Abstract
Objective
To determine the prevalence of asymptomatic brain ischaemic in the presence of vascular disease in other arterial territories.
Design
Studies up to January 2011 were identified through comprehensive search strategies. Arcsine transformation for meta-analysis was used to calculate the standardized mean difference (SMD) and 95% confidence intervals (CI).
Setting
A systematic review and meta-analysis were performed.
Participants
For each study, the proportion of patients positive for SBI in the presence of other systemic vascular disease was extracted and analyzed.
Main outcome measures
Using a random-effects model, a pooled effect estimate interpreted as a percentage prevalence of disease was calculated.
Results
SBI in the presence of acute ischaemic stroke was found in 23% (SMD 0.99; P < 0.001; 95% CI 0.88–1.10); a 35% prevalence was found in patients with coronary artery disease (SMD 1.26; P < 0.001; 95% CI 0.95–1.58); and a 14% prevalence in patients with peripheral artery disease (SMD 0.48; P < 0.002; 95% CI 0.42–0.54), although the data-set in the latter is smaller.
Conclusions
Patients with systemic vascular disease are at an increased risk of silent brain infarction.
Introduction
The presence of silent brain infarction (SBI) is known to more than double the risk of subsequent stroke and dementia.1,2 Screening and treating high-risk patients could reduce such future risk.3
If reliable quantification of prevalence of SBI in other vascular diseases can be established, then appropriate assessment and perhaps more aggressive therapeutic treatments could be initiated to reduce the incidence of stroke and dementia in this high-risk population.
We sought to perform three comprehensive meta-analyses of SBI in the presence of acute ischaemic stroke, coronary artery disease (CAD) and peripheral artery disease (PAD), representative of the three major beds for vascular disease to identify the prevalence of SBI in order to better determine the associated risk of future vascular events.
Methods
Data sources
Studies up to and including 1 January 2011 were identified through searches in PubMed, Google Scholar, Embase and MEDLINE. The following search terms were used: ‘silent brain infarction in stroke’, ‘asymptomatic stroke and cerebral infarction’ and ‘silent cerebral infarction in stroke’ with and/or as Boolean operators. Further searches were performed looking specifically at terms linking SBI with other vascular disease locations, such as ‘silent brain infarction in peripheral vascular disease’, ‘silent brain infarction in coronary artery disease’ and ‘silent brain infarction in vascular disease’. The retrieved studies were examined to assess their appropriateness for inclusion. The references of all identified publications were manually reviewed for additional studies and the PubMed ‘relevant articles’ function was used. Table 1 provides details on the studies used including author, date, study title and mean age.
Table 1.
Silent brain infarction in acute ischaemic stroke, CAD and PAD
| Disease | Study | Study title | N | Mean age (range), years | SBI (%) |
|---|---|---|---|---|---|
| AIS | Boon et al. (1994)16 | Silent brain infarction in 755 consecutive patients with first-ever supratentorial ischemic stroke. Relationships with index-stroke subtype, vascular risk factors and mortality | 755 | 71 | 27 |
| Brainin et al. (1995)13 | Silent brain infarcts and transient ischemic attacks: a three-year study of first-ever ischemic stroke patients: the Klosterneuburg stroke data bank | 728 | 68 ± 10 | 11 | |
| Chodosh et al. (1988)17 | Silent stroke in the NINCDS stroke data bank | 1203 | 69.1 | 11 | |
| Corea et al. (2001)18 | Silent infarcts in stroke patients: patient characteristics and effect on 2-year outcome | 202 | 70.05 | 25.7 | |
| Corea et al. (2002)19 | Brain CT scan in acute stroke patients: silent infarcts and relation to outcome | 191 | 76 | 37.8 | |
| Coutts et al. (2005)20 | Silent ischemia in minor stroke and TIA patients identified on MR imaging | 143 | – | 9.8 | |
| Davis et al. (1996)21 | Silent cerebral infarction in patients enrolled in the TOAST study | 629 | 65 | 22.7 | |
| Giele et al. (2004)9 | Silent brain infarcts in patients with manifest vascular disease | 308 | 58 | 17 | |
| Herderschee et al. (1992)22 | Silent stroke in patients with TIA or minor ischemic stroke | 2329 | 13 | ||
| Jorgensen et al. (1994)14 | Silent infarction in acute stroke patients: prevalence, localization, risk factors and clinical significance: the Copenhagen Stroke Study | 322 | 73 ± 12 | 32.5 | |
| Kang et al. (2006)23 | Silent ischemic lesion recurrence on MRI predicts subsequent clinical vascular events | 104 | – | 33.7 | |
| Kase et al. (1989)24 | Prevalence of silent stroke in patients presenting with initial stroke: the Framingham Study | 124 | 46 | 10 | |
| Liebetrau et al. (2004)25 | Silent and symptomatic infarcts on cranial computerized tomography in relation to dementia and mortality: a population-based study in 85-year-old subjects | 239 | All 85 | 8.6 | |
| Minn et al. (2005)6 | Significance of silent infarcts in acute ischaemic stroke patients aged 80 years or older | 50 | ≥ 80 | 76 | |
| Oh et al. (2010)26 | The prevalence and risk factor analysis of silent brain infarcts in patients with first-ever stroke | 395 | 63.8 | 33.4 | |
| Ong et al. (2009)10 | Impact of silent infarction on the outcome of stroke patients | 226 | 68 ± 13 | 20 | |
| Ricci et al. (1993)27 | Silent brain infarction in patients with first-ever stroke. A community based study in Umbria, Italy | 209 | 71 | 38.3 | |
| Vermeer et al. (2002)11 | Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study | 1077 | 75 | 24 | |
| Vermeer et al. (2003)2 | Silent brain infarcts and white matter lesions increase stroke risks in the general population: the Rotterdam Scan Study | 668 | 71 ± 7 | 14 | |
| CAD | Geerlings et al. (2010)28 | Brain volumes and cerebrovascular lesions on MRI in patients with atherosclerotic disease. The SMART study | 1044 | 58 ± 10 years | 10 |
| Giele et al. (2004)9 | Silent brain infarcts in patients with manifest vascular disease | 308 | 18–79 | 17 | |
| Hara et al. (1994)7 | Silent cerebral infarction associated with coronary artery disease | 50 | – | 80 | |
| Hoshide et al. (2001)29 | Different patterns of silent cerebral infarct in patients with coronary artery disease or hypertension | 107 | 62 | 46 | |
| Kozdag et al. (2008)30 | Silent cerebral infarction in chronic heart failure: ischemic and nonischemic dilated cardiomyopathy | 72 | 50–74 | 39 | |
| Nadareishvili et al. (1999)31 | Cerebral microembolism in acute myocardial Infarction | 112 | 68 ± 11 years | 15 | |
| Ozeren et al. (1998)32 | Silent cerebral lesions on MRI in subjects with CAD | 72 | 43 | ||
| Pardo et al. (1998)33 | Silent brain infarctions in patients with coronary artery disease. A Spanish population survey | 100 | 34–82 | 30 | |
| Selvetella et al. (2003)34 | Left ventricular hypertrophy is associated with asymptomatic cerebral damage in hypertensive patients | 195 | 67 ± 1 years | 55 | |
| Siachos et al. (2005)35 | Silent strokes in patients with heart failure | 117 | 51 | 34 | |
| Uekita et al. (2003)36 | Cervical and intercranial atherosclerosis and silent brain infarction in Japanese patients with CAD | 133 | – | 58 | |
| PAD | Geerlings et al. (2010)28 | Brain volumes and cerebrovascular lesions on MRI in patients with atherosclerotic disease. The SMART study | 1044 | 58 ± 10 years | 5 |
| Giele et al. (2004)9 | Silent brain infarcts in patients with manifest vascular disease | 58 | 18–79 | 21 |
TIA, transient ischaemic attack; NINCDS, National Institute of Neurological and Communicative Disorders and Stroke; CT, computed tomography; SBI, silent brain infarction; CAD, coronary artery disease; PAD, peripheral artery disease; AIS, acute ischaemic stroke; TOAST, Trial of Org 10172 in Acute Stroke Treatment; MRI, magnetic resonance imaging; SMART, Second Manifestation of ARTerial disease study; SMART-MR, Second Manifestation of ARTerial disease in Magnetic Resonance
Search strategy and selection criteria
Studies were included if they were based on populations which included patients with symptomatic ischaemic stroke, PAD and ischaemic heart disease. SBI lesions were recorded in some studies and defined in size between 3 and 5 mm in diameter.
The cardiac studies used a variety of techniques to identify CAD such as electrocardiogram, single-photon emission computed tomography, troponin, echocardiogram and scintography. Studies using only troponin as a measure of CAD were excluded from the analysis.
Study selection
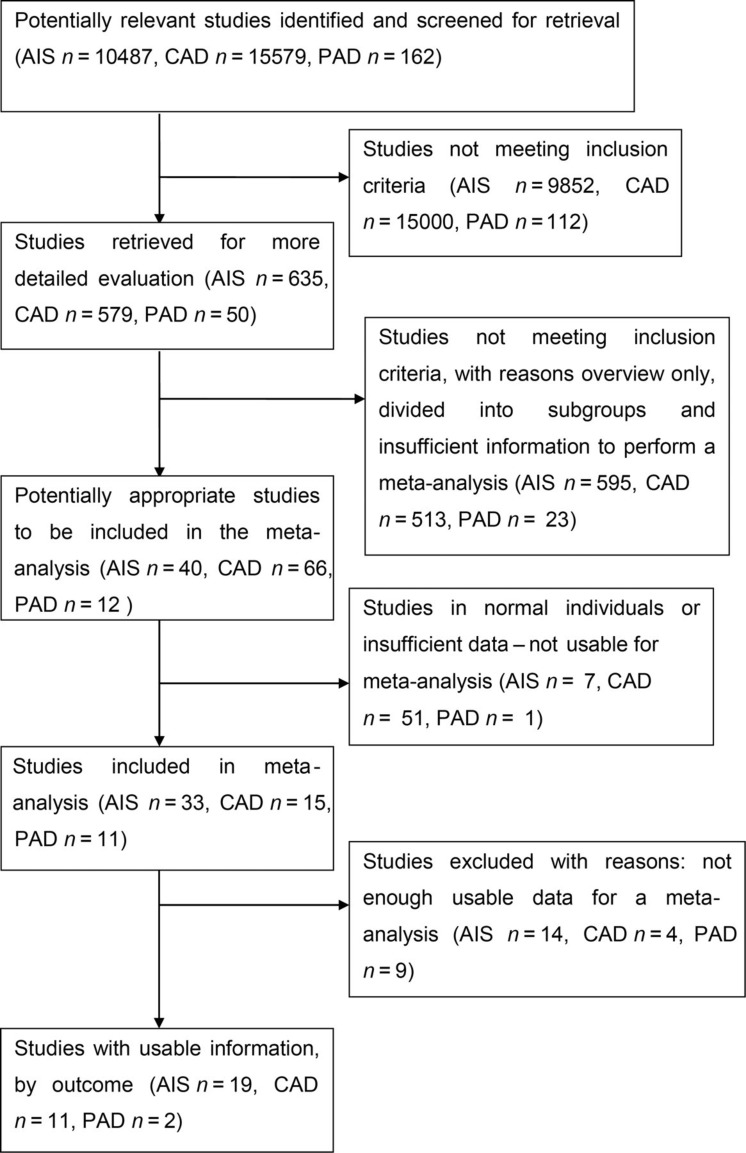
Study selection was performed independently by two reviewers and disagreements were resolved by consensus and by the opinion of a third reviewer when necessary. Inclusion criteria included: (1) studies in populations of acute ischaemic stroke, CAD and PAD; (2) studies where the presence of SBI was measured using computed tomography (CT) and/or magnetic resonance imaging (MRI); and (3) subjects were >18 years of age. Exclusion criteria included: (1) subjects were <18 years age; (2) studies that focused on populations with no history of any vascular disease; and (3) studies where cases of stroke had a background of metabolic disease or other non-vascular origin. Figure 1 shows the prism statement and search strategy for the systematic review and identifies the pathway to study identification for the meta-analysis in all three arms.
Figure 1.
Search strategy flow chart – silent brain infarction (SBI) in acute ischaemic stroke (AIS), carotid artery disease (CAD) and peripheral artery disease (PAD)
Data extraction and analysis
Data for analysis were extracted from each study by two reviewers, results compared and for each study, a pooled odds ratio (OR) and 95% confidence interval (CI) was calculated using a random-effects analysis model.4 The strength of risk of SBI versus no risk of SBI was considered statistically significant with an OR >1 and a P value of <0.05. For each meta-analysis, an I 2 test for heterogeneity was performed, with significance set at P < 0.05.5
A one-sided arcsine transformation was used for the meta-analyses.5 For each study, the proportion of patients who were positive for asymptomatic SBI from the total population of patients was recorded. The standardized mean difference (SMD) and standard error for each proportion were then calculated and the results combined using the generic inverse variance approach in Review Manager version 5.1.1 (The Nordic Cochrane Centre, Copenhagan, Denmark). Pooled data were first analysed with a fixed-effects model, and if heterogeneity was detected by T 2 tests for heterogeneity, including visual inspection of forest plots, a random-effects model was used. This produced an SMD with 95% CI for each study and a pooled effect size for all studies with 95% CI that was weighted to the size of the individual studies. The concluding result for the prevalence of SBI in patients with symptomatic vascular disease was interpreted as a percentage.
Results
We identified 635 relevant studies in SBI in stroke, 579 in SBI in CAD and 50 in SBI in PAD. Reference lists were searched of relevant studies, and papers containing extractable data were selected, resulting in a total of 19 studies for SBI in ischaemic stroke, 11 for SBI in CAD and 2 for SBI in PAD. Table 1 provides study information of sample size and mean age for the individual populations studied.
SBI in the presence of acute ischaemic stroke
Our initial search produced 10,487 potential studies. Most studies were excluded because they were not population-specific, there was no measurement of SBI or it was measured in a non-vascular population. Of the relevant 635 studies, 19 met our inclusion criteria.
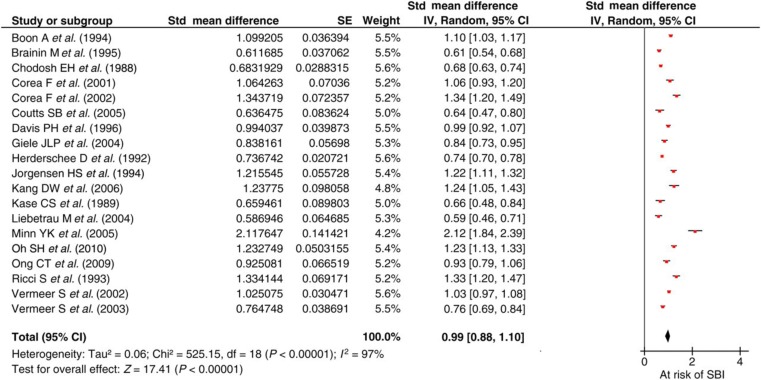
Figure 2 shows a relative risk of SBI in the presence of acute ischaemic stroke of 23% with an SMD of 0.99 (95% CI 0.88–1.10). There is significant heterogeneity of the population due to the small study sample size (n = 50) by Minn et al. 6 However, following iterative analysis excluding this study, the results were broadly similar.
Figure 2.
Silent brain infarction (SBI) in the presence of acute ischaemic stroke
SBI in the presence of CAD
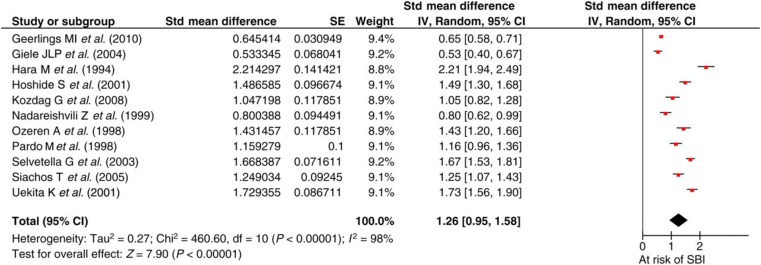
The initial search produced 15,579 potential studies, of which 579 were reviewed and eventually 11 studies were analysed. Figure 3 demonstrates a 35% prevalence of SBI in patients with CAD with an SMD of 1.26 (95% CI 0.95–1.58). There was significant heterogeneity in the population (P = 0.00001) due to the small sample size in the study (n = 50) by Hara et al. 7 but an iterative analysis deleting this study showed similar overall results.
Figure 3.
Silent brain infarction (SBI) in the presence of coronary artery disease
SBI in the presence of PAD
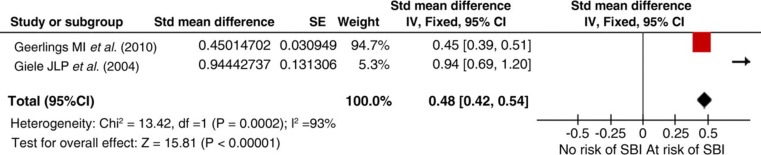
The initial search identified 162 potential studies, of which 12 were reviewed but finally only two met our inclusion criteria. Pooled results show a 14% (SMD 0.48; 95% CI 0.42–0.54) prevalence of SBI in the presence of PAD (Figure 4). The two studies, however, had significantly different sample sizes, n = 1044 and 58, respectively.
Figure 4.
Silent brain infarction (SBI) in the presence of peripheral artery disease
Discussion
SBI is an independent risk factor for stroke, stroke recurrence8 and dementia independent of any other vascular risk factors.1,2 Our three independent meta-analyses have quantified the prevalence of risk for SBI in each of the major vascular diseases, with the greatest risk occurring in CAD, followed by a previous cerebral ischaemic event. It is not surprising that SBI risk also occurs with PAD, but quantifying that risk is more difficult as the number of studies was small by comparison with the other vascular beds.
Several studies have examined the incidence of SBI and its relation to risk factors for stroke,1,2 with an increased two- to three-fold risk in the presence of SBI on MRI in elderly populations. Risk factors for SBI are considered to be comparable to those for stroke; therefore, cardiovascular patients may also be at high risk of silent infarcts with similar risk factors such as ageing, carotid artery stenosis, hypertension, diabetes mellitus and retinal artery stenosis. Age and hypertension are independently and strongly associated with SBI2,9,10 and Vermeer et al. 2 identified absolute risk of stroke within four years at 11.7% for participants with SBI compared with 2.3% for those without SBI.
SBI is thought to be associated with a higher mortality, although results have varied,10 with the Rotterdam Scan Study11 finding a prevalence of 20% of SBI in a normal, healthy population aged between 60 and 90 years. The prevalence was strongly affected by age9 and increased from 8% in the participants aged 60–64 years to 35% in the oldest group (85–90 years). The Framingham Offspring cohort was investigated for SBI with no history of stroke or transient ischaemic attack and 10.7% had at least one SBI which were largely located in the basal ganglia (52%).12 Furthermore, patients with vascular disease are at risk of SBI at a younger age,9 and intervention thresholds may need to be reduced for commencing therapeutic regimens for the prevention of stroke in these high-risk populations.
The prognosis of stroke and its outcome does not appear to be influenced by the presence of SBI.13,14 However, the risk of stroke for people with one or more silent infarcts is increased by 2–10-fold,1,9 therefore influencing the prevalence of stroke in an ageing population. However, screening for SBI in the presence of other vascular diseases may not be appropriate for both cost and patient wellbeing.
As with any meta-analysis, a number of limitations need to be considered. Firstly, there was variation in the mode of imaging for demonstrating brain infarction with some studies using CT (55%) and others using MRI (45%). The latter is well-known to be more sensitive and it is therefore likely that our study is underestimating the relative risk in view of the 55% of studies using CT. The presence of CAD was inconsistently defined across the included criteria. To counter this, we used hard point criteria which involved at least two positive test results to ensure a secure diagnosis. Also, the data on PAD are very small. In practical terms, it may not be useful to screen elderly patients using brain imaging to identify the presence of SBI; however, SBI may explain certain subtle physical and cognitive decline in patients with multiple vascular disease and this knowledge would be helpful in the management of those patients. Finally, while considerable effort was made in our search strategy to identify all relevant papers regardless of size and outcome to reduce publication bias, this cannot be ever completely eliminated.
It is suggested that physicians need to approach all manifestations of atherothrombotic vascular disease whether clinically symptomatic or silent as one pathological entity that intermittently affects different vascular territories.15 Our work adds support to the need for a global and more aggressive approach to vascular disease management.
SBI is prevalent following involvement of atherosclerosis in systemic vascular beds. Often unnoticed and untreated, this condition may account for undetected cognitive and functional decline as well as unexpected ischaemic stroke.
DECLARATIONS
Competing interests
PS, as the Editor of JRSM Cardiovascular Disease, was not involved in the review process of this manuscript or the final decision
Funding
JS was funded in part by the Hammersmith Hospitals Research Fund
Ethical approval
Not required
Guarantor
PS
Contributorship
JS undertook the search and analysis and wrote the first draft. PS conceived the idea, oversaw the analysis and reviewed the manuscript. PB analysed the data and reviewed the manuscript. All authors contributed to the final version of the manuscript
Acknowledgements
PS and PB hold senior Department of Health Fellowships
References
- 1. Bernick C, Kuller L, Dulberg C, et al. Silent MRI infarcts and the risk of future stroke: the Cardiovascular Health Study. Neurology 2001;57:1222–9 [DOI] [PubMed] [Google Scholar]
- 2. Vermeer S, Hollander M, Van Dijk E, Hofman A, Koudstaal PJ, Breteler M Silent brain infarcts and white matter lesions increase stroke risks in the general population: the Rotterdam Scan Study. Stroke 2003;34:392–6 [DOI] [PubMed] [Google Scholar]
- 3. Vermeer SE, Longstreth WT Jr, Koudstaal PJ Silent brain infarcts: a systematic review. Lancet Neurol 2007;6:611–9 [DOI] [PubMed] [Google Scholar]
- 4. Mantel N, Haenszel W Statistical aspects of the analysis of data from the retrospective analysis of disease. J Nat Cancer Inst 1959;22:719–48 [PubMed] [Google Scholar]
- 5. Kulinskaya E, Morgenthaler S, Staudte R Meta Analysis: A Guide to Calibrating and Combining Statistical Evidence. New York: SAGE Publications, 2008. [Google Scholar]
- 6. Minn YK, Cho SJ, Lee JH, et al. Significance of silent infarcts in acute ischaemic stroke patients aged 80 years or older. Cerebrovasc Dis 2005;20:92–5 [DOI] [PubMed] [Google Scholar]
- 7. Hara M, Ito K, Nawata T, et al. Silent cerebral infarction associated with coronary artery disease. Cardiology 1994;85:171–4 [DOI] [PubMed] [Google Scholar]
- 8. European Atrial Fibrillation Trial (EAFT) study group Stroke prevention in atrial fibrillation. Lancet 1993;342:1255–62 [PubMed] [Google Scholar]
- 9. Giele JLP, Witkamp TD, Mali WPTM, Van der Graaf Y Silent brain infarcts in patients with manifest vascular disease. Stroke 2004;35:742–6 [DOI] [PubMed] [Google Scholar]
- 10. Ong CT, Sung KC, Sung SF, Wu CS, Hsu YC, Su YH Impact of silent infarction on the outcome of stroke patients. J Formos Med Assoc 2009;108:224–30 [DOI] [PubMed] [Google Scholar]
- 11. Vermeer S, Koudstaal P, Oudkerk M, Hofman A, Breteler M Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke 2002;33:21–5 [DOI] [PubMed] [Google Scholar]
- 12. Das R, Seshadri S, Beiser A, et al. Prevalence and correlates of silent brain infarction in the Framingham Offspring Study. Stroke 2008;39:2929–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brainin M, McShane LM, Steiner M, Dachenhausen A, Seiser A Silent brain infarcts and transient ischemic attacks: a three-year study of first-ever ischemic stroke patients: the Klosterneuburg stroke data bank. Stroke 1995;26:1348–52 [DOI] [PubMed] [Google Scholar]
- 14. Jorgensen HS, Nakayama H, Raaschou HO, Gam J, Olsen TS Silent infarction in acute stroke patients: prevalence, localization, risk factors and clinical significance: the Copenhagen Stroke Study. Stroke 1994;25:97–104 [DOI] [PubMed] [Google Scholar]
- 15. Fuster V, Moreno PR Atherothrombosis as a systemic, often silent disease. Nat Clin Pract Cardiovasc Med 2005;2:431 [DOI] [PubMed] [Google Scholar]
- 16. Boon A, Lodder J, Heuts-Van Raak L, Kessels F Silent brain infarction in 755 consecutive patients with first-ever supratentorial ischemic stroke. Relationships with index-stroke subtype, vascular risk factors and mortality. Stroke 1994;25:2384–90 [DOI] [PubMed] [Google Scholar]
- 17. Chodosh EH, Foulkes MA, Kase CS, et al. Silent stroke in the NINCDS stroke data bank. Neurology 1988;38:1674–9 [DOI] [PubMed] [Google Scholar]
- 18. Corea F, Henon H, Pasquier F, Leys D, the Lille Stroke/Dementia study group Silent infarcts in stroke patients: patient characteristics and effect on 2-year outcome. J Neurol 2001;248:271–8 [DOI] [PubMed] [Google Scholar]
- 19. Corea F, Tambasco N, Luccioli R, Ciorba E, Parnetti L, Gallai V Brain CT scan in acute stroke patients: silent infarcts and relation to outcome. Clin Exp Hypertens 2002;24:669–76 [DOI] [PubMed] [Google Scholar]
- 20. Coutts SB, Hill D, Simon JE, Sohn CH, Scott JN, Demchuk AM, for the VISION group Silent ischemia in minor stroke and TIA patients identified on MR imaging. Neurology 2005;65:513–7 [DOI] [PubMed] [Google Scholar]
- 21. Davis PH, Bendixen BH, Adams HP, Woolson RF, Culebras A Silent cerebral infarction in patients enrolled in the TOAST study. Neurology 1996;40:942–8 [DOI] [PubMed] [Google Scholar]
- 22. Herderschee D, Hijdra A, Algra A, Koudstaal PJ, Kappelle LJ, Van Gijn J Silent stroke in patients with TIA or minor ischemic stroke. Stroke 1992;23:1220–4 [DOI] [PubMed] [Google Scholar]
- 23. Kang DW, Latour LL, Lattimore SU, Warach S Silent ischemic lesion recurrence on MRI predicts subsequent clinical vascular events. Arch Neurol 2006;63:1730–3 [DOI] [PubMed] [Google Scholar]
- 24. Kase CS, Wolf PA, Chodosh EH, et al. Prevalence of silent stroke in patients presenting with initial stroke: the Framingham Study. Stroke 1989;20:850–2 [DOI] [PubMed] [Google Scholar]
- 25. Liebetrau M, Steen B, Hamann GF, Skoog I Silent and symptomatic infarcts on cranial computerized tomography in relation to dementia and mortality: a population-based study in 85-year-old subjects. Stroke 2004;34:1816–20 [DOI] [PubMed] [Google Scholar]
- 26. Oh SH, Kim NK, Kim SH The prevalence and risk factor analysis of silent brain infarcts in patients with first-ever stroke. J Neurol Sci 2010;293:97–101 [DOI] [PubMed] [Google Scholar]
- 27. Ricci S, Celani MG, La Rosa F, Righetti E, Duca E, Caputo N Silent brain infarction in patients with first-ever stroke. A community based study in Umbria, Italy. Stroke 1993;24:647–51 [DOI] [PubMed] [Google Scholar]
- 28. Geerlings MI, Appelman AP, Vincken KL, et al. SMART study group Brain volumes and cerebrovascular lesions on MRI in patients with atherosclerotic disease. The SMART study. Atherosclerosis 2010;210:130–6 [DOI] [PubMed] [Google Scholar]
- 29. Hoshide S, Kario K, Mitsuhashi T, et al. Different patterns of silent cerebral infarct in patients with coronary artery disease or hypertension. Am Heart J 2001;14:509–15 [DOI] [PubMed] [Google Scholar]
- 30. Kozdag G, Ciftci E, Ural D, et al. Silent cerebral infarction in chronic heart failure: ischemic and nonischemic dilated cardiomyopathy. Vasc Health Risk Manag 2008;4:463–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nadareishvili Z, Choudary Z, Joyner C, Brodie D, Norris JW Cerebral microembolism in acute myocardial Infarction. Stroke 1999;30:2679–82 [DOI] [PubMed] [Google Scholar]
- 32. Ozeren A, Acarturk E, Koc F, Demir M, Sarica Y, Eroghu H Silent cerebral lesions on MRI in subjects with CAD. Jpn Heart J 1998;39:611–8 [DOI] [PubMed] [Google Scholar]
- 33. Pardo M, Fuster L, Nuez T Silent brain infarctions in patients with coronary artery disease. A Spanish population survey. J Neurol 1998;245:93–7 [DOI] [PubMed] [Google Scholar]
- 34. Selvetella G, Notte A, Maffei A, Calistri V, Scamardella V Left ventricular hypertrophy is associated with asymptomatic cerebral damage in hypertensive patients. Stroke 2003;34:1766–70 [DOI] [PubMed] [Google Scholar]
- 35. Siachos T, Vanbakel A, Feldman DS Silent strokes in patients with heart failure. J Card Fail 2005;11:485–90 [DOI] [PubMed] [Google Scholar]
- 36. Uekita K, Hasebe N, Funayama N Cervical and intercranial atherosclerosis and silent brain infarction in Japanese patients with CAD. Cerebrovasc Dis 2003;16:61–8 [DOI] [PubMed] [Google Scholar]