Abstract
We report on a 36-year-old Caucasian woman who presented to the emergency department with post-traumatic retroperitoneal bleeding diagnosed by computed tomography. After clinical stabilization of the patient, selective arterial embolization was performed. The angiomyolipoma's feeding artery was successfully treated with an 8-mm Amplatzer Vascular Plug Type II. The upper pole of the left kidney, which was supplied by a separate upper renal artery, was conserved. Consequently, the renal angiomyolipoma became necrotic and surgical resection of the entire renal angiomyolipoma was performed. This case illustrates a simple and effective application of an Amplatzer Vascular Plug occluder for vessel embolization, without additional coiling.
Keywords: Hemorrhage, outcome, interlocking detachable coil, Amplatzer plug renal angiomyolipoma, selective arterial embolization
Renal angiomyolipoma (AML) is a common benign renal neoplasm composed of variable amounts of adipose tissue, thick-walled blood vessels, and smooth muscle tissue originating from perivascular epithelial cells (1, 2). The incidence of renal AML is approximately 0.3–3% (3). Often, the tumor is found incidentally during diagnostic CT, ultrasound, or MRI examinations. CT or ultrasound reliably confirms the diagnosis (4, 5). There are two types of renal AML: isolated renal AML (80% of cases) and multiple AML lesions associated with tuberous sclerosis (20% of cases) (6, 7). Most renal AML are asymptomatic. The management of AML is based on risk stratification for bleeding. Most studies have focused on a 4-cm cut-off as an indication for nephron-sparing surgery. Recently, selective angiographic embolization (SAE) has been accepted as an alternative, minimally invasive therapeutic approach with similar long-term outcome (8–10). We report on a case of massive retroperitoneal hemorrhage caused by a left-sided solitary giant AML.
Case report
A 36-year-old Caucasian woman fell on her left side during a parking maneuver with her motor scooter. Shortly after the incident, the patient developed acute left flank pain and hematuria associated with tachycardia, hypotension, and diaphoresis. The pain worsened and she was brought to the emergency department of our institution.
On physical examination the patient was in acute distress with a tenderness of palpation of the left flank. The patient was otherwise healthy with no know history of benign or malignant medical conditions. On laboratory analysis, hemoglobin (Hb 9.8 mg/dL) and hematocrit (HCT 36%) were reduced. Conventional CT with i.v. contrast injection (Figs. 1 and 2) demonstrated a left renal mass containing fat with an extended retroperitoneal hematoma. The diagnosis of a massive retroperitoneal hemorrhage from a ruptured AML was made. Furthermore, CT revealed a duplicated left renal artery.
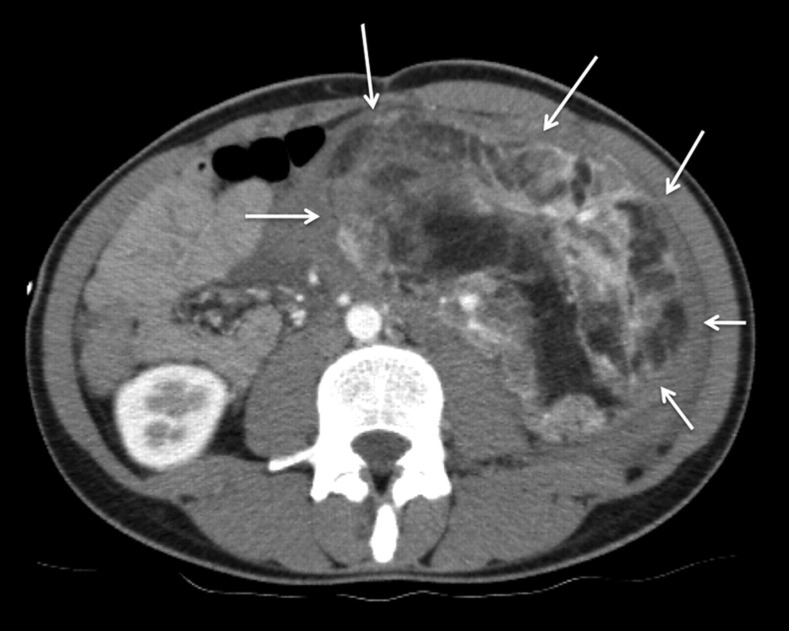
Fig. 1.
Axial CT of the lower abdomen demonstrates a well-defined tumor mass of 8.5 × 14 cm containing adipose and soft tissue elements consistent with a renal angiomyolipoma (small white arrows)
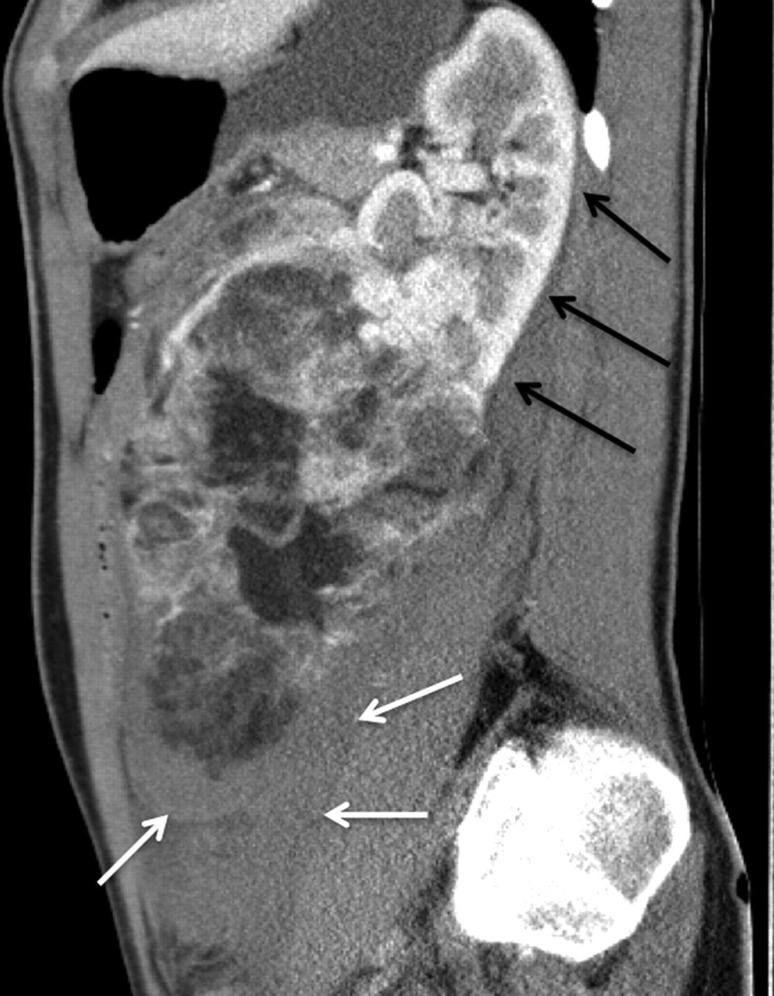
Fig. 2.
Sagittal CT image demonstrates the longitudinal extend (17 cm) of the giant angiomyolipoma. The upper part of the left kidney shows regular renal parenchyma without tumor infiltration (black arrows). There is a significant retroperitoneal hematoma (small white arrows)
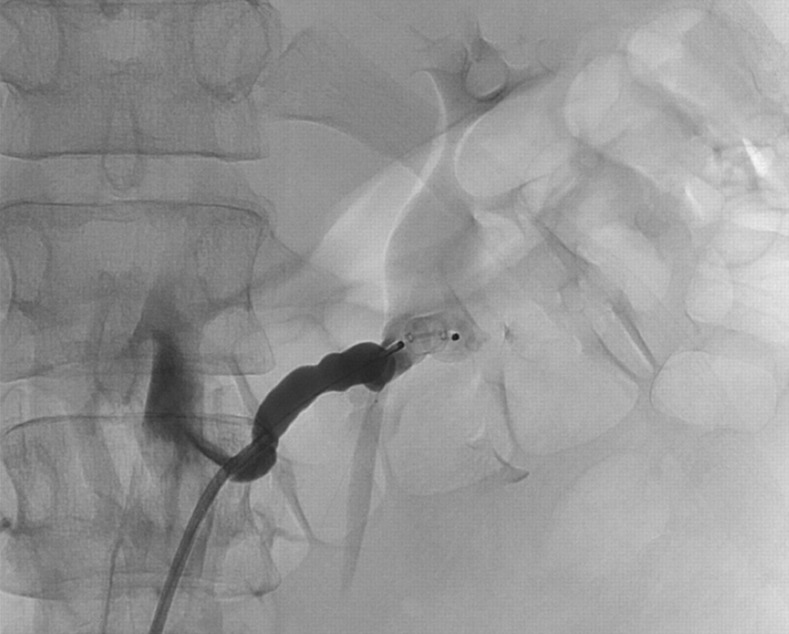
After initial stabilization of the patient, she was then referred to our interventional radiology department for SAE. Using a right common femoral artery approach a diagnostic flush aortogram was performed to exclude extrarenal feeders to the tumor. A selective catheterization of the upper and lower pole left renal artery revealed that the upper renal artery was exclusively supplying the renal parenchyma not affected by the AML with no significant feeding of the tumor (Fig. 3) whereas the lower renal artery solely supplied the giant AML (Fig. 4). The diameter of the lower left artery was 6.5 mm. Embolization of the tumor-feeding lower left renal artery was performed with an 8-mm Amplatzer Vascular Plug (AVP; AGA Medical, Golden Valley, MN, USA). The AVP was deployed through a long 6-F envoy-guiding catheter (Codman & Shurtleff, Raynham, MA, USA) with 0.070” ID (1.8 mm). An instant and complete occlusion of the lower left renal artery was achieved (Fig. 5).
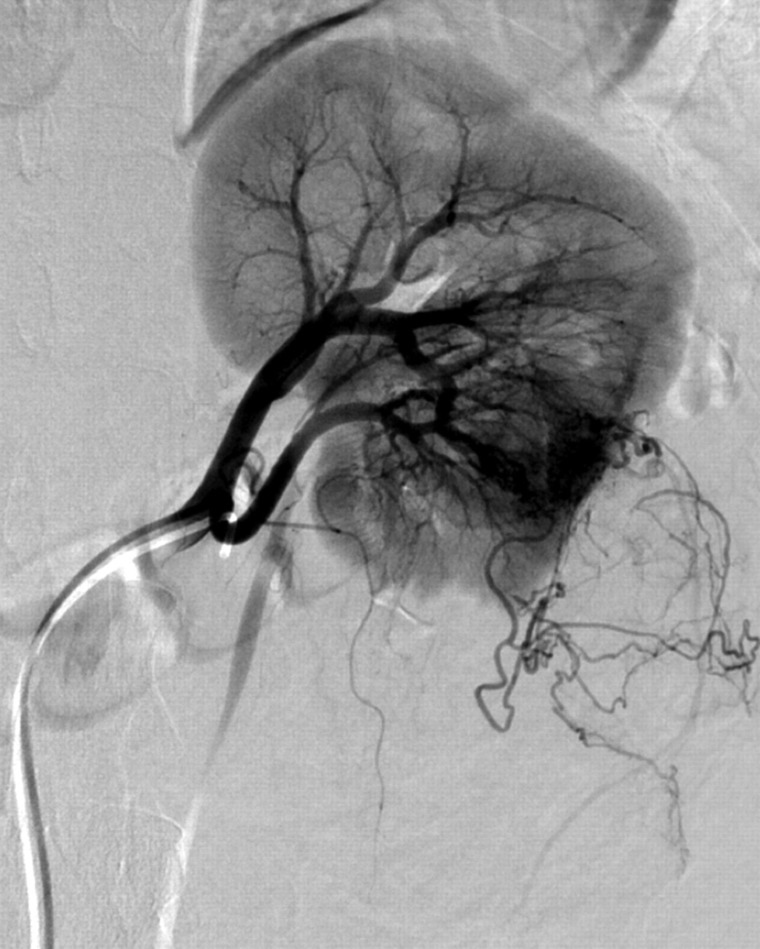
Fig. 3.
Selective angiogram of the left upper renal artery supplying approximately two-thirds of the regular renal parenchyma. There are no significant feeders to the angiomyolipoma
Fig. 4.
Selective angiogram of the left lower renal artery which is exclusively supplying the angiomyolipoma tumor mass
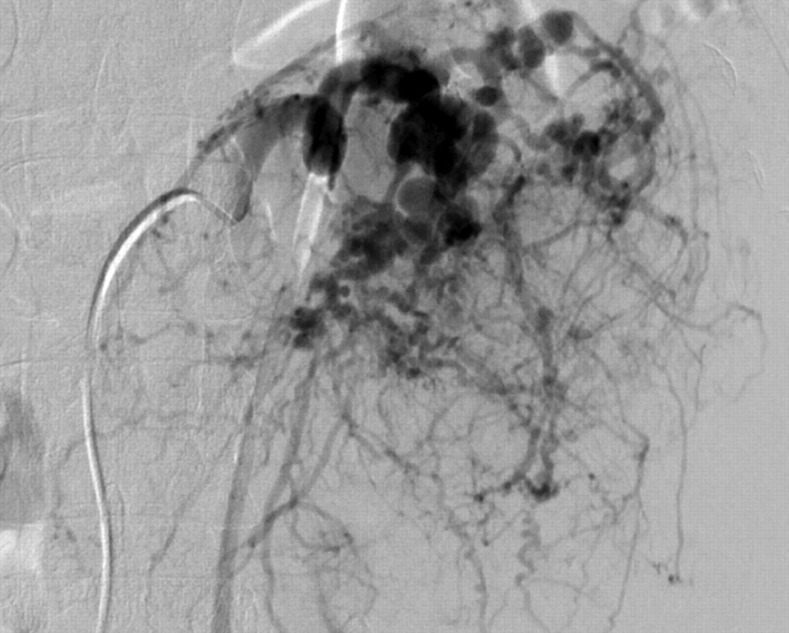
Fig. 5.
Implantation of an Amplatzer Vascular Plug Type II in the left lower renal artery. There is an abrupt and complete occlusion of the AML supplying vessel
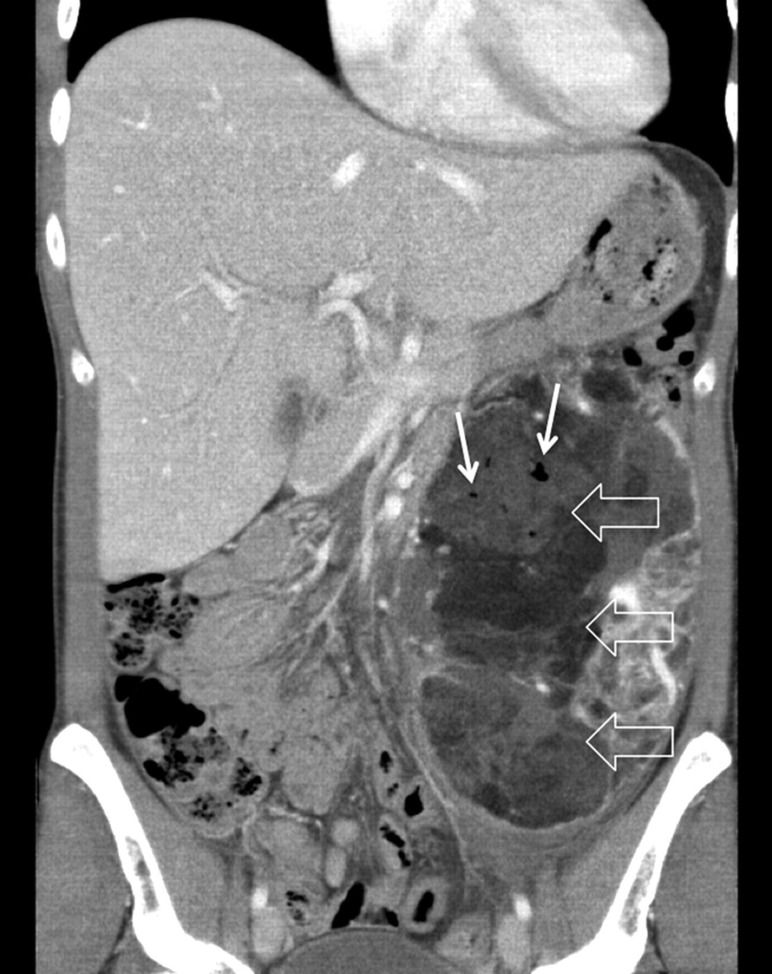
Immediately after embolization the patient complained of left-sided abdominal pain, which was treated with a single dose of 50 mg pethidine i.v. As a consequence of tumor devascularization the patient developed post-embolization syndrome characterized by acute pain, malaise, nausea, severe night sweats, and temperatures of up to 39°C 10 days following the procedure. A follow-up CT scan showed necrosis of AML with signs of abscess formation (Fig. 6) 14 days post embolization. A nephron-sparing surgical resection of the residual AML was performed, preserving the healthy upper pole of the left kidney, which was supplied by the separate upper renal artery. The patient was discharged from hospital 4 days later.
Fig. 6.
Coronal view of the CT demonstrates an extended necrosis (large white arrows) of the angiomyolipoma tumor mass 10 days after the selective arterial embolization. The air bubbles are indicative for an abscess formation (small white arrows)
Discussion
Predictive factors for bleeding complications in patients with renal AML are tumor size (10), presence of symptoms (11), and presence of tuberous sclerosis (4). Different embolization techniques for the treatment of AML have been described. The ultimate goal of every SAE is to achieve complete tumor devascularization and to preserve healthy renal parenchyma. Ramon et al. utilized a mixture of 20 mL ethanol and 1 mL (one bottle) of 45–150 µm PVA particles for SAE (10). Lee et al. describe a superselective approach using a coaxial microcatheter: First, the targeted tumor vessel was tapped with microcoils (12). Then, gelfoam cubes were intermittently injected followed by further microcoils to form a “gelfoam sandwich” (13). In our case, only one tumor-feeding artery – the lower left renal artery – was identified. In order to achieve an effective inflow occlusion of the renal artery we chose an AVP type II occluder. AVP occluders can be considered as a special type of interlocking detachable coil. They are Nitinol-based devices, which are pre-shaped for specific indications, such as the primary occlusion of atrial septal defects, patent foramen ovale, patent arterial ducts, or specific closure of ventricular septal defects (14). The flexible self-expanding cylindrical Nitinol device and the multitude of different designs and sizes of AVP occluders enable an application of these devices in the treatment of additional conditions.
Recent studies and case reports have demonstrated that AVP occluders can be used for therapeutic embolization of various vascular lesions. There is a large body of literature on the application of the same AVP type II used in our case for various conditions: the occlusion of a spontaneous mesocaval shunt (15), to treat a type II endoleak after endovascular thoraco-abdominal aortic aneurysm repair (16, 17), and to occlude an arteriovenous hemodialysis access (18). Generally, the AVP should be oversized by approximately 20% of the vessel diameter. In our case, the tumor-supplying feeder had a relatively large vessel diameter of 6.5 mm. Therefore we chose an 8-mm AVP II. In contrast to regular endovascular coiling, the embolization effect is immediate and complete instantaneously after coil detachment, clinically apparent in our case by the onset of left flank pain shortly after SAE caused by the devascularization of the AML. Tumor shrinkage over time without surgical resection of the residual tumor debris seemed unlikely, due to its size. Ten days post embolization, the residual tumor was removed surgically. According to recent literature, this approach represents the state-of-the-art treatment (12). By now AVP type 4 is available which allows delivery through a standard 0.038 diagnostic catheter (e.g. 4 or 5 F catheters) and therefore eliminates the need for catheter exchange. This facilitates the embolization procedere further and also allows embolization of smaller vessels. AVP type 4 is available up to a maximum diameter of 8 mm.
Renal AML is a fairly common incidental finding in the kidney. Due to the tumor's adipose components, CT diagnoses are very accurate. Renal AML containing calcifications are very rare (19). Patients with asymptomatic lesions require no treatment. Some AML may progress rapidly and grow up to 4 cm, annually (20). Renal AML associated with tuberous sclerosis grow fast and have an increased risk of spontaneous hemorrhage (4, 21). In our case, we could not find an association with tuberous sclerosis. Gaikwad et al. have described a case of lethal spontaneous hemorrhage of a giant renal AML with a maximum diameter of 15 cm and a thrombosed pseudoaneurysm and an extensive perinephric hematoma (22). Another case report illustrates the spontaneous rupture of a renal AML in a pregnant woman ensuing in hemorrhagic shock (23). The patient underwent emergency nephrectomy; the postoperative follow-up was uneventful. Occasionally, the first clinical manifestations of renal AML can present as Wunderlich's syndrome, which is defined as a spontaneous non-traumatic renal hemorrhage confined to the subcapsular and surrounding perirenal adipose tissue (24).
In conclusion, the spontaneous or traumatic hemorrhage of a renal AML is a potentially life-threatening condition. In our case, the application of an AVP Type II was proven to be effective for SAE of the main and sole feeder to a giant renal AML located in the lower pole of the left kidney. The AVP Type II significantly reduced the time to vessel occlusion, without the need for additional coils and represents an alternative to an emergency nephrectomy.
Footnotes
Conflict of interest:None.
REFERENCES
- 1. Schieven LW, Smedts F, Hopman AH, et al. Fine needle aspiration using improved agar microbiopsy is highly concordant with renal mass final diagnosis and subclassification. J Urol 2009;182:2590–3 [DOI] [PubMed] [Google Scholar]
- 2. Faraji H, Nguyen BN, Mai KT Renal epithelioid angiomyolipoma: a study of six cases and a meta-analytic study. Development of criteria for screening the entity with prognostic significance. Histopathology 2009;55:525–34 [DOI] [PubMed] [Google Scholar]
- 3. Zhang JQ, Fielding JR, Zou KH Etiology of spontaneous perirenal hemorrhage: a meta-analysis. J Urol 2002;167:1593–6 [DOI] [PubMed] [Google Scholar]
- 4. Nelson CP, Sanda MG Contemporary diagnosis and management of renal angiomyolipoma. J Urol 2002;168:1315–25 [DOI] [PubMed] [Google Scholar]
- 5. Yamakado K, Tanaka N, Nakagawa T, et al. Renal angiomyolipoma: relationships between tumorsize, aneurysm formation, and rupture. Radiology 2002;225:78–82 [DOI] [PubMed] [Google Scholar]
- 6. Casper KA, Donnelly LF, Chen B, et al. Tuberous sclerosis complex: renal imaging findings. Radiology 2002;225:451–6 [DOI] [PubMed] [Google Scholar]
- 7. Rakowski SK, Winterkorn EB, Paul E, et al. Renal manifestations of tuberous sclerosis complex: Incidence, prognosis, and predictive factors. Kidney Int 2002;70:1777–82 [DOI] [PubMed] [Google Scholar]
- 8. De Luca S, Terrone C, Rossetti SR Management of renal angiomyolipoma: a report of 53 cases. BJU Int 1999;83:215–8 [DOI] [PubMed] [Google Scholar]
- 9. Rimon U, Duvdevani M, Garniek A, et al. Large renal angiomyolipomas: digital subtraction angiographic grading and presentation with bleeding. Clin Radiol 2006;61:520–6 [DOI] [PubMed] [Google Scholar]
- 10. Ramon J, Rimon U, Garniek A, et al. Renal angiomyolipoma: long-term results following selective arterial embolization. Eur Urol 2009;55:1155–61 [DOI] [PubMed] [Google Scholar]
- 11. Unlü C, Lamme B, Nass P, et al. Retroperitoneal haemorrhage caused by a renal angiomyolipoma. Emerg Med J 2006;23:464–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee SY, Hsu HH, Chen YC, et al. Embolization of renal angiomyolipomas: short-term and long-term outcomes, complications, and tumorshrinkage. Cardiovasc Intervent Radiol 2009;32: 1171–8 [DOI] [PubMed] [Google Scholar]
- 13. Kaufmann JA Vascular Interventions. : Vascular and Interventional Radiology. Philadelphia, PA: SAGE Publications, 2004:83 [Google Scholar]
- 14. de Giovanni JV The use of Amplatzer devices to occlude vascular fistulae. J Interv Cardiol 2001;14:45–8 [DOI] [PubMed] [Google Scholar]
- 15. Boixadera H, Tomasello A, Quiroga S, et al. Successful Embolization of a Spontaneous Mesocaval Shunt Using the Amplatzer Vascular Plug II. Cardiovasc Intervent Radiol 2010;33:1044–8 [DOI] [PubMed] [Google Scholar]
- 16. Meyer C, Probst C, Strunk H, et al. Second-generation Amplatzer Vascular Plug (AVP) for the treatment of subsequent subclavian backflow type II endoleak after TEVAR. Cardiovasc Intervent Radiol 2009;32:1264–7 [DOI] [PubMed] [Google Scholar]
- 17. Grenon SM, Gagnon J, Hsiang Y, et al. Occlusion of the common and internal iliac arteries for aortoiliac aneurysm repair: experience with the Amplatzer vascular plug. Can J Surg 2009;52:e276–80 [PMC free article] [PubMed] [Google Scholar]
- 18. Powell S, Narlawar R, Odetoyinbo T, et al. Early Experience with the Amplatzer Vascular Plug II for Occlusive Purposes in Arteriovenous Hemodialysis Access. Cardiovasc Intervent Radiol 2010;33: 150–6 [DOI] [PubMed] [Google Scholar]
- 19. Merran S, Vieillefond A, Peyromaure M, et al. Renal angiomyolipoma with calcification: CT-pathology correlation. Br J Radiol 2004;77: 782–3 [DOI] [PubMed] [Google Scholar]
- 20. Steiner MS, Goldman SM, Fishman EK, et al. The natural history of renal angiomyolipoma. J Urol 1993;150:1782–86 [DOI] [PubMed] [Google Scholar]
- 21. Künzi T, Walther F, Marti HP, et al. Intrarenal arterial aneurysms with haematuria in a patient with tuberous sclerosis complex. Nephrol Dial Transplant 2005;20:2268–70 [DOI] [PubMed] [Google Scholar]
- 22. Gaikwad AB, Madathil MB, Kothari AS Giant renal angiomyolipoma with fatal hemorrhage due to a large pseudoaneurysm. J Clin Ultrasound 2008;36:174–6 [DOI] [PubMed] [Google Scholar]
- 23. Wang HB, Yeh CL, Hsu KF Spontaneous rupture renal angiomyolipoma with hemorrhagic shock. Intern Med 2009;48:1111–2 [DOI] [PubMed] [Google Scholar]
- 24. Albi G, del Campo L, Tagarro D Wünderlich's syndrome: causes, diagnosis and radiological management. Clin Radiol 2002;57:840–5 [PubMed] [Google Scholar]