Abstract
The cardiovascular system is regulated by the autonomic nervous system, the renin–angiotensin–aldosterone system, nitric oxide (NO) and other factors including neuropeptides. Research in neurohumoral factors has led to the development of many cardiovascular drugs. Adrenomedullin (ADM), initially isolated from the adrenal gland, has diverse physiological and pathophysiological functions in the cardiovascular system. It is produced in many organs and tissues including the vasculature. ADM has numerous actions, including vasodilation, natriuresis, antiapoptosis and stimulation of NO production. It might play a protective role in various cardiovascular pathologies, and its plasma level is elevated in patients with hypertension and heart failure. Administration of ADM is a possible therapeutic approach for treating cardiovascular diseases. A number of studies have investigated the infusion of ADM in humans, which seems to be benficial in heart failure and myocardial infarction. Instead of ADM infusion, augmentation of its endogenous level is another possible strategy. Gene therapy is feasible in animal models, but its application in humans is limited. At present, the most promising clinical application of ADM is the use of the plasma level of mid-regional proadrenomedullin as a biomarker in cardiovascular diseases. It is a good marker of prognosis and survival in patients with coronary aretery disease or heart failure.
Introduction
The cardiovascular system is regulated by various neurohumoral components, including the autonomic nervous system, renin–angiotensin–aldosterone system and various neuropeptide hormones.1 The development of many cardiovascular drugs, such as angiotensin receptor blockers, beta-blockers and endothelin antagonists, are all based on neurohormonal blockade. The evaluation of novel neurohormones could lead to further advances in cardiovascular medicine and the development of new therapeutic options.
Adrenomedullin (ADM) was discovered in 1993 in human pheochromocytoma tissue and subsequently found to be a circulating hormone. This paved the way for subsequent ADM research, which encompasses different fields such as cardiovascular diseases, the metabolic syndrome, inflammation and carcinogenesis.
The effects of ADM on the cardiovascular system have been extensively studied. ADM is released from the vascular wall and acts as an autocrine or a paracrine hormone to regulate vascular tone and blood pressure. It is a powerful vasodilator and reduces blood pressure. It may also be involved in the different stages of the cardiovascular continuum as well as in the haemodynamic changes in septic shock. The present review covers advances in ADM research and their potential implications in the therapy of cardiovascular diseases.
Methodology
We performed a search for articles in English on PubMed using ‘adrenomedullin’ as the search term. The titles and abstracts of the retrieved articles were scanned for keywords including ‘cardiovascular’, ‘heart’, ‘cardiac’, ‘myocardial’, ‘circulatory’, ‘circulation’, ‘vascular’, ‘blood pressure’, ‘hypertension’, ‘stroke’, ‘ischemia’ and ‘atherosclerosis’.
Synthesis and secretion of ADM
ADM can be detected in the culture media of adrenal medullary cells, glomerular mesangial cells, cardiac myocytes, vascular endothelial and smooth muscle cells. Among these cells, vascular endothelial and smooth muscle cells actively synthesize and secrete ADM. As there are also receptors on these cells,2 ADM may function as an autocrine or a paracrine hormone in the vasculature.
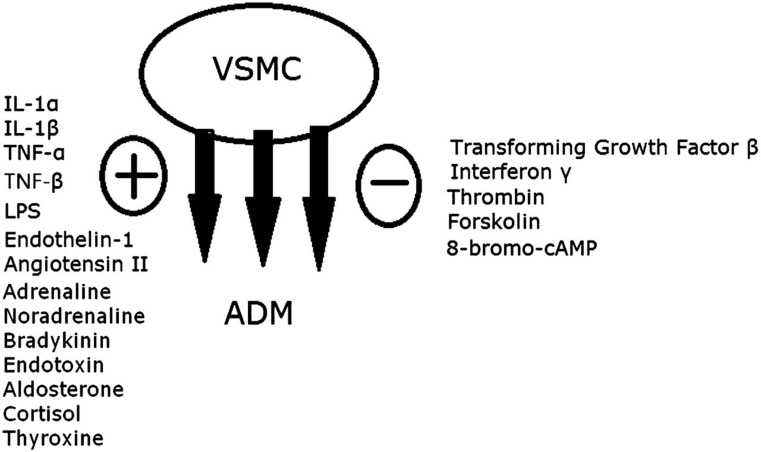
There are multiple factors that stimulate ADM production and release (Figure 1). In vitro studies showed that cytokines such as tumour necrosis factor-α, -β, interleukin-1α, -β and lipopolysaccharide can strongly stimulate the release of ADM.3 Other circulating hormones such as steroids, thyroxine, angiotensin II, noradrenaline and bradykinin can also increase ADM production.
