Abstract
We report a case of multiple benign metastasizing leiomyoma (BML) lung nodules showing faint or non-avid uptake of F-18 fluorodeoxyglucose (FDG) (respective 1-hour early and 2-hour delayed maximum standardized uptake values; 1.3 or less and 1.2 or less) in a 50-year-old woman with a history of hysterectomy for uterine leiomyoma at the age of 38 years. When multiple lung nodules show faint or non-avid FDG uptake in a patient with a history of hysterectomy for uterine leiomyoma, BML should be included in the differential diagnosis.
Keywords: FDG, uterus, benign metastasizing leiomyoma, lung, nodule
Benign metastasizing leiomyoma (BML) is a rare disease occurring predominantly in women of reproductive age and usually develops several years after hysterectomy or myomectomy for fibroids (1, 2). It is characterized by benign, slow-growing, smooth muscle tumors outside the uterus. The lungs are the most common site of metastasis (1, 2). The F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) or PET/computed tomography (CT) findings of lung BMLs have been previously reported in three cases (3, 4). We report the FDG-PET/CT findings of multiple BMLs in a case. To our knowledge, this is the fourth case of lung BML in which FDG-PET findings are described in English literature and the first case in which dual time-point imaging was performed.
Case report
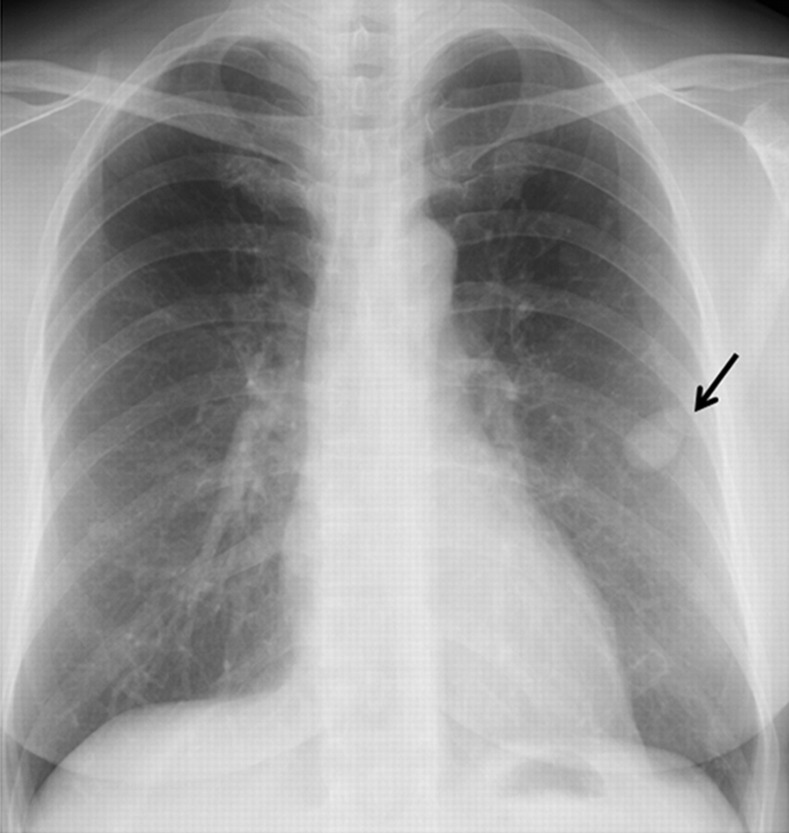
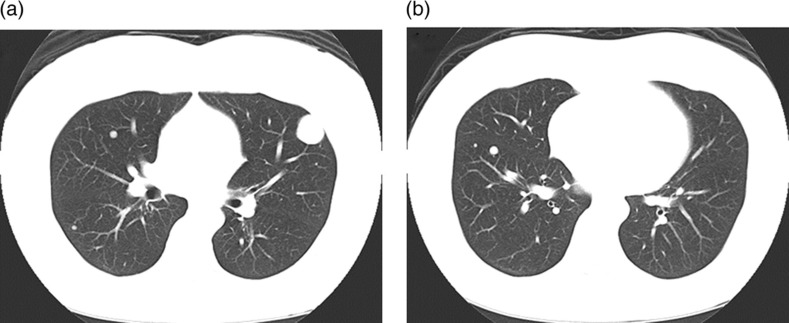
A 50-year-old woman with a history of hysterectomy for uterine leiomyoma 12 years ago was examined for multiple small lung nodules, including the largest one (30 × 25 mm in size) which were incidentally discovered on chest radiographs at a private hospital (Fig. 1). Unenhanced CT images showed multiple peripheral parenchymal nodules of variable sizes with smooth margins (Fig. 2).
Fig. 1.
Frontal chest radiograph demonstrates multiple lung nodules. The largest lung nodule (arrow) is noted in the left lung
Fig. 2.
CT images show multiple well-demarcated round lung nodules of various sizes
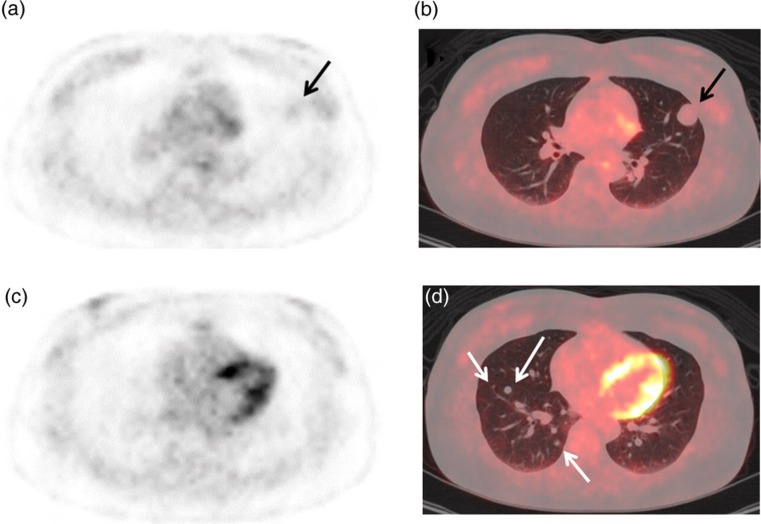
FDG-PET was performed using a PET/CT system (GE Discovery STE, GE Healthcare, Milwaukee, WI, USA) to identify primary malignancy and metastatic lesions. A whole-body imaging from the head to the thigh and chest imaging were performed 1 hour (early) and 2 hours (delayed), respectively, after intravenous injection of FDG (3.7 MBq/kg body weight). At PET/CT, her blood glucose level was 93 mg/dl. Faint FDG uptake was noted in the largest nodule (Fig. 3a and b), and its early and delayed maximum standardized uptake values (SUVmaxs) were 1.3 and 1.2, respectively. In this connection, early and delayed SUVmaxs were 0.5 and 0.5 in the lung field, 1.5 and 1.4 in the thoracic muscle, and 2.4 and 1.6 in the mediastinum at the Fig. 3a tomographic level, respectively. The remaining smaller nodules ≤ 8 × 8 mm in diameter showed no visible uptake (early SUVmax 0.5–0.8; delayed SUVmax 0.4–0.6) (Fig. 3c and d). There was no other pathologic FDG uptake to suspect primary malignancy and metastatic lesions in the whole body.
Fig. 3.
Early 1 hour FDG-PET emission (a, c) and fused PET/CT (b, d) images show no visible FDG uptake in the multiple small lung nodules (white arrows, early SUVmax 0.5–0.8, delayed SUVmax 0.4–0.6) except the largest nodule which has faint FDG uptake (black arrows, early SUVmax 1.3, delayed SUVmax 1.2)
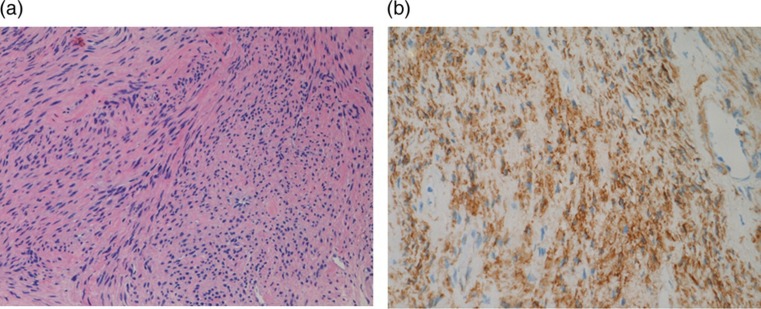
CT-guided needle lung biopsy was performed for the largest nodule to obtain a pathological diagnosis of the lung nodules. Microscopic examinations showed an interlacing pattern by spindle cells having elongated nuclei without cellular atypia (Fig. 4a). The Ki67 proliferative index was less than 1%. Tumor cells were positive for alpha-smooth muscle actin (Fig. 4b) and both estrogen and progesterone receptors and negative for CD31, CD34, AE1/AE3, EMA, HMB45, and S-100 protein on immunohistochemical stains. The pathological findings of the tumor confirmed the diagnosis of benign metastasizing leiomyoma. The patient selected careful observation as a management and the chest radiographs showed no change to date, 1 year after the biopsy.
Fig. 4.
(a) Microscopic examination of the lesion (HE stain, ×20) showed interlacing bundles of spindle cells with elongated nuclei. (b) These cells are positive for alpha-smooth muscle actin on immunohistochemical stain (×20)
Discussion
BML is a rare condition where benign smooth muscle tumors with similar histological appearance to uterine leiomyoma are present at distant sites, and it usually occurs after hysterectomy for uterine leiomyoma (1, 2). Metastases most often affect the lungs, and less other sites (2, 5). Lung BML lesions usually produce no symptoms, and are often discovered incidentally on chest imaging performed for other indications (1). Typical imaging findings include multiple, well-circumscribed, bilateral pulmonary nodules from several millimeters to centimeters in size (1, 2). Management options include careful observation, resection, and hormonal therapy (6).
FDG-PET is based on the differential uptake of FDG by metabolizing cells and is transported into cells on the basis of their rate of glycolysis. Because of increased avidity of neoplastic cells for glucose, FDG accumulates at higher rates in tumor cells than in non-neoplastic cells. PET scans can visualize active neoplastic lesions as areas of focal hypermetabolism (7). Therefore, FDG-PET is a sensitive modality for the diagnosis and staging of several types of malignancies (8). FDG-PET is also used for evaluation of pulmonary malignancy, and the sensitivity and specificity of FDG-PET for detection of malignant nodules are about 90% and 78%, respectively (9).
FDG-PET findings of lung BLM have been reported in one case (3) and two cases (4). These multiple nodules exhibited faint or non-avid uptake like ours. Although the early nodule SUVmax was unknown in the first report (3), it ranged between 0.2 and 2.2 (nodule size 4 × 4 mm to 30 × 48 mm) in one case and between 0.7 and 1.5 (nodule size 7 × 7 mm to 14 × 15 mm) in another case in the second report (4). In our case, early nodule SUVmax ranged between 0.5 and 1.3 (nodule size ≤8 × 8 mm to 30 × 25 mm). The largest metastatic nodule showed the highest SUVmax in the three cases, respectively. Thus, the FDG visibility may depend on not only metabolic activity, but also tumor size of BML. An accepted practice in the evaluation of pulmonary nodules is that those which demonstrate a maximum SUV of greater than 2.5 are suspicious for malignancy in the appropriate clinical setting (10). The early SUVmaxs of these multiple BML lung nodules ranged between 0.2 and 2.2, which were in the above mentioned SUVmax range of benign lung tumors.
The previous two reports did not mention the FDG delayed imaging findings and the change of SUVmax from early to delayed imaging of their BMLs. Although limitations of dual time-point PET in the assessment of lung nodules with low FDG avidity has been reported (10), in our case, the SUVmax did not increase during dual time-point imaging. FDG faint or non-avid uptake in multiple lung nodules was reported in bronchioloalveolar cell carcinomas, carcinoid tumors, and metastases from various types of extra-pulmonary malignancy including uterine leiomyosarcoma and may be related to both tumor low metabolic activity and limited PET resolution for small tumors less than 1 cm in diameter (11–13). The FDG uptake in BMLs may be also related to both of the low metabolic activity and tumor size.
In conclusion, when multiple hypometabolic pulmonary nodules are noted in a patient with a history of hysterectomy for uterine leiomyoma on FDG-PET or PET/CT, BML should be included in the differential diagnosis.
Footnotes
Conflict of interest:None.
REFERENCES
- 1. Lee AY, Poder L, Qayyum A, et al. Imaging malignant and apparent malignant transformation of benign gyneaecological disease. Clin Radiol 2010;65:1031–7 [DOI] [PubMed] [Google Scholar]
- 2. Fasih N, Prasad Shanbhogue AK, Macdonald DB, et al. Leiomyomas beyond the uterus: unusual locations, rare manifestations. Radiographics 2008;28:1931–48 [DOI] [PubMed] [Google Scholar]
- 3. di Scioscio V, Feraco P, Miglio L, et al. Benign metastasizing leiomyoma of the lung: PET findings. J Thorac Imaging 2009;24:41–4 [DOI] [PubMed] [Google Scholar]
- 4. Lin X, Fan W, Lang P, et al. Benign metastasizing leiomyoma identified using 18F-FDG PET/CT. Int J Gynaecol Obstet 2010;110:154–6 [DOI] [PubMed] [Google Scholar]
- 5. Jo JH, Lee JH, Kim DC, et al. A case of benign metastasizing leiomyoma with multiple metastasis to the soft tissue, skeletal muscle, lung and breast. Korean J Intern Med 2006;21:199–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoetzenecker K, Ankersmit HJ, Aigner C, et al. Consequences of a wait-and-see strategy for benign metastasizing leiomyomatosis of the lung. Ann Thorac Surg 2009;87:613–4 [DOI] [PubMed] [Google Scholar]
- 7. Nabi HA, Zubeldia JM Clinical application of 18F-FDG in oncology. J Nucl Med Technol 2002;30:3–9 [PubMed] [Google Scholar]
- 8. Rigo P, Paulus P, Kaschten BJ, et al. Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. Eur J Nucl Med 1996;23:1641–74 [DOI] [PubMed] [Google Scholar]
- 9. Hellwig D, Baum RP, Kirsch C FDG-PET, PET/CT and conventional nuclear medicine procedures in the evaluation of lung cancer: a systematic review. Nuklearmedizin 2009;48:59–69 [PubMed] [Google Scholar]
- 10. Cloran FJ, Banks KP, Song WS, et al. Limitations of dual time point PET in the assessment of lung nodules with low FDG avidity. Lung Cancer 2010;68:66–71 [DOI] [PubMed] [Google Scholar]
- 11. Chang JM, Lee HJ, Goo JM, et al. False positive and false negative FDG-PET scans in various thoracic disease. Korean J Radiol 2006;7:57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fortes DL, Allen MS, Lowe VJ, et al. The sensitivity of 18F-fluorodeoxyglucose positron emission tomography in the evaluation of metastatic pulmonary nodules. Eur J Cardiothorac Surg 2008;34:1223–7 [DOI] [PubMed] [Google Scholar]
- 13. Kao YH, Saad U, Tan AE, et al. Fluorine-18-fluorodeoxyglucose PET/CT for the evaluation of suspected recurrent uterine leiomyosarcomas. Acta Radiol 2011;52:463–6 [DOI] [PubMed] [Google Scholar]