample (e. g Human Inflammatory Multiplex kit; Meso Scale Discovery, Gaithersburg, MD, USA).
Cell pellet analysis
Pellets are re-suspended in 100 μL RPMI media. This cell suspension (10 μL) is then mixed with 10 μL trypan blue, and the cells are counted by a manual haemocytometer and assessed for viability. Results are expressed as total cells (n) or cells per fluid volume (n/mL), with viability typically being >95% for the 24-hour blister, and >90% for the 72-hour blister. The range of total cell count during our current research is from 1.5 × 104 to 3.0 × 106.
One of the challenges of assessing the differential is a low cell count (n < 50,000). To assess the profiles, cell suspensions can undergo a cytospin and slides stained using a haemotoxylin and eosin, Kimura's4 or a May–Grunwald–Giemsa stain.6 Based upon this, Dinh et al. 6 reported that the 24-hour blister cells consisted of 74% neutrophils, 22% monocytes, 3% lymphocytes and 1% eosinophils.
Furthermore, cells can be differentiated by flow cytometry, whereby fluorescent antibodies are used to identify receptor expression (for example, anti-CD3 for T lymphocytes, anti-CD19 for B lymphocytes, anti-CD56 for natural killer cells, anti-CD16 for neutrophils and anti-CD14 for monocyte/macrophage populations). In addition, receptor expression can be assessed for cell activation using anti-CD11b/CD62L4 for expression of class II major histocompatibility complex using anti-human leukocyte antigen (HLA) DR, or for the expression of 15-epi-lipoxin A4 receptor expression using anti-formyl peptide receptor-like 1 (FPRL1).5
Adverse events
During our recent testing, we observed a single episode of blister infection (1 in 104 blisters). To avoid further events, study participants are advised to keep the blister area clean and covered for 24–48 hours following fluid aspiration. Forearm blisters also result in a discoloured mark, which matches the size of the blister. Two such marks are evident in Figure 2. Complete resolution of these marks can occur within a matter of weeks, though this can take over a year in some cases. Marks appear to take longer to resolve in more pigmented skin. Participants should be made aware of these factors prior to consent.
Discussion
The majority of what we know about the innate and adaptive immune-mediated responses to injury/infection has been garnered from experiments on rodents. In an age when the emphasis is on ‘translational medicine’, the skin blister represents a reproducible model to tease out the processes that drive immune responses in humans and ask whether defective pathways in the evolution of the response or its resolution contribute to the aetiology of chronic inflammatory diseases. By combining cantharidin blister fluid volume along with cell numbers and phenotype, investigators can determine the nature, severity and duration of acute inflammatory responses in both healthy and diseased humans and the potential efficacy of putative anti-inflammatory drugs.
DECLARATIONS
Competing interests
None declared
Funding
WJJ is supported by a British Heart Foundation MBPhD Studentship (No. FS/10/044/28294) and DWG by a Wellcome Trust Senior Fellowship
Ethical approval
Ethical approval was obtained from UCL Research Ethics (Project ID No. 2907/002)
Guarantor
DWG
Contributorship
WJJ wrote the paper, and DWG provided intellectual insight and guidance on the subject matter
Acknowledgements
The funding of the research from the British Heart Foundation and the Wellcome Trust
References
- 1. Willerson JT, Ridker PM Inflammation as a cardiovascular risk factor. Circulation 2004:109:II-2–10 [DOI] [PubMed] [Google Scholar]
- 2. Michel O, Nagy AM, Schroeven M, Néve J, Fondu P, Sergysels R Dose-response relationship to inhaled endotoxin in normal subjects. Am J Respir Crit Care Med 1997;156:1157–64 [DOI] [PubMed] [Google Scholar]
- 3. Follin P Skin chamber technique for study of in vivo exudated human neutrophils. J Immunol Methods 1999;232:55–65 [DOI] [PubMed] [Google Scholar]
- 4. Day RM, Harbord M, Forbes A, Segal AW Cantharidin blisters: a technique for investigating leukocyte trafficking and cytokine production at sites of inflammation in humans. J Immunol Methods 2001;257:213–20 [DOI] [PubMed] [Google Scholar]
- 5. Morris T, Stables M, Colville-Nash P, et al. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc Natl Acad Sci USA 2010;107:8842–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dinh PHD, Corraza F, Mestdagh K, Kassengera Z, Doyen V, Michel O Validation of the cantharidin-induced skin blister as an in vivo model of inflammation. Br J Clin Pharmacol 2011;72:912–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morris T, Stables M, Hobbs A, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol 2009;183:2089–96 [DOI] [PubMed] [Google Scholar]