Figure 1.
Factors stimulating (+) or inhibiting (−) ADM secretion from vascular smooth muscle cell (VSMC). IL, interleukin; LPS, lipopolysaccharide; TNF, tumour necrosis factor
Receptor and signal transduction
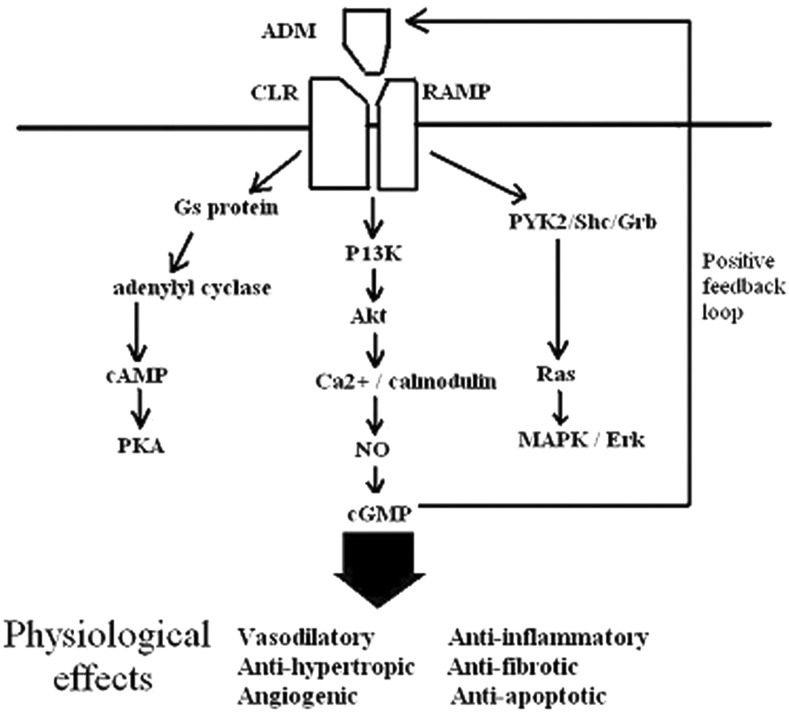
Animal studies have revealed widespread distribution of ADM receptors and binding sites4 in various organs such as the heart, brain, lungs and kidneys. In particular, specific receptors of ADM are expressed in the vascular endothelium and smooth muscle cells.2 The biological activity of ADM is exerted through the calcitonin receptor-like receptor (CLR) and a specific receptor-activity modifying protein. ADM binds to these receptor complexes and activates the second messenger signal, resulting in an increase in cAMP and nitric oxide (NO) synthesis. ADM also acts on various intracellular signal transduction pathways such as protein kinase B phosphorylation and protein tyrosine kinase activation (Figure 2).5
Figure 2.
ADM major signalling pathways and downstream effects. Akt, protein kinase B; Erk, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; PKA, protein kinase A
Adrenomedullin-2
After the discovery of ADM, another member of the family was identified, called adrenomedullin-2 (ADM-2) or intermedin.6 The mRNA is expressed in the kidneys, stomach, ovaries, pancreas and the lymphoid tissues. Intravenous injection of synthetic ADM-2 can lower arterial blood pressure more potently than ADM and induce antidiuresis and antinatriuresis in mice.6 A recent study has shown that ADM-2 produced from human endothelial cells may contribute to the regulation of vascular function.7 Therefore, ADM-2 may act as a potential marker for cardiovascular stress and play a protective role in human endothelium and modulate vascular behaviour in conjunction with ADM.
Biological actions of ADM in cardiovascular system
ADM plays diverse roles in different organs (Table 1). An important finding is the elevation of plasma ADM levels in patients with congestive heart failure,5 which may be a neurohormonal response. This suggests that ADM may have diagnostic value and may even be used as a therapeutic agent.
Table 1.
Biological functions of adrenomedullin
| