Abstract
Background
The standardized uptake value (SUV) is the most common estimate of metabolic activity used in clinical positron emission tomography (PET). Several biological and technological factors influence the accurate SUV calculation.
Purpose
To assess another potential source of variability of the SUV, the variations in urinary excretion of fluorodeoxyglucose (FDG).
Material and Methods
Twenty patients with various malignancies scheduled for PET/CT with 18F-FDG were included in the present study. The activity in urine voided immediately before image acquisition was measured and decay corrected. An estimation of FDG content in the urinary bladder was made during imaging, and the two components of urinary FDG were added. The urinary output of FDG, and the quantity of FDG divided by the time to measurements, was estimated.
Results
The excretion of FDG in urine was between 5.7% and 15.2% of injected dose (decay corrected), and from 0.06% to 0.3%/min after injection, a five-fold difference in clearance.
Conclusion
About 10% of injected dose is excreted in urine at 70 min post injection, but the urinary FDG excretion was found to be highly variable, yet another uncertainty affecting the SUV measurements.
Keywords: PET, tissue characterization, molecular imaging
There is a generalized sentiment that quantitative data have a higher scientific value than qualitative data, not only in nuclear medicine, and not only in medicine, but more than 150 years ago Lord Kelvin posted that “if you cannot measure it, you cannot improve it”. The difficulty in comparing data of the latter type is only partially responsible for this. The presentiment that functional imaging is best expressed as numbers is common within our field. In an editorial DiChiro and Brooks stated, with regards to our attitude towards standardized uptake values (SUV) rather than visual assessment: “How sweet is the siren song of mathematics that it can blind people to what is before their very eyes” (1).
Although visual assessment is the main tool for interpreting positron emission tomography (PET), the number of factors influencing the SUV is impressive (2). However, SUV remains an adjunct without which it is difficult to imagine PET. The use of SUV is now so universal it is difficult to find any publications not using this (or related values like lean body mass SUV). New PET/CT systems give us SUV with at least two decimals, and cut-off values are regularly used in recommendations and guidelines. Although we always keep reminding ourselves that SUV is but a semi-quantitative measure, it is the best we have, and it is used for all it is worth, and even more.
While exploring the urinary excretion of FDG, it became apparent that this was highly variable. The results give reason to believe that in patients with normal kidney function, and blood glucose levels within the accepted range for performing PET, the excretion of FDG during the hour between administration and imaging varies between 5% and 15%. This variation is yet another reason to be cautious about the exactitude of SUV. The urinary excretion is surely dependant on the hydration of the patients, but probably also on numerous other factors. This comes in addition to all the other factors that are described as influencing SUV (2). And of course, the FDG excreted through the kidneys do not enter the cells and do not participate in the intracellular accumulation of FDG.
Therefore, a study was initiated in order to document the potential source of inexactitude of the SUV value secondary to variations in the urinary excretion of FDG.
Material and Methods
The regional ethics committee was notified of the study, but considered this to be basically quality assurance and hence had no objections to the performance of the study. All patients gave their informed consent.
Patient population
Twenty male patients (median age, 60 years; body weight, 83.4 ± 15.2 kg) with various malignancies (45% gastrointestinal, 55% others) were included in the present study. Plasma glucose levels were measured and found to be adequate in all patients (mean, 5.5 ± 0.7 mmol/L; range, 4.5–6.9 mmol/L).
Procedure
PET imaging was performed with the radiolabeled glucose analogue 18-F-FDG, and a Siemens BioGraph 40 True point PET/CT scanner (Siemens, Erlangen, Germany) was used. Each patient underwent a PET/CT scan (4 min/bed position, starting caudally) according to the standard procedure. After fasting for more than 4 h, the patients were administered 356 ± 16 MBq of 18F-FDG by intravenous injection. After supine resting for 71 ± 23 min (range, 48–123 min), the patients were asked to void in a urinary bottle, immediately before image acquisition. The study was performed in men only. The reason for this was that any urinary contamination, as could be produced with women collecting urine in a basin or bedpan, could render the interpretation of the PET examination of the pelvis more difficult.
FDG measurements
The urine was collected in a urinary bottle, and the total volume and the time of voiding was recorded. A 10 mL syringe was filled with urine, and the radioactivity in the syringe was measured in a well counter (ISOMED2000; Dresden, Germany). The radioactivity was then decay corrected, and related to the injected dose.
The post-void residual urine and its FDG content was derived from attenuation corrected images, acquired approximately 10 min after voiding. An isocontour ROI at a fixed threshold (20% from the SUVmax) was drawn around the bladder. The SUVmax and the SUVmean of the voxels falling within the 80% peak-voxel-intensity isocontour were generated. Estimated bladder activity was calculated according to the following formula:
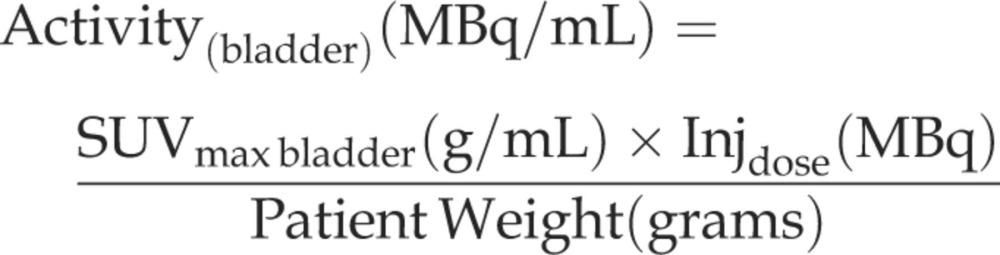 |
The measured activity multiplied with the ROI volume was used as total bladder FDG activity, and added to the FDG urine activity. The total urinary activity was measured and decay corrected to the time of injection, and the variation in urinary output of FDG was estimated.
The images were examined for paravenous injection. Finally, the time when the urinary sample was obtained was recorded, and correlated with the total urinary FDG, both the excreted FDG and the residual bladder FDG. Finally, all the activity measurements used in these estimations were decay corrected to the injection time.
Results
The excretion of FDG in urine was between 2% and 20% of the injected dose (ID) at a mean latency time of 71 min, with a mean of 9% ID (Table 1). The mean FDG activity in urine was 0.18 ± 0.11 MBq/mL (range, 0.03–0.46 MBq/mL) (Table 2).
Table 1.
Patient characteristics and urinary output of FDG
| Patient no. | Age | Urinary volume (mL) | Urine (MBq/mL) | Dose excreted (%) |
|---|---|---|---|---|
| 1 | 61 | 100 | 0.034 | 2.8 |
| 2 | 69 | 145 | 0.1236 | 11.0 |
| 3 | 64 | 235 | 0.1262 | 20.7 |
| 4 | 48 | 245 | 0.0239 | 12.8 |
| 5 | 21 | 125 | 0.2108 | 14.7 |
| 6 | 45 | 250 | 0.0729 | 12.7 |
| 7 | 58 | 290 | 0.0454 | 7.3 |
| 8 | 31 | 110 | 0.2151 | 11.0 |
| 9 | 47 | 510 | 0.0393 | 9.6 |
| 10 | 74 | 135 | 0.0836 | 5.0 |
| 11 | 68 | 420 | 0.0324 | 8.0 |
| 12 | 47 | 115 | 0.1808 | 11.0 |
| 13 | 67 | 185 | 0.0917 | 6.8 |
| 14 | 83 | 70 | 0.1715 | 5.8 |
| 15 | 26 | 295 | 0.0815 | 9.6 |
| 16 | 52 | 145 | 0.0749 | 4.4 |
| 17 | 54 | 305 | 0.0708 | 9.4 |
| 18 | 82 | 165 | 0.0745 | 5.8 |
| 19 | 63 | 230 | 0.114 | 10.4 |
| 20 | 72 | 250 | 0.0153 | 1.8 |
The volume and concentration of FDG in the urine recovered before the PET examination, related to injected dose
Table 2.
