Abstract
Platelets are integral to normal haemostatic function and act to control vascular haemorrhage with the formation of a stable clot. The fibrinogen receptor (glycoprotein IIb/IIIa [GPIIb/IIIa]) is the most abundant platelet integrin and, by binding fibrinogen, facilitates irreversible binding of platelets to the exposed extracellular matrix and enables the cross-linking of adjacent platelets. The vital role of GPIIb/IIIa requires tight control of both its synthesis and function. After transcription from distinct domains on chromosome 17, the two subunits of the heterodimer are carefully directed through organelles with intricate regulatory steps designed to prevent the cellular expression of a dysfunctional receptor. Similarly, exquisite control of platelet activation via bidirectional signalling acts to limit the inappropriate and excessive formation of platelet-mediated thrombus. However, the enormous diversity of genetic mutations in the fibrinogen receptor has resulted in a number of allelic variants becoming established. The Pro33 polymorphism in GPIIIa is associated with increased cardiovascular risk due to a pathological persistence of outside-in signalling once fibrinogen has dissociated from the receptor. The polymorphism has also been associated with the phenomenon of aspirin resistance, although larger epidemiological studies are required to establish this conclusively. A failure of appropriate receptor function due to a diverse range of mutations in both structural and signalling domains, results in the bleeding diathesis Glanzmann's thrombasthaenia. GPIIb/IIIa inhibitors were the first rationally designed anti-platelet drugs and have proven to be a successful therapeutic option in high-risk primary coronary intervention. As our understanding of bidirectional signalling improves, more subtle and directed therapeutic strategies may be developed.
Introduction
Platelets were first discovered over 130 years ago by Bizzozero,1,2 but it was not until the early 20th century that they were correctly identified as being derived from megakaryocytes, having been variously hypothesized as being fragments of leukocytes, extruded red cell nuclei and albuminous precipitants to name but a few.3 Platelets are central to the formation of thrombus following vascular injury,4 and have increasingly become a target for pharmaceuticals directed at cardiovascular disease prevention.
The platelet fibrinogen receptor is integral to the formation of platelet-mediated thrombus, as it represents the final common pathway of platelet activation, adhesion and aggregation. It is formed from two subunits of glycoprotein IIb (GPIIb; integrin α IIb) and glycoprotein IIIa (GPIIIa; integrin β 3), and is the most abundant integrin on the platelet surface. Quantification of fibrinogen receptor expression using monoclonal antibodies has revealed that each platelet expresses approximately 80,000 such receptors on its surface,5 with an additional internal pool that can be transported to the surface during platelet activation.6
Despite the receptor's colloquial name, it is neither specific to platelets nor to fibrinogen, and the receptor is also found – unsurprisingly – on megakaryoctes7 and also on some transformed cells. In addition to binding fibrinogen,8 it also has the capacity to bind a number of other soluble and immobilized ligands, of which von Willebrand factor appears to have the most significant role in haemostasis.9
This review highlights key points involved in the synthesis, function and regulation of the fibrinogen receptor in haemostasis, with reference also to the pathological fibrinogen receptor and to pharmacological attempts to manipulate its function.
Synthesis of the fibrinogen receptor
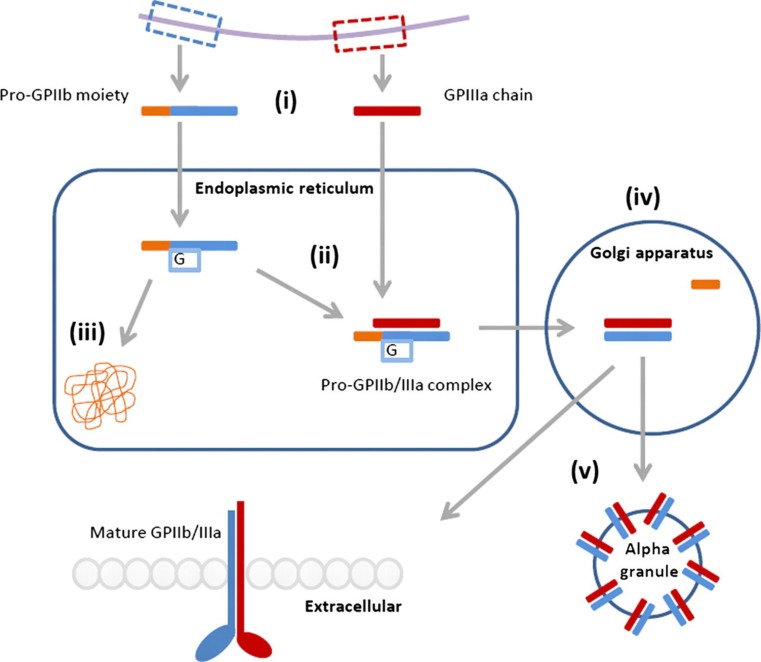
The fibrinogen receptor is formed from two calcium-dependent heterodimers10,11 that are encoded on the long arm of chromosome 17q21.32 with independent regulatory domains12 (Figure 1). The synthesis of the two subunits differs, with GPIIb synthesized as a pro-GPIIb moiety composed of a heavy and a light chain connected by disulfide bonds,13,14 while GPIIIa is synthesized as a single, mature chain.15
Figure 1.
Synthesis of the fibrinogen receptor. (i) The subunits are transcribed from independent domains of chromosome 17q21.32, resulting in a pro-GPIIb moiety with heavy and light chains and a mature GPIIIa chain. (ii) The pro-GPIIb moiety is glycosylated and associates with the mature GPIIIa chain within the endoplasmic reticulum. (iii) Uncomplexed subunits are degraded by the calnexin cycle. (iv) Proteolytic cleavage of the pro-GPIIb subunit within the Golgi apparatus yields the mature GPIIb/IIIa. (v) Mature GPIIb/IIIa is transported to the plasma membrane or stored in alpha granules. G, glycoslation
Within the endoplasmic reticulum, the pro-GPIIb moiety associates with the mature GPIIIa after the latter has undergone post-translational glycosylation.7 The formation of a pro-GPIIb/IIIa complex is a prerequisite for surface expression of either subunit,16 as demonstrated in transfection experiments using COS cells.17 Within the endoplasmic reticulum, high mannose N-linked oligosaccharides are added to the pro-GPIIb precursor15 and uncomplexed subunits are degraded in a proteasome-dependent manner controlled by the calnexin cycle.18
The pro-GPIIb/IIIa complex is then transported to the Golgi apparatus where the mannose chains of the pro-GPIIb are converted into complex oligosaccharides, and proteolytic cleavage of the pro-GPIIb subunit yields the disulfide-linked light and heavy chains. This mature complex is then transported to the cell surface or stored within alpha granules.
The fibrinogen receptor in primary haemostasis
The central role of platelets is to terminate haemorrhage following vascular injury,4 although recently significant roles in atherosclerosis,19 immune response,20 inflammation,21,22 tumour metastasis23 and angiogenesis24 have been identified. The involvement of the fibrinogen receptor in these diverse processes is currently poorly characterized although, as the primary mediator of platelet adhesion, and hence platelet localization, it is likely that the fibrinogen receptor participates to some extent. Indeed, the generation of integrin β 3-null mice in the investigation of Glanzmann's thrombasthaenia (GT) has revealed impaired neovascularization of the retina,25 failure of the coronary capillaries to mature,26 accelerated atherosclerosis,27 cardiac hypertrophy and inflammation28 and promotion of tumour growth29 in such mice. Detailed discussion on these secondary roles of platelets is outside the scope of this review, but these have been comprehensively reviewed elsewhere.30–32
In the resting state, platelets circulate without interacting with the vessel wall or each other, but following vessel injury and exposure of the extracellular matrix, a plethora of different platelet receptors interact with each other in a coordinated manner to facilitate tethering, rolling and adhesion to the vessel wall and a cascade of intricate signalling processes; these result in the recruitment and aggregation of platelets to form a stable, haemostatic plug. The role of platelets in primary haemostasis has been comprehensively reviewed by Broos et al.,33 but we will briefly summarize the three main processes in which the fibrinogen receptor is involved: (i) adhesion to the vessel wall, (ii) platelet aggregation and (iii) bidirectional signalling.
Adhesion to the vessel wall
In order for platelets to adhere to the exposed extracellular matrix, they must be able to form bonds that are stronger than the rheological forces opposing their interaction. GPIIb/IIIa irreversibly binds immobilized fibrinogen34 thus achieving stable adherence to the vessel wall. However, within arterial vessels there are high wall shear forces due to rapidly flowing blood, and the slow rate of bond formation by GPIIb/IIIa is insufficient to facilitate stable adhesion. To resolve this problem, the glycoprotein Ib-V-IX complex on platelets slows their flow rate along the vessel wall by binding von Willebrand factor in a process that exhibits fast association and disassociation.34 Once slowed, GPIIb/IIIa has sufficient time to form the irreversible bonds required.
Platelet aggregation
Once bound to the extracellular matrix, the now activated platelet must recruit and bind other platelets in order to form a haemostatic plug. Selective fusion of cytoplasmic granules with the plasma membrane results in release of a diverse complement of proteins,35 many of which act as soluble platelet agonists in an autocrine and paracrine manner. The signalling steps that lead to activation of GPIIb/IIIa are summarized below, but they result in the receptor binding soluble fibrinogen that is required for the stable cross-linking of adjacent platelets.36,37
The mechanism of aggregation is also affected by shear. At elevated shear rates (1000–10,000 s−1) platelets bind to each other initially via von Willebrand factor receptors, as with adhesion to the vessel wall.33 At even higher shear rates (>10,000 s−1), platelet aggregation can occur in the absence of activation in the presence of soluble von Willebrand factor.33 These aggregates however are unstable as, without activation of GPIIb/IIIa, irreversible platelet–platelet binding does not occur.
Bidirectional signalling
Some integrins are constitutively active but others require signalling events to transform them from a quiescent to an active state, thus enabling them to modulate their function in a temporal and spatial manner. GPIIb/IIIa is in the latter category, as tight regulation of its activation prevents uncontrolled platelet aggregation and inappropriate intravascular thrombus formation.
In response to a stimulus, structural rearrangement transforms the integrin from a closed, low-affinity, bent conformation to an open, high-affinity, extended conformation by a switchblade-like movement of the integrin legs38 in a process that is regulated by both inside-out and outside-in signalling.
Inside-out signalling
Inside-out signalling refers to processes that act on the cytoplasmic domain of integrins (the cytoplasmic tails [CTs]) and result in changes in the extracellular domain, facilitating structural rearrangement of the ligand-binding site. In the context of affinity maturation of the GPIIb/IIIa receptor, platelet agonists (such as adenosine diphosphate [ADP] or thrombin) and adhesion receptors (such as the GPIb-V-IX receptor for von Willebrand factor) activate cytoplasmic proteins that interact with the CTs of GPIIb/IIIa.
The CTs of the alpha and beta subunits are intimately connected via hydrophobic and electrostatic bonds that require complete disruption and the subsequent unclasping of CTs for full activation of the receptor.39 Unclasping of these cytoplasmic tails is facilitated by talin-H that is formed following activation of these cytoplasmic pathways and displaces the binding of the alpha-CT from the beta-CT.40 Once unclasped the CTs, which have no intrinsic enzymatic activity of their own, offer previously hidden binding sites to over 20 cytoplasmic proteins that are involved in perpetuating platelet activation.41
Outside-in signalling
Outside-in signalling, as the name suggests, is the converse of inside-out signalling and results in spreading of the platelet. Ligand interaction via the extracellular domain results in changes within the CTs. Ligand binding may follow previous activation of the receptor by inside-out signalling and conformational change of the binding site, or may occur in isolation, as quiescent GPIIb/IIIa maintains a low-affinity for its ligand.
Clustering of integrins is the visible extracellular manifestation of outside-in signalling as, via a variety of ligand- and activation-dependent mechanisms, integrin density increases where soluble ligand is present at the extracellular surface. Clustering activates cytosolic tyrosine kinases (e.g. the Src-family tyrosine kinases via interaction with GPIIIa CT), thus enabling adhesion-dependent protein phosphorylation.42 The process of clustering is necessary for optimal receptor function in addition to avidity modulation.43
In addition to this visible process of clustering, arachidonic acid is liberated from the plasma membrane in conjunction with ADP signalling, thus providing substrate for cyclooxygenase (COX) and consequent generation of thromboxane (TXA2).44
Inhibition of integrin activity
The change in conformation of a GPIIb/IIIa receptor to either the quiescent or active state is not an all-or-nothing phenomenon within an individual platelet, but rather a dynamic equilibrium which is shifted towards one or other state by the interactions of pro-thrombotic and antithrombotic mediators acting on extracellular ligand-binding sites. Inhibitory signals prevent inappropriate activation and limit thrombus formation to the site of vascular injury.
We have already mentioned that the G-protein-coupled soluble agonists and adhesion receptors act as promoters of the active, pro-thrombotic state. The molecules primarily responsible for promoting the quiescent state are nitric oxide (NO) and prostacyclin (PGI2), via the generation of cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP), respectively.45
PGI2 is synthesized by inducible COX-2 within the vascular endothelium46 and is up-regulated in a paracrine fashion by TXA2 released from platelet granules.47 Elevation of cAMP levels leads to phosphorylation of proteins that inhibit the inside-out activation of GPIIb/IIIa by inhibiting cytoskeletal re-arrangement.48
Endothelial NO synthase (eNOS) is integral to platelet function and, with the short biological half-life of NO; its actions are restricted to the immediate vicinity of its synthesis.49 NO has been found to mediate a number of antiplatelet actions. It inhibits the adhesion of platelets to the endothelium,50,51 suppresses platelet aggregation52 and stimulates disaggregation.53
NO acts to inhibit platelet activation via a number of mechanisms which all appear to be cGMP-mediated.54 In the context of the fibrinogen receptor, cGMP inhibits activation of phosphoinositide 3-kinase (PI3-K),55 which, via inside-out signalling, normally promotes a conformational change in GPIIb/IIIa that facilitates its binding to fibrinogen.56 NO also inhibits GPIb-mediated myosin light chain (MLC) phosphorylation and GPIIb/IIIa activation.57
Dynamic recruitment of fibrinogen receptors
The expression of fibrinogen receptors on the platelet surface is a dynamic not a static process. Alpha granules contain a pool of fibrinogen receptors that are translocated to the plasma membrane on activation.6,58–60 Differential packaging of proteins within alpha granules enables this to be carried out in a selective manner, with translocation of other glycoproteins and secretion of fibrinogen contributing to amplification of signal transduction.61
The expression of GPIIb/IIIa exhibits a positive relationship with ADP-induced aggregation in both healthy subjects and patients with acute coronary syndromes prior to therapy with oral ADP receptor antagonists.62 The expression was, however, also found to be related to mean platelet volume, and therefore may not actually represent increased receptor density. A future direction of study should be to investigate whether platelets are able to synthesize GPIIb/IIIa de novo in response to stimuli as is the case with the regulatory protein B-cell lymphoma-3 to control clot retraction.63
A pathological fibrinogen receptor
The formation of thrombus in response to vascular injury is not due to a pathological fibrinogen receptor per se, as it is an appropriate response to the generation of pro-inflammatory and pro-aggregatory factors released into the intravascular compartment. There are, however, instances when aggregation is enhanced or suppressed due to intrinsic properties of the integrin. We will discuss the common Pro33 polymorphism, aspirin resistance and GT as examples of these occurrences.
Pro33 polymorphism in GPIIIa
The platelet fibrinogen receptor is polymorphic with a number of recognized stable allelic variants based on single amino acid substitutions.64 The GPIIIa Pro33 polymorphism is one of the more heavily studied variants after it was associated with neonatal alloimmune thrombocytopaenia. A modification of the PlA1 epitope to PlA2 involves an amino acid substitution of proline for leucine at position 33 within the extracellular domain adjacent to the ligand-binding site. Pro33 has a Caucasian population frequency of 0.15,65 and in 1996 was noted to cluster with acute coronary thrombosis, especially in patients whose primary event occurred under the age of 60 years.66
Studies since then have yielded inconsistent results, but overall it has been found that carriers of the PlA2 allele are at increased risk of coronary artery disease (odds ratio [OR], 1.10; 95% confidence interval [CI], 1.03–1.18), with the risk being greatest in those <60 years old (OR, 1.21; 95% CI, 1.05–1.38) and in those with in-stent re-stenosis (OR, 1.31; 95% CI, 1.10–1.56).67 Morphological studies using magnetic resonance imaging have demonstrated a decrease in the thickness of the fibrous caps covering atherosclerotic plaques68 in individuals with this polymorphism, and this has previously been associated with increased risk of plaque rupture.69 Platelet function studies, as with the epidemiological findings, have often yielded contradictory results, but overall the polymorphism appears to associate with increased platelet aggregability70 and platelet activation.68
The pro-thrombotic phenotype seen in Pro33 individuals is not secondary to an increase in receptor expression but rather appears to be the result of a change in integrin signalling.71 Data regarding the affinity of fibrinogen binding and alteration of GPIIb/IIIa clustering are inconclusive, but the Pro33 isoform demonstrates increased serine/threonine phosphorylation of extracellular signal-regulated kinase and MLC kinase (MLCK).72
MLCK-mediated phosphorylation of MLCs is necessary for platelet shape change, secretion and the clustering of integrins.73 Myosin phosphatase (MP) normally dephosphorylates MLCK thus reducing platelet activation. In platelets from individuals with the Pro33 polymorphism, MP is itself deactivated by phosphorylation resulting in persistent activation of MLCK.72 The result is ongoing actin re-organization within the platelet via postoccupancy outside-in signalling, and this leads to an increased level of platelet activation and hence increased cardiovascular risk.
Aspirin resistance
Aspirin had been a popular synthetic analgesic agent for almost 90 years before its antiplatelet effects were found to confer a significant survival advantage postmyocardial infarction74 and in the secondary prevention of cardiovascular disease.75 A proportion of patients, however, fail to respond appropriately to aspirin in a heterogeneous phenomenon known as aspirin resistance.
The concept of aspirin resistance originated with the clinical observation that a number of patients on aspirin therapy continued to have platelet-mediated thrombotic events.76 This was perhaps unsurprising considering often suboptimal medication adherence, variable drug pharmacokinetics and the action of aspirin on only a single pathway of platelet activation. However, it became evident that in some individuals there was a biochemical component of aspirin resistance that could not be overcome with increasing serum levels of the drug. A failure to suppress TXA2 production or to inhibit platelet aggregation in response to the agonist arachidonic acid currently forms the basis of the generally accepted definition for biochemical, or laboratory, aspirin resistance.77
The reported prevalence of aspirin resistance varies significantly between studies as, despite an accepted biochemical definition, in vitro measurements of platelet function are performed using disparate assays with large inter-assay variability.78 Two recent systematic reviews have, however, demonstrated some agreement, with the mean prevalence of aspirin resistance being identified as 24%79 and 28%.80 In the second study, examination of the relationship of aspirin resistance to clinical events found that a significantly increased proportion of those classified as aspirin-resistant suffered a cardiovascular event (OR, 3.85; 95% CI, 3.08–4.80; P < 0.001).80 Investigations into the aetiology of this phenomenon have indicated that the fibrinogen receptor, or rather its GPIIIa component, may be involved in many cases.
A number of candidate genes have been investigated as potential causes of aspirin resistance, and the same PlA1/A2 single-nucleotide polymorphism in GPIIIa as described in the previous section has been identified as the strongest candidate in a large systematic review of 50 polymorphisms within 11 genes.81 The PlA1/A2 polymorphism was found to be significantly associated with aspirin resistance in healthy subjects (OR, 2.36; 95% CI, 1.24–4.49; P = 0.009), but not so when combined with patients with cardiovascular disease (OR, 1.14; 95% CI, 0.84–1.54; P = 0.40). The authors of this study identify significant heterogeneity within the studies analysed and suggest caution in the interpretation of their results. However, recent preliminary work from our laboratory analysing the platelet proteome has shown GPIIIa expression to differ between aspirin-sensitive and resistant individuals (unpublished data, Timothy Goodman, 2011).
The data obtained from investigation of the Pro33 population, many of whom were healthy subjects not taking aspirin, suggest a possible mechanism for aspirin resistance. Oral antiplatelet agents act primarily by dampening the inside-out signalling that leads to GPIIb/IIIa activation. Aspirin achieves this by irreversibly acetylating intracellular COX-1 thereby inhibiting TXA2 production, and the ADP antagonists bind the extracellular ADP receptors thus preventing activation of their G-protein-coupled receptors. The Pro33 polymorphism appears to result in sustained outside-in signalling yet, as previously stated, aspirin is generally considered to be involved in dampening inside-out signalling. The distinction between signalling direction within the cytoplasm is somewhat of an artificial construct, as many processes are involved in both, and during the process of thrombus formation there is simultaneous outside-in and inside-out signalling. Aspirin is known to acetylate platelet proteins involved in platelet aggregation other than COX-1, and so it is possible that the Pro33 isoform is more susceptible to acetylation resulting in alteration of function.82
An alternative hypothesis is that increased intracellular arachidonic acid, the substrate for COX-1, may act to inhibit MP within platelets, as has previously been seen in muscle.83 This would lead to persistent platelet activation via postoccupancy outside-in signalling as described above.
Studies into aspirin resistance have been handicapped by a combination of small sample size and a lack of standardization in its evaluation and definition.84 Estimates of prevalence of this phenomenon are still widely debated and, until larger epidemiological studies utilizing a network biology approach to target both putative mechanisms and pharmacogenetics85 are undertaken, much about this clinically important phenomenon will remain elusive.
Glanzmann's thrombasthaenia
GT was first identified in 1918, and subsequently characterized as a bleeding diathesis involving mucous and cutaneous bleeding in the context of normal platelet count and lifespan, prolonged bleeding time, deficient clot retraction and absent platelet aggregation.86 The hypothesis that these observations were secondary to deficiencies in the cell membrane was confirmed with the finding of plasma membrane glycoprotein abnormalities.87–90 The phenotype of GT is due to defects/deficiencies in either the alpha or beta subunits of the fibrinogen receptor resulting in either a reduction in receptor expression or, more commonly, expression of a defective receptor. The elucidation of GPIIb/IIIa synthesis, structure and function has occurred in parallel to the investigation of GT and is reviewed in its historical context by Coller and Shattil.91
Recent platelet aggregation studies have revealed a decreased response to the soluble agonists ADP, arachidonic acid, collagen and epinephrine, but a normal response to ristocetin,92 in patients with GT. The intracellular pool of fibrinogen within alpha granules is also reduced due to defects in fibrinogen transport and storage.93,94
GT is caused by a diverse set of genetic mutations that are mainly non-sense mutations, out-of-frame and in-frame small deletions and insertions within either the ITGA2B or ITGB3 genes.95 There is increased prevalence among certain ethnic groups, with a predilection for those populations with a high frequency of consanguinity.96 Over 100 distinct mutations have been identified to date but, due to the low prevalence of specific mutations and significant genetic heterogeneity, it has not yet been possible to assign individual phenotypes.95 Standard mutagenic techniques are not applicable in nucleated platelets but, by utilizing alternative techniques, knockout models have enabled certain mouse phenotypes to be categorized, many of which are characterized based on detection of the beta-3-mediated defects in the other cell types in which it is expressed. Genetic mutations have been identified throughout the structure of the fibrinogen receptor and result in defects in ligand binding, structural rearrangement, outside-in and inside-out signalling. They are too numerous to elaborate here, but are reviewed elsewhere.95
Targeting the platelet fibrinogen receptor
In the United Kingdom, almost one-third of deaths are due to cardiovascular disease, with coronary heart disease and stroke accounting for the majority of cases.97 Coronary thrombosis is the common pathway in transmural myocardial infarction,98 and platelets are the primary effectors of this.99
Many different agents to inhibit platelet aggregation have been developed including inhibitors of TXA2 synthesis, ADP receptor antagonists and phosphodiesterase inhibitors. None of these targets however cause complete inhibition of platelet aggregation (IPA),100,101 as individually they each modulate only a single pathway of platelet activation. The potential to inhibit the final common pathway of platelet aggregation may, in contrast, result in more complete and faster IPA.102
Intravenous GPIIb/IIIa inhibitors
The intravenous GPIIb/IIIa inhibitors (GPI) were the first rationally designed antiplatelet drugs which act to block the ligand-binding site, thus preventing both outside-in signalling and the aggregatory outcome of inside-out signalling. There are three intravenous GPI that are currently approved for clinical use: Abciximab (a monoclonal antibody fragment), Eptifibatide (a cyclic peptide) and Tirofiban (a peptidomimetic molecule). All three agents act by competing for receptor occupancy with the soluble receptor agonists, and have comparable efficacy in terms of cardiovascular outcomes.103,104
In the context of clinical outcomes, the addition of Abciximab to standard pharmacological therapy during elective coronary stenting, reduced the composite endpoint of death, myocardial infarction and the need for urgent revascularization at both 30 days (hazard ratio [HR], 0.48; 95% CI, 0.33–0.69; P < 0·001)105 and six months (HR, 0.47; 95% CI, 0.33–0.68; P < 0.001).106 The mortality benefit is greatest in patients with elevated troponin,107 and is independent of both coronary lesion morphology108 and interventional technique.109 Increased major bleeding is a recognized adverse effect of intravenous GPI, but this can be limited by minimizing the duration of therapy before primary coronary intervention without offsetting its therapeutic effectiveness.110
Oral GPIIb/IIIa inhibitors
The period soon after myocardial infarction is extremely high risk in the context of platelet-mediated thrombosis. This period of high risk continues for a number of weeks after the event as platelets retain predilection for spontaneous activation.111,112 It would be impractical to maintain patients on intravenous therapy for such a protracted time, and so oral GPI were developed in an attempt to offer prolonged treatment during this period.
Meta-analysis of over 33,000 patients involved in phase III trials of oral GPI demonstrated an increase in mortality in individuals prescribed these agents compared with placebo (OR, 1.37; 95% CI, 1.13–1.66; P = 0.001).113 This increase in mortality may be due to either toxic effects or a paradoxical increase in platelet activation secondary to partial agonist action due to suboptimal GPIIb/IIIa blockade.114 The second hypothesis had been previously mooted to explain lack of positive outcomes associated with prolonged Abciximab infusions in the GUSTO IV study, where placebo demonstrated no significant increase in death or myocardial infarction compared with 24 hours (OR, 1.0; 95% CI, 0.83–1.24) or 48 hours (OR, 1.1; 95% CI, 0.94–1.39) Abciximab infusion, at 30 days.115 Consequentially, the oral GPI (Xemilofiban, Orbofiban and Sibrafiban) were not approved for clinical use.
GPIIb/IIIa inhibitors in clinical practice
The role for GPI in clinical practice is limited, and the current recommendations from the European Society of Cardiology are for intravenous GPI to be used in high-risk primary coronary intervention among patients who are already treated with dual antiplatelet therapy.116 However, many of the early trials of GPI were without the benefit of either drug-eluting stents or dual antiplatelet therapy, and so the clinical benefits attributed to GPI are likely to be attenuated with the provision of what is now standard therapy. Also, as more potent ADP receptor antagonists and direct thrombin inhibitors are licensed, the role for GPI as an adjuvant antiplatelet agent may diminish.
Despite being the first rationally designed antiplatelet agent, GPI are still a relatively blunt tool as they act by blocking ligand binding rather than modulating signalling. As our understanding of bidirectional signalling evolves, it may prove possible to target specific aspects of outside-in signalling with new agents and this may lead to a possible resurrection of the possibilities for oral therapy.
Therapy for Glanzmann's thombasthenia
In contrast to the prevention of thrombus formation from pathological activation of the fibrinogen receptor, the prevention of bleeding from a pathological lack of appropriate activation is considerably more challenging. Therapy for GT has historically involved multiple platelet transfusions with the associated problems of transfusion antibody formation. The availability of recombinant factor VIIa117 and bone marrow transplantation118 have been important recent advances in care. Advanced genetic techniques to increase de novo expression of functional fibrinogen receptors are in early phases of development, but offer the potential for cure without the mortality associated with bone marrow transplantation.
Conclusions
The platelet fibrinogen receptor is central to the formation of a platelet-mediated thrombus in response to vascular injury, and is responsible for much pathology via both appropriate and defective responses to stimuli. GPIIb/IIIa synthesis and heterodimer formation are carefully marshalled through organelles designed to transport only functional GPIIb/IIIa to the cell surface. However, the enormous diversity of genetic mutations has resulted in a number of allelic variants becoming established within populations, resulting in the diametrically opposed outcomes of increased cardiovascular risk and bleeding diatheses.
The PlA2 allelic variant and consequent Pro33 polymorphism in GPIIIa appears to confer increased cardiovascular risk both in the context of increased platelet aggregability and of resistance to aspirin. The increased cardiovascular risk of Pro33 appears to be less than that for conventional risk factors but, until a large epidemiological study with prolonged follow-up is performed, the true magnitude of the effect will remain unclear. Similarly, regarding the role of Pro33 in aspirin resistance, larger studies are needed with the added caveat that standardization of the definition of aspirin resistance is required – the subject of aspirin resistance has, since it was first reported, been plagued by under-powered studies using diagnostic tests with high intra- and intersubject variability.
Since the emergence of intravenous GPI and the therapeutic failure of oral GPI, the fibrinogen receptor has been somewhat sidelined as a therapeutic target as the newer, more potent, ADP antagonists and direct thrombin inhibitors have entered clinical practice. As our understanding of the extracellular domain of GPIIb/IIIa and its contribution towards outside-in signalling is further elucidated using modern techniques, the opportunity for more advanced rationally designed GPI may present itself. On the other side, advances in gene therapy and stem cell manipulation may facilitate a cure for GT.
In conclusion, while much has been learnt about the fibrinogen receptor and its contribution to both thrombotic and haemorrhagic pathology, there still remain important questions that will require modern technological methods and large epidemiological studies to answer.
DECLARATIONS
Competing interests
None
Funding
The authors receive funding from Guy's and St Thomas' Charity
Ethical approval
N/A
Guarantor
CNF and AF
Contributorship
CNF performed the literature review. CNF and AF wrote the paper
Acknowledgements
None
References
- 1. Bizzozero G Su di un nuovo elemento morfologico del sangue dei mammiferi e della sua importanza nella trombosi e nella coagulazione. L'Osservatore 1881;17:785–7 [Google Scholar]
- 2. Bizzozero G Ueber einen neuen Formbestandtheil des Blutes und dessen Rolle bei der Thrombose und der Bluntgerinnung. Virchows Arch Pathol Anat Physiol 1882;1882:261–332 [Google Scholar]
- 3. Wright J The histogenesis of the blood platelets. J Morphol 1910;21:263 [Google Scholar]
- 4. Pinniger JL, Prunty FT Some observations on the blood-clotting mechanism; the role of fibrinogen and platelets, with reference to a case of congenital afibrinogenaemia. Br J Exp Pathol 1946;27:200–10 [PMC free article] [PubMed] [Google Scholar]
- 5. Wagner CL, Mascelli MA, Neblock DS, et al. Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood 1996;88:907–14 [PubMed] [Google Scholar]
- 6. Wencel-Drake JD, Plow EF, Kunicki TJ, et al. Localization of internal pools of membrane glycoproteins involved in platelet adhesive responses. Am J Pathol 1986;124:324–34 [PMC free article] [PubMed] [Google Scholar]
- 7. Duperray A, Berthier R, Chagnon E, et al. Biosynthesis and processing of platelet GPIIb-IIIa in human megakaryocytes. J Cell Biol 1987;104:1665–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marguerie GA, Plow EF, Edgington TS Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem 1979;254:5357–63 [PubMed] [Google Scholar]
- 9. Reininger AJ Function of von Willebrand factor in haemostasis and thrombosis. Haemophilia 2008;14(Suppl. 5):11–26 [DOI] [PubMed] [Google Scholar]
- 10. Jennings LK, Phillips DR Purification of glycoproteins IIb and III from human platelet plasma membranes and characterization of a calcium-dependent glycoprotein IIb-III complex. J Biol Chem 1982;257:10458–66 [PubMed] [Google Scholar]
- 11. Kunicki TJ, Pidard D, Rosa JP, Nurden AT The formation of Ca ++ -dependent complexes of platelet membrane glycoproteins IIb and IIIa in solution as determined by crossed immunoelectrophoresis. Blood 1981;58:268–78 [PubMed] [Google Scholar]
- 12. Thornton MA, Poncz M, Korostishevsky M, et al. The human platelet alphaIIb gene is not closely linked to its integrin partner beta3. Blood 1999;94:2039–47 [PubMed] [Google Scholar]
- 13. Phillips DR, Agin PP Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem 1977;252:2121–6 [PubMed] [Google Scholar]
- 14. Bray PF, Rosa JP, Lingappa VR, et al. Biogenesis of the platelet receptor for fibrinogen: evidence for separate precursors for glycoproteins IIb and IIIa. Proc Natl Acad Sci USA 1986;83:1480–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosa JP, McEver RP Processing and assembly of the integrin, glycoprotein IIb-IIIa, in HEL cells. J Biol Chem 1989;264:12596–603 [PubMed] [Google Scholar]
- 16. Duperray A, Troesch A, Berthier R, et al. Biosynthesis and assembly of platelet GPIIb-IIIa in human megakaryocytes: evidence that assembly between pro-GPIIb and GPIIIa is a prerequisite for expression of the complex on the cell surface. Blood 1989;74:1603–11 [PubMed] [Google Scholar]
- 17. O'Toole TE, Loftus JC, Plow EF, et al. Efficient surface expression of platelet GPIIb-IIIa requires both subunits. Blood 1989;74:14–8 [PubMed] [Google Scholar]
- 18. Mitchell WB, Li J, French DL, Coller BS AlphaIIbbeta3 biogenesis is controlled by engagement of alphaIIb in the calnexin cycle via the N15-linked glycan. Blood 2006;107:2713–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Massberg S, Brand K, Gruner S, et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med 2002;196:887–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shiraki R, Inoue N, Kawasaki S, et al. Expression of toll-like receptors on human platelets. Thromb Res 2004;113:379–85 [DOI] [PubMed] [Google Scholar]
- 21. Farr M, Wainwright A, Salmon M, Hollywell CA, Bacon PA Platelets in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int 1984;4:13–7 [DOI] [PubMed] [Google Scholar]
- 22. Thornton P, McColl BW, Greenhalgh A, et al. Platelet interleukin-1alpha drives cerebrovascular inflammation. Blood 2010;115:3632–9 [DOI] [PubMed] [Google Scholar]
- 23. Gasic GJ, Gasic TB, Stewart CC Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci USA 1968;61:46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Italiano JE Jr, Richardson JL, Patel-Hettd S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 2008;111:1227–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hodivala-Dilke KM, McHugh KP, Tsakiris DA, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest 1999;103:229–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weis SM, Lindquist JN, Barnes LA, et al. Cooperation between VEGF and beta3 integrin during cardiac vascular development. Blood 2007;109:1962–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weng S, Zemany L, Standley KN, et al. Beta3 integrin deficiency promotes atherosclerosis and pulmonary inflammation in high-fat-fed, hyperlipidemic mice. Proc Natl Acad Sci USA 2003;100:6730–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ren J, Avery J, Zhao H, et al. Beta3 integrin deficiency promotes cardiac hypertrophy and inflammation. J Mol Cell Cardiol 2007;42:367–77 [DOI] [PubMed] [Google Scholar]
- 29. Reynolds LE, Wyder L, Lively JC, et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med 2002;8:27–34 [DOI] [PubMed] [Google Scholar]
- 30. Nurden AT Platelets, inflammation and tissue regeneration. Thromb Haemost 2011;105(Suppl. 1):S13–33 [DOI] [PubMed] [Google Scholar]
- 31. Lievens D, von Hundelshausen P Platelets in atherosclerosis. Thromb Haemost 2011;106:827–38 [DOI] [PubMed] [Google Scholar]
- 32. Jain S, Harris J, Ware J Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol 2010;30:2362–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Broos K, Feys HB, De Meyer SF, Vanhoorelbeke K, Deckmyn H Platelets at work in primary hemostasis. Blood Rev 2011;25:155–67 [DOI] [PubMed] [Google Scholar]
- 34. Savage B, Saldivar E, Ruggeri ZM Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 1996;84:289–97 [DOI] [PubMed] [Google Scholar]
- 35. Rendu F, Brohard-Bohn B The platelet release reaction: granules' constituents, secretion and functions. Platelets 2001;12:261–73 [DOI] [PubMed] [Google Scholar]
- 36. Mustard JF, Packham MA, Kinlough-Rathbone RL, Perry DW, Regoeczi E Fibrinogen and ADP-induced platelet aggregation. Blood 1978;52:453–66 [PubMed] [Google Scholar]
- 37. Weisel JW, Nagaswami C, Vilaire G, Bennett JS Examination of the platelet membrane glycoprotein IIb-IIIa complex and its interaction with fibrinogen and other ligands by electron microscopy. J Biol Chem 1992;267:16637–43 [PubMed] [Google Scholar]
- 38. Kamata T, Handa M, Ito S, et al. Structural requirements for activation in alphaIIb beta3 integrin. J Biol Chem 2010;285:38428–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ma YQ, Yang J, Pesho MM, et al. Regulation of integrin alphaIIbbeta3 activation by distinct regions of its cytoplasmic tails. Biochemistry 2006;45:6656–62 [DOI] [PubMed] [Google Scholar]
- 40. Vinogradova O, Velyvis A, Velyviene A, et al. A structural mechanism of integrin alpha(IIb)beta(3) “inside-out” activation as regulated by its cytoplasmic face. Cell 2002;110:587–97 [DOI] [PubMed] [Google Scholar]
- 41. Liu S, Calderwood DA, Ginsberg MH Integrin cytoplasmic domain-binding proteins. J Cell Sci 2000;113(Part 20):3563–71 [DOI] [PubMed] [Google Scholar]
- 42. Arias-Salgado EG, Lizano S, Sarkar S, et al. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci USA 2003;100:13298–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hato T, Pampori N, Shattil SJ Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin alphaIIb beta3. J Cell Biol 1998;141:1685–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jin J, Quinton TM, Zhang J, Rittenhouse SE, Kunapuli SP Adenosine diphosphate (ADP)-induced thromboxane A(2) generation in human platelets requires coordinated signaling through integrin alpha(IIb)beta(3) and ADP receptors. Blood 2002;99:193–8 [DOI] [PubMed] [Google Scholar]
- 45. Smolenski A Novel roles of cAMP/cGMP-dependent signaling in platelets. J Thromb Haemost 2012;10:167–76 [DOI] [PubMed] [Google Scholar]
- 46. Ruan CH, So SP, Ruan KH Inducible COX-2 dominates over COX-1 in prostacyclin biosynthesis: mechanisms of COX-2 inhibitor risk to heart disease. Life Sci 2011;88:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caughey GE, Cleland LG, Gamble JR, James MJ Up-regulation of endothelial cyclooxygenase-2 and prostanoid synthesis by platelets. Role of thromboxane A2. J Biol Chem 2001;276:37839–45 [DOI] [PubMed] [Google Scholar]
- 48. Aszodi A, Pfeifer A, Ahmad M, et al. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO J 1999;18:37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gkaliagkousi E, Ferro A Nitric oxide signalling in the regulation of cardiovascular and platelet function. Front Biosci 2011;16:1873–97 [DOI] [PubMed] [Google Scholar]
- 50. Radomski MW, Palmer RM, Moncada S Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987;2:1057–8 [DOI] [PubMed] [Google Scholar]
- 51. Radomski MW, Palmer RM, Moncada S The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem Biophys Res Commun 1987;148:1482–9 [DOI] [PubMed] [Google Scholar]
- 52. Benjamin N, Dutton JA, Ritter JM Human vascular smooth muscle cells inhibit platelet aggregation when incubated with glyceryl trinitrate: evidence for generation of nitric oxide. Br J Pharmacol 1991;102:847–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Radomski MW, Palmer RM, Moncada S The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol 1987;92:639–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dangel O, Mergia E, Karlisch K, et al. Nitric oxide-sensitive guanylyl cyclase is the only nitric oxide receptor mediating platelet inhibition. J Thromb Haemost 2010;8:1343–52 [DOI] [PubMed] [Google Scholar]
- 55. Pigazzi A, Heydrick S, Folli F, et al. Nitric oxide inhibits thrombin receptor-activating peptide-induced phosphoinositide 3-kinase activity in human platelets. J Biol Chem 1999;274:14368–75 [DOI] [PubMed] [Google Scholar]
- 56. Zhang J, Shattil SJ, Cunningham MC, Rittenhouse SE Phosphoinositide 3-kinase gamma and p85/phosphoinositide 3-kinase in platelets. Relative activation by thrombin receptor or beta-phorbol myristate acetate and roles in promoting the ligand-binding function of alphaIIbbeta3 integrin. J Biol Chem 1996;271:6265–72 [DOI] [PubMed] [Google Scholar]
- 57. Roberts W, Michno A, Aburima A, Naseem KM Nitric oxide inhibits von Willebrand factor-mediated platelet adhesion and spreading through regulation of integrin alpha(IIb)beta(3) and myosin light chain. J Thromb Haemost 2009;7:2106–15 [DOI] [PubMed] [Google Scholar]
- 58. Woods VL Jr, Wolff LE, Keller DM Resting platelets contain a substantial centrally located pool of glycoprotein IIb-IIIa complex which may be accessible to some but not other extracellular proteins. J Biol Chem 1986;261:15242–51 [PubMed] [Google Scholar]
- 59. Cramer EM, Savidge GF, Vainchenker W, et al. Alpha-granule pool of glycoprotein IIb-IIIa in normal and pathologic platelets and megakaryocytes. Blood 1990;75:1220–7 [PubMed] [Google Scholar]
- 60. Niiya K, Hodson E, Bader R, et al. Increased surface expression of the membrane glycoprotein IIb/IIIa complex induced by platelet activation. Relationship to the binding of fibrinogen and platelet aggregation. Blood 1987;70:475–83 [PubMed] [Google Scholar]
- 61. Sehgal S, Storrie B Evidence that differential packaging of the major platelet granule proteins von Willebrand factor and fibrinogen can support their differential release. J Thromb Haemost 2007;5:2009–16 [DOI] [PubMed] [Google Scholar]
- 62. Yakushkin VV, Zyuryaev IT, Khaspekova SG, et al. Glycoprotein IIb-IIIa content and platelet aggregation in healthy volunteers and patients with acute coronary syndrome. Platelets 2011;22:243–51 [DOI] [PubMed] [Google Scholar]
- 63. Weyrich AS, Denis MM, Schwertz H, et al. mTOR-dependent synthesis of Bcl-3 controls the retraction of fibrin clots by activated human platelets. Blood 2007;109:1975–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Newman PJ, Valentin N Human platelet alloantigens: recent findings, new perspectives. Thromb Haemost 1995;74:234–9 [PubMed] [Google Scholar]
- 65. Simsek S, Faber NM, Bleeker PM, et al. Determination of human platelet antigen frequencies in the Dutch population by immunophenotyping and DNA (allele-specific restriction enzyme) analysis. Blood 1993;81:835–40 [PubMed] [Google Scholar]
- 66. Weiss EJ, Bray PF, Tayback M, et al. A polymorphism of a platelet glycoprotein receptor as an inherited risk factor for coronary thrombosis. N Engl J Med 1996;334:1090–4 [DOI] [PubMed] [Google Scholar]
- 67. Di Castelnuovo A, de Gaetano G, Donati MB, Iacoviello L Platelet glycoprotein receptor IIIa polymorphism PLA1/PLA2 and coronary risk: a meta-analysis. Thromb Haemost 2001;85:626–33 [PubMed] [Google Scholar]
- 68. Kucharska-Newton AM, Monda KL, Campbell S, et al. Association of the platelet GPIIb/IIIa polymorphism with atherosclerotic plaque morphology: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis 2011;216:151–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kolodgie FD, Burke AP, Farb A, et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol 2001;16:285–92 [DOI] [PubMed] [Google Scholar]
- 70. Feng D, Lindpaintner K, Larson MG, et al. Increased platelet aggregability associated with platelet GPIIIa PlA2 polymorphism: the Framingham Offspring study. Arterioscler Thromb Vasc Biol 1999;19:1142–7 [DOI] [PubMed] [Google Scholar]
- 71. Vijayan KV, Bray PF Molecular mechanisms of prothrombotic risk due to genetic variations in platelet genes: Enhanced outside-in signaling through the Pro33 variant of integrin beta3. Exp Biol Med (Maywood) 2006;231:505–13 [DOI] [PubMed] [Google Scholar]
- 72. Vijayan KV, Liu Y, Sun W, Ito M, Bray PF The Pro33 isoform of integrin beta3 enhances outside-in signaling in human platelets by regulating the activation of serine/threonine phosphatases. J Biol Chem 2005;280:21756–62 [DOI] [PubMed] [Google Scholar]
- 73. Kamm KE, Stull JT Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem 2001;276:4527–30 [DOI] [PubMed] [Google Scholar]
- 74. ISIS-2 Collaborative Group Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet 1988;2:349–60 [PubMed] [Google Scholar]
- 75. Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 2005;353:2373–83 [DOI] [PubMed] [Google Scholar]
- 76. Bhatt DL, Topol EJ Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov 2003;2:15–28 [DOI] [PubMed] [Google Scholar]
- 77. Hankey GJ, Eikelboom JW Aspirin resistance. Lancet 2006;367:606–17 [DOI] [PubMed] [Google Scholar]
- 78. Lordkipanidze M, Pharand C, Schampaert E, et al. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease. Eur Heart J 2007;28:1702–8 [DOI] [PubMed] [Google Scholar]
- 79. Hovens MM, Snoep JD, Eikenboom JC, et al. Prevalence of persistent platelet reactivity despite use of aspirin: a systematic review. Am Heart J 2007;153:175–81 [DOI] [PubMed] [Google Scholar]
- 80. Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR Aspirin ‘resistance’ and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ 2008;336:195–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Goodman T, Ferro A, Sharma P Pharmacogenetics of aspirin resistance: a comprehensive systematic review. Br J Clin Pharmacol 2008;66:222–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. O'Kane P, Xie L, Liu Z, et al. Aspirin acetylates nitric oxide synthase type 3 in platelets thereby increasing its activity. Cardiovasc Res 2009;83:123–30 [DOI] [PubMed] [Google Scholar]
- 83. Gong MC, Fuglsang A, Alessi D, et al. Arachidonic acid inhibits myosin light chain phosphatase and sensitizes smooth muscle to calcium. J Biol Chem 1992;267:21492–8 [PubMed] [Google Scholar]
- 84. Fitzgerald R, Pirmohamed M Aspirin resistance: effect of clinical, biochemical and genetic factors. Pharmacol Ther 2011;130:213–25 [DOI] [PubMed] [Google Scholar]
- 85. Ross S, Anand SS, Joseph P, Paré G Promises and challenges of pharmacogenetics: an overview of study design, methodological and statistical issues. J R Soc Med Cardiovasc Dis 2012;1:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Caen J, Castaldi P, Leclerc J, et al. Congenital bleeding dosorders with long bleeding time and normal platelet count. I. glanzmann's thrombasthenia. Am J Med 1966;41:4–26 [Google Scholar]
- 87. Nurden AT, Caen JP An abnormal platelet glycoprotein pattern in three cases of Glanzmann's thrombasthenia. Br J Haematol 1974;28:253–60 [DOI] [PubMed] [Google Scholar]
- 88. Phillips DR, Agin PP Platelet membrane defects in Glanzmann's thrombasthenia. Evidence for decreased amounts of two major glycoproteins. J Clin Invest 1977;60:535–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jamieson GA, Okumura T, Fishback B, et al. Platelet membrane glycoproteins in thrombasthenia, Bernard-Soulier syndrome, and storage pool disease. J Lab Clin Med 1979;93:652–60 [PubMed] [Google Scholar]
- 90. Hagen I, Nurden A, Bjerrum OJ, Solum NO, Caen J Immunochemical evidence for protein abnormalities in platelets from patients with Glanzmann's thrombasthenia and Bernard-Soulier syndrome. J Clin Invest 1980;65:722–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Coller BS, Shattil SJ The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood 2008;112:3011–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sebastiano C, Bromberg M, Breen K, Hurford MT Glanzmann's thrombasthenia: report of a case and review of the literature. Int J Clin Exp Pathol 2010;3:443–7 [PMC free article] [PubMed] [Google Scholar]
- 93. Zucker M, Pert J, Wilgartner M Platelet function in a patient with thrombasthenia. Blood 1966;28:524–34 [PubMed] [Google Scholar]
- 94. Belloc F, Heilmann E, Combrie R, Boisseau MR, Nurden AT Protein synthesis and storage in human platelets: a defective storage of fibrinogen in platelets in Glanzmann's thrombasthenia. Biochim Biophys Acta 1987;925:218–25 [DOI] [PubMed] [Google Scholar]
- 95. Nurden AT, Fiore M, Nurden P, Pillois X Glanzmann thrombasthenia: a review of ITGA2B and ITGB3 defects with emphasis on variants, phenotypic variability, and mouse models. Blood 2011;118:5996–6005 [DOI] [PubMed] [Google Scholar]
- 96. George JN, Caen JP, Nurden AT Glanzmann's thrombasthenia: the spectrum of clinical disease. Blood 1990;75:1383–95 [PubMed] [Google Scholar]
- 97. Scarborough P, Wickramasinghe K, Bhatnagar P, Rayner M Trends in coronary heart disease, 1961–2011. See http://www.bhf.org.uk/research/statistics.aspx (last checked 3 May 2012) [Google Scholar]
- 98. DeWood MA, Spores J, Hensley GR, et al. Coronary arteriographic findings in acute transmural myocardial infarction. Circulation 1983;68(Pt 2):I39–49 [PubMed] [Google Scholar]
- 99. Jeong MH, Owen WG, Staab ME, et al. Porcine model of stent thrombosis: platelets are the primary component of acute stent closure. Cathet Cardiovasc Diagn 1996;38:38–43 [DOI] [PubMed] [Google Scholar]
- 100. Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol 2003;41:961–5 [DOI] [PubMed] [Google Scholar]
- 101. Payne CD, Li YG, Small DS, et al. Increased active metabolite formation explains the greater platelet inhibition with prasugrel compared to high-dose clopidogrel. J Cardiovasc Pharmacol 2007;50:555–62 [DOI] [PubMed] [Google Scholar]
- 102. Coller BS, Folts JD, Smith SR, Scudder LE, Jordan R Abolition of in vivo platelet thrombus formation in primates with monoclonal antibodies to the platelet GPIIb/IIIa receptor. Correlation with bleeding time, platelet aggregation, and blockade of GPIIb/IIIa receptors. Circulation 1989;80:1766–74 [DOI] [PubMed] [Google Scholar]
- 103. Gurm HS, Tamhane U, Meier P, et al. A comparison of abciximab and small-molecule glycoprotein IIb/IIIa inhibitors in patients undergoing primary percutaneous coronary intervention: a meta-analysis of contemporary randomized controlled trials. Circ Cardiovasc Interv 2009;2:230–6 [DOI] [PubMed] [Google Scholar]
- 104. Valgimigli M, Biondi-Zoccai G, Tebaldi M, et al. Tirofiban as adjunctive therapy for acute coronary syndromes and percutaneous coronary intervention: a meta-analysis of randomized trials. Eur Heart J 2010;31:35–49 [DOI] [PubMed] [Google Scholar]
- 105. EPISTENT Investigators Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. Lancet 1998;352:87–92 [DOI] [PubMed] [Google Scholar]
- 106. Lincoff AM, Califf RM, Moliterno DJ, et al. Complementary clinical benefits of coronary-artery stenting and blockade of platelet glycoprotein IIb/IIIa receptors. Evaluation of platelet IIb/IIIa inhibition in stenting investigators. N Engl J Med 1999;341:319–27 [DOI] [PubMed] [Google Scholar]
- 107. Heeschen C, Hamm CW, Goldmann B, et al. Troponin concentrations for stratification of patients with acute coronary syndromes in relation to therapeutic efficacy of tirofiban. PRISM Study Investigators. Platelet Receptor Inhibition in Ischemic Syndrome Management. Lancet 1999;354:1757–62 [DOI] [PubMed] [Google Scholar]
- 108. Ellis SG, Lincoff AM, Miller D, et al. Reduction in complications of angioplasty with abciximab occurs largely independently of baseline lesion morphology. EPIC and EPILOG Investigators. Evaluation of 7E3 for the revention of ischemic complications. Evaluation of PTCA to improve long-term outcome with abciximab GPIIb/IIIa receptor blockade. J Am Coll Cardiol 1998;32:1619–23 [DOI] [PubMed] [Google Scholar]
- 109. Bhatt DL, Lincoff AM, Califf RM, et al. The benefit of abciximab in percutaneous coronary revascularization is not device-specific. Am J Cardiol 2000;85:1060–4 [DOI] [PubMed] [Google Scholar]
- 110. Stone GW, Bertrand ME, Moses JW, et al. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007;297:591–602 [DOI] [PubMed] [Google Scholar]
- 111. Trip MD, Cats VM, van Capelle FJ, Vreeken J Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med 1990;322:1549–54 [DOI] [PubMed] [Google Scholar]
- 112. Ault KA, Cannon CP, Mitchell J, et al. Platelet activation in patients after an acute coronary syndrome: results from the TIMI-12 trial. Thrombolysis in myocardial infarction. J Am Coll Cardiol 1999;33:634–9 [DOI] [PubMed] [Google Scholar]
- 113. Chew DP, Bhatt DL, Sapp S, Topol EJ Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists: a meta-analysis of phase III multicenter randomized trials. Circulation 2001;103:201–6 [DOI] [PubMed] [Google Scholar]
- 114. Scarborough RM, Kleiman NS, Phillips DR Platelet glycoprotein IIb/IIIa antagonists. What are the relevant issues concerning their pharmacology and clinical use? Circulation 1999;100:437–44 [DOI] [PubMed] [Google Scholar]
- 115. Simoons ML Effect of glycoprotein IIb/IIIa receptor blocker abciximab on outcome in patients with acute coronary syndromes without early coronary revascularisation: the GUSTO IV-ACS randomised trial. Lancet 2001;357:1915–24 [DOI] [PubMed] [Google Scholar]
- 116. Hamm CW, Bassand JP, Agewall S, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999–3054 [DOI] [PubMed] [Google Scholar]
- 117. Poon MC Clinical use of recombinant human activated factor VII (rFVIIa) in the prevention and treatment of bleeding episodes in patients with Glanzmann's thrombasthenia. Vasc Health Risk Manag 2007;3:655–64 [PMC free article] [PubMed] [Google Scholar]
- 118. Flood VH, Johnson FL, Boshkov LK, et al. Sustained engraftment post bone marrow transplant despite anti-platelet antibodies in Glanzmann thrombasthenia. Pediatr Blood Cancer 2005;45:971–5 [DOI] [PubMed] [Google Scholar]