Abstract
Crouzon syndrome with acanthosis nigricans (CAN) is a very rare condition with an approximate prevalence of 1 per 1 million newborns. We add the first report on prenatal 2D and 3D ultrasound findings in CAN. In addition we present the postnatal 3D CT findings. The diagnosis was confirmed by molecular testing.
Keywords: Crouzon syndrome with acanthosis nigricans, Crouzon syndrome, craniosynostosis
Crouzon syndrome with acanthosis nigricans (CAN) is caused by a specific Ala391Glu mutation in the FGFR3 gene on chromosome 4. The entity was initially described by Meyer et al. in 1995 (1) and is quite rare with an estimated prevalence of 1 per 1 million newborns (2).
To date only 35 cases have been documented in the literature (3). We here describe the first prenatal 2D and 3D ultrasound and postnatal 3D CT findings in a sporadic case of CAN.
Case report
A healthy 31-year-old woman had infertility treatment with ovulation induction and intrauterine homolog insemination at a private fertility clinic. In early pregnancy she experienced acute abdominal pain. At laparoscopy a ruptured tubal pregnancy was found, indicating a heterotopic pregnancy, and a salpingectomy was performed.
She had a first trimester scan which produced a combined risk for Down syndrome of 1:507. She chose to have a chorionic villus sample (CVS) taken, which showed a normal karyotype. At 19 + 6 weeks she had a normal anomaly scan. At 27 + 3 weeks she was referred to the fetal medicine unit (FMU) for a growth scan. This ultrasound showed intrauterine growth restriction (estimated fetal weight 24.8% compared to the normative mean value) and a small head circumference (227.8 mm). The anomaly scan was repeated and found to be normal. Fetal biometry was performed every second week showing normal growth, although the head circumference remained below the 5th centile.
At 35 + 5 weeks the woman was referred for an expert scan at the FMU because of an abnormally-shaped cranium (Fig. 1). 2D and 3D scans were performed showing brachycephaly, hypertelorism, ocular proptosis, and a beaked nose (Fig. 2). No hand or foot anomalies were seen. These findings gave rise to the suspicion of Crouzon syndrome. The fetal DNA saved from the CVS was analyzed for mutations in the FGFR2 gene (Crouzon, Apert, and Pfeiffer syndrome) and reflex tested for Muenke syndrome in the FGFR3 gene. No mutations were found.
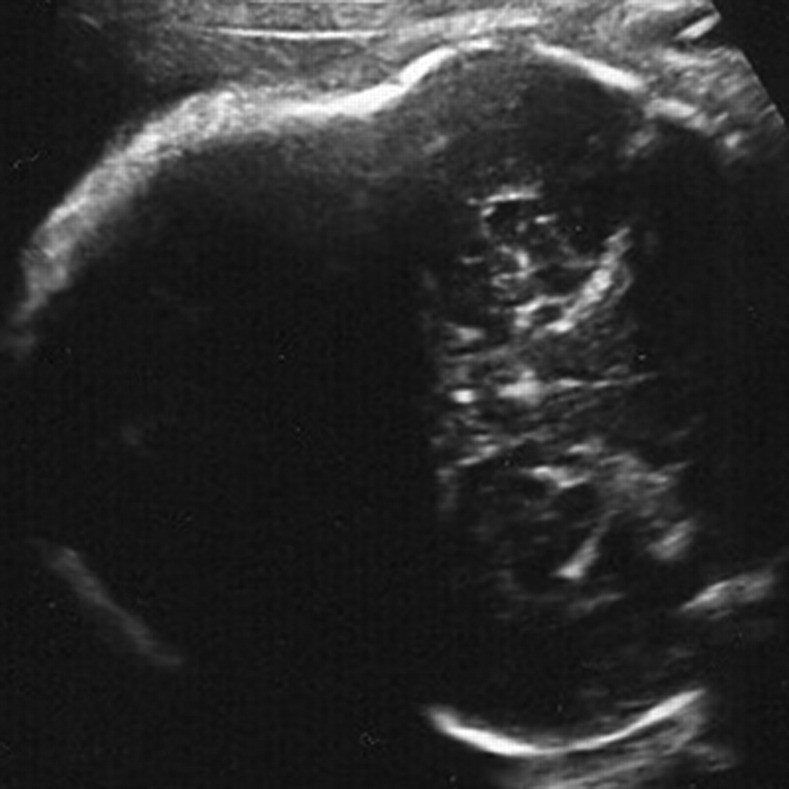
Fig. 1.
2D UL of the cranium, showing clowerleaf shaped cranium
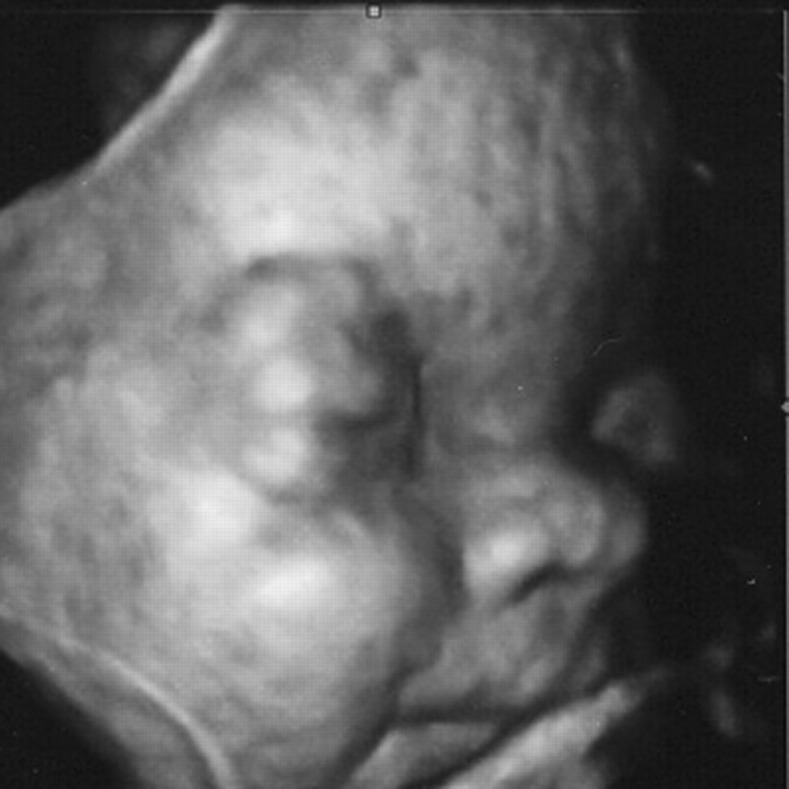
Fig. 2.
3D UL of the face, showing ocular proptosis and beaked nose
At 39 + 6 weeks a Cesarean section was performed on maternal request and a boy was delivered (weight, 3.156 g; length, 51 cm; Apgar, 10/1 and 10/5; and normal cord blood gas values). Craniofacial abnormalities were evident, but choanal atresia was not suspected. At 7 days of age he developed intermittent obstructed nasal airways with compromised breastfeeding. Acceptable weight gain was obtained after introduction of formula milk. The boy was referred to the Copenhagen Craniofacial Unit at Rigshospitalet, University of Copenhagen.
At clinical examination at 5 weeks of age, he had pronounced oxycephaly (Kleeblatt-Schädel) and ocular proptosis. In addition he had pronounced maxillary hypoplasia and severe breathing difficulties with clinical suspicion of apnea. No skin changes such as hyperkeratotic papules or pigmentations were noted. He gradually developed hydrocephalus and at age 9 weeks a 3D craniofacial CT scan was done. The CT scan was performed using a Toshiba Aquillion helical CT-scanner (Toshiba, Zoetermeer, The Netherlands) (slice thickness, 0.5 mm; pixel spacing, 0.39/0.39; KVp, 120; X-ray tube current, 60mA; exposure, 24 mAs).
The 3D CT scan (Figs. 3 and 4) revealed a brachycephalic skull shape (the cephalic index, which describes the relationship between the long and short axis of the cranium was 85, normal values are 75–83) with protrusion of the forehead and flattened occiput. The skull was wide and the temporal regions were bulging. Both coronal and lambdoid sutures were prematurely fused. A wide midline defect of the skull extended from the nasal root to the occiput. The skull was paper thin with extremely increased digital markings, especially in the posterior portion of the skull. Markedly increased digital markings on CT may suggest that intracranial pressure (ICP) is increased or that ICP has been increased at an earlier stage. The synchondroses of the cranial base were patent and the cranial base angle was flat (platybasia). The nasal root was depressed; the orbital roofs were short and the distance between the orbits was increased (hypertelorism); the maxilla was hypoplastic, especially in the sagittal and vertical dimensions. Choanal height was decreased and the nasopharyngeal airway was constricted. The mandible was unremarkable.
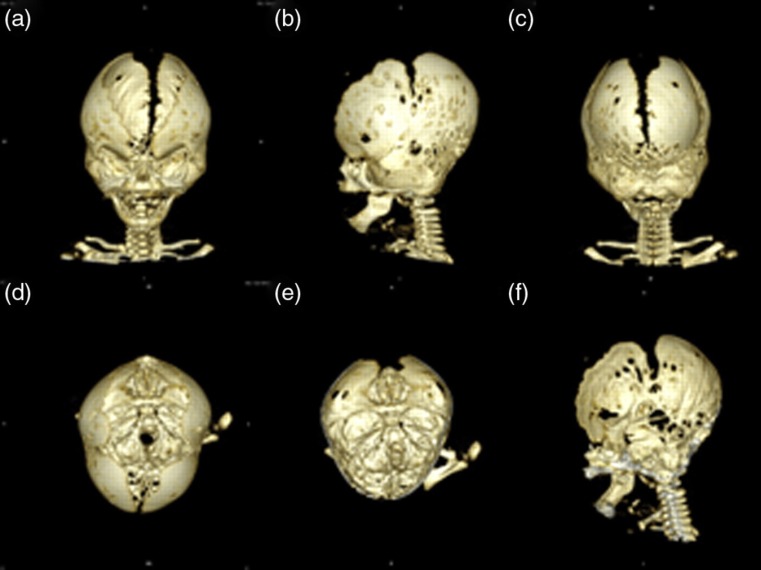
Fig. 3.
3D reconstruction of the craniofacial skeleton based on the CT scanning performed at 9 weeks of age. (a) Frontal view. Note the wide midline defect and the bulging temporal regions. (b) Left, lateral view. Note the fusion of the coronal suture. (c) Occipital view. Note the fusion of the lambdoid sutures. (d) Top view. Note the wide midline defect. (e) Top view of the internal cranial base and occiput. Note the increased digital markings in the occipital region. Also note the patent synchondroses of the cranial base. (f) Mid-sagittal cut through the skull. Note the flattened cranial base angle (platybasia) secondary to increased intracranial pressure
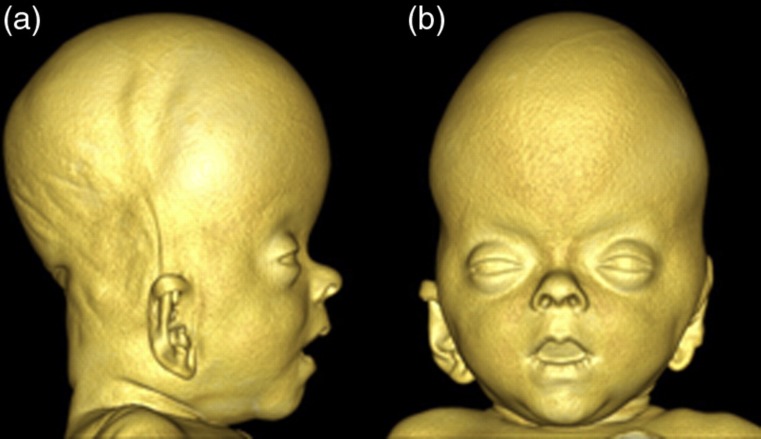
Fig. 4.
3D reconstruction of the craniofacial soft-tissues based on the CT scanning performed at 9 weeks of age. (a) Profile view of the head. Note the extreme head shape with decreased length and increased height. Also note the protrusion of the forehead and the depressed nasal bridge. (b) Frontal view of the head. Note the bulging temporal regions and the hypertelorism
Since the child developed signs of increased intracranial pressure a ventriculoperitoneal shunt was placed.
Due to the patient's physical findings consistent with the diagnosis of Crouzon syndrome, a full mutation screening for the more uncommon Crouzon mutations in the FGFR2 and FGFR3 gene was performed. It identified the heterozygous c.1172C > A; p.Ala391Glu mutation in the FGFR3 gene, which is responsible for Crouzon syndrome with acanthosis nigricans.
Further treatment involves multidisciplinary control and coordination by the Copenhagen Craniofacial Unit. Midface distraction will be performed at 7–10 years of age.
Discussion
Crouzon syndrome with acanthosis nigricans was initially described by Meyer et al. in 1995 (1), and is quite rare with an estimated prevalence of 1 per 1 million newborns (2). The majority of cases are sporadic, but familial cases have been reported in the literature in a pattern, consistent with an autosomal dominant condition (1).
Most authors differentiate between Crouzon syndrome associated with a FGFR2 mutation by referring to it as classic Crouzon syndrome, and the condition associated with a FGFR3 mutation by referring to it as Crouzon syndrome with acanthosis nigricans (CAN) (4). Cohen (5) has argued that CAN is independent of Crouzon syndrome because of the differences in the phenotype and because of the highly specific mutation in the FGFR3 gene. He has proposed, that the condition should be named Crouzonodermoskeletal syndrome.
Arnaud-Lopez et al. have described the phenotype of CAN based on two new cases and 33 cases reported in the literature (3). In addition to the craniofacial anomalies seen in Crouzon syndrome, they found that all the individuals developed acanthosis nigricans within the first decade. Acanthosis nigricans is a skin disorder with velvety hyperpigmentated skin. It is usually seen in body folds as neck, axillae, eyelids, perioral, inguinal, and perianal regions. The authors found, that hyperpigmentations were more widespread in CAN than in cases with acanthosis nigricans alone. In addition, they found melanocytic nevi in 25% of the cases, choanal atresia or stenosis in 41%, hydrocephalus in 43%, Chiari malformations in 23%, oral abnormalities in 34%, and vertebral abnormalities in 20% of the cases. Mental retardation was found in only two (6%) of the 35 cases. Hearing loss was less frequent than in individuals with classic Crouzon syndrome and speech delay was found in only three (9%) of the 35 cases.
Because the p.Ala391Glu mutation in the FGFR3 gene is allelic to mutations seen in achondroplasia, Schweitzer et al. (6) performed whole body skeletal radiographs in six patients with Crouzon syndrome with acanthosis nigricans to identify possible skeletal findings. They found changes such as narrow sacrosciatic notches, short vertebral bodies (anterior-posterior direction), and broad, short metacarpals and phalanges in all six cases; these changes are also seen in individuals with achondroplasia. Images of the skeletal findings are all shown in the reference. They suggested that CAN should be suspected in an individual with the phenotype of Crouzon syndrome with choanal atresia and hydrocephalus and subtle skeletal findings even before acanthosis nigricans appear.
In the present case the newborn showed severe craniofacial dysmorphology, but there were no immediate signs of choanal atresia or hydrocephalus. However, CT scanning revealed reduced choanal height and constriction of the nasopharyngeal airway. In addition hydrocephalus developed gradually within a few weeks of birth and a shunt operation was successfully performed. Up till now the patient has shown no signs of skin involvement and no skeletal findings have been seen.
3D CT imaging has proved helpful in determining the extent of the craniosynostosis, facilitating treatment planning (7). In addition, in the present case the CT scan also revealed important information on hydrocephalus, increased digital markings (increased intracranial pressure), flattening of the cranial base, and constriction of the nasopharyngeal airway.
In conclusion, Crouzon syndrome may be suspected prenatally on the basis of 2D and 3D ultrasound findings. The diagnosis may be confirmed by molecular testing. If a FGFR2 mutation is not found, a p.Ala391Glu FGFR3 mutation should be suspected and tested for. A p.Ala391Glu FGFR3 mutation gives rise to CAN which share craniofacial characteristic with classic Crouzon syndrome. 3D CT imaging is a useful tool in determining the extent of the craniofacial anomalies and thereby in facilitating treatment planning.
References
- 1. Meyers GA, Orlow SJ, Munro IR, et al. Fibroblast growth factor receptor 3 (FGFR3) transmembrane mutation in Crouzon syndrome with acanthosis nigricans. Nature Genet 1995;11:462–4 [DOI] [PubMed] [Google Scholar]
- 2. Cohen MM Jr. Crouzon syndromes. : Cohen MM Jr, MacLean RE, Craniosynostosis: Diagnosis, Evaluation, and Management. 2nd edn. New York, NY: SAGE Publications; 2000:361–5 [Google Scholar]
- 3. Arnaud-Lopez L, Fragoso R, Mantilla-Capacho J, et al. Crouzon with acanthosis nigricans: further delineation of the syndrome. Clin Genet 2007;72:405–10 [DOI] [PubMed] [Google Scholar]
- 4. Nagase T, Nagase M, Hirose S, et al. Crouzon syndrome with acanthosis nigricans: case report and mutational analysis. Cleft Palate Craniofac J 2000;37:78–82 [DOI] [PubMed] [Google Scholar]
- 5. Cohen MM Jr. Let's call it ‘Crouzonodermoskeletal syndrome’ so we won't be prisoners of our own conventional terminology. Am J Med Genet 1999;84:74 [PubMed] [Google Scholar]
- 6. Schweitzer DN, Graham JM Jr, Lachman RS, et al. Subtle radiographic findings of achondroplasia in patients with Crouzon syndrome with acanthosis nigricans due to an ala391-to-glu substitution in FGFR3. Am J Med Genet 2001;98:75–91 [PubMed] [Google Scholar]
- 7. Lowe LH, Booth TN, Joglar JM, et al. Midface anomalies in children. Radiographics 2000;20:907–22 [DOI] [PubMed] [Google Scholar]