Estimation of bladder content at examination
| Bladder volume | SUVmean bladder | SUVmax bladder | Estimated urinary FDG concentration (MBq/mL) | Estimated dose in bladder (MBq) |
|---|---|---|---|---|
| 443.2 | 17.3 | 26.3 | 0.10 | 44.3 |
| 152.5 | 23.2 | 35.7 | 0.18 | 27.5 |
| 132.2 | 11.6 | 18.3 | 0.09 | 11.9 |
| 135.7 | 20.7 | 41.7 | 0.19 | 25.8 |
| 9.9 | 17.6 | 43.2 | 0.22 | 2.2 |
| 122.6 | 3.8 | 6.5 | 0.03 | 3.7 |
| 290.2 | 17.4 | 28.2 | 0.11 | 31.9 |
| 98.9 | 21 | 35 | 0.13 | 12.9 |
| 146.6 | 14.4 | 22.8 | 0.09 | 13.2 |
| 69.3 | 21.4 | 35.1 | 0.12 | 8.3 |
| 80.9 | 13.3 | 22.9 | 0.09 | 7.3 |
| 144.6 | 31.2 | 52.2 | 0.23 | 33.3 |
| 266.4 | 11.5 | 17.2 | 0.10 | 26.6 |
| 108.9 | 25.6 | 42.6 | 0.27 | 29.4 |
| 164.3 | 5.9 | 10.4 | 0.05 | 8.2 |
| 49.2 | 38.3 | 66.5 | 0.22 | 10.8 |
| 165.8 | 7.3 | 12.7 | 0.05 | 8.3 |
| 221.8 | 7.6 | 13.3 | 0.07 | 15.5 |
| 216.3 | 10 | 16.8 | 0.07 | 15.1 |
| 444.6 | 6.5 | 13.2 | 0.04 | 17.7 |
The bladder volume and FDG urinary content defined by SUVmean and SUVmax, and the estimated activity in the urine
The variation in amount of FDG “not available for tissue absorption” varied from 5.7% to 15.2% of injected dose. When this value was related to time by dividing the amount of non-available FDG by the time in minutes after injection, the FDG excretion varied from 0.06% to 0.31% per minute (Table 3).
Table 3.
Estimation of urinary output of FDG
| FDG bladder | FDG voided | Total urinary FDG | Time after injection (min) | Urinary FDG (%) | Clearance |
|---|---|---|---|---|---|
| 44.3 | 3.4 | 47.7 | 68 | 15.2 | 0.22 |
| 27.5 | 17.9 | 45.4 | 102 | 14.6 | 0.14 |
| 11.9 | 29.7 | 41.6 | 123 | 14.4 | 0.12 |
| 25.8 | 5.9 | 31.7 | 57 | 10 | 0.18 |
| 2.2 | 26.4 | 28.6 | 119 | 8.5 | 0.07 |
| 3.7 | 18.2 | 21.9 | 96 | 6.9 | 0.07 |
| 31.9 | 13.2 | 45.1 | 51 | 14 | 0.27 |
| 12.9 | 23.7 | 36.6 | 59 | 11.7 | 0.2 |
| 13.2 | 20.0 | 33.2 | 59 | 10.9 | 0.18 |
| 8.3 | 11.3 | 19.6 | 48 | 6 | 0.13 |
| 7.3 | 13.6 | 20.9 | 84 | 6.4 | 0.08 |
| 33.3 | 20.8 | 54.1 | 58 | 17.7 | 0.31 |
| 26.6 | 17 | 43.6 | 53 | 13.1 | 0.25 |
| 29.4 | 12 | 31.4 | 76 | 9.2 | 0.12 |
| 8.2 | 24 | 32.2 | 55 | 9.9 | 0.18 |
| 10.8 | 10.9 | 21.7 | 51 | 6.6 | 0.13 |
| 8.3 | 21.6 | 29.9 | 59 | 9.2 | 0.16 |
| 15.5 | 12.3 | 27.8 | 58 | 8 | 0.14 |
| 15.1 | 26.2 | 41.3 | 56 | 12.3 | 0.22 |
| 17.6 | 3.8 | 21.5 | 88 | 5.7 | 0.06 |
Total urinary FDG as percentage of injected dose found in urine, and “clearance”, expressed as percentage of injected dose (MBq)/min
Discussion
The variation in the urinary excretion of FDG is considerable. The study demonstrated a five-fold variation in the clearance of FDG, and showing this to be yet another factor that should be taken into account when addressing SUV values.
A large number of factors have already been described as affecting SUV values, such as size and type of region of interest used, relative calibration between PET scanner and dose calibrator, scan acquisition and reconstruction parameters (3, 4), and blood glucose levels (4–6).
The measurements in this evaluation were unsophisticated, and in particular the validity of bladder content of FDG is questionable. The aim of this paper was not to exactly define the variations in the urinary output of FDG, but rather to demonstrate that the urinary excretion of FDG is highly variable from patient to patient, and this is clearly demonstrated albeit the imperfection of the methodology.
It is also true that the uptake of FDG is most important during the initial phase, with high blood levels of FDG, and at a stage when the difference in excretion has not yet played a major role. However, it is probable that the highest rate of excretion of FDG is related to the blood serum level of the agent, so the largest percentage differences may occur in the initial stages.
The variations in urinary FDG excretion was found to be 5.7–15.2% of injected dose (decay corrected), and from 0.06–0.3% /min after injection, a five-fold variation of FDG excreted in the urine. This variation is higher than expected. In addition, all the patients in this study had normal glucose levels, and normal kidney function. It is not improbable that the variations in urinary excretion of FDG could be influenced by impaired glucose tolerance or reduced kidney function.
The role of vastly different excretion is not a major issue, but, as a number of factors, yet another one to consider when the level of certitude attributable to SUV is being discussed.
Bearing this in mind, strict cut-off values based on SUV seems even more hazardous, and not only dependent on the PET scanner and factors that are constant at a given center, or monitored as blood glucose, but also factors dependent on the individual patient.
We will try to continue the study in the same patients coming for repeated examination, it is not unlikely that the variations observed my also be applicable to within patient measurements, thus considerable care should be taken when small changes in SUV values are defined as therapeutic effect or not.
In conclusion, variation in urinary excretion of FDG is yet another reason to be cautious when considering SUV as an exact value.
Footnotes
Conflict of interest:None.
REFERENCES
- 1. DiChiro G, Brooks RA PET quantitation: blessing and curse. J Nucl Med 1988;29:1603 [PubMed] [Google Scholar]
- 2. Boellaard R Standards for PET image acquisition and quantitative data analysis. J Nucl Med 2009;50 (Suppl. 1):11S–20S [DOI] [PubMed] [Google Scholar]
- 3. Hoekstra CJ, Hoekstra OS, Stroobants SG, et al. Methods to monitor response to chemotherapy in non–small cell lung cancer with 18F-FDG PET. J Nucl Med 2002;43:1304–9 [PubMed] [Google Scholar]
- 4. Westerterp M, Pruim J, Oyen W, et al. Quantification of FDG PET studies using standardised uptake values in multi-centre trials: effects of image reconstruction, resolution and ROI definition parameters. Eur J Nucl Med Mol Imaging 2007;34:392–404 [DOI] [PubMed] [Google Scholar]
- 5. Hoekstra CJ, Paglianiti I, Hoekstra OS, et al. Monitoring response to therapy in cancer using [F-18]-2-fluoro-2-deoxy-D-glucose and positron emission tomography: an overview of different analytical methods. Eur J Nucl Med 2000;27:731–43 [DOI] [PubMed] [Google Scholar]
- 6. Weber WA Chaperoning drug development with PET. J Nucl Med 2006;47:735–7 [PubMed] [Google Scholar]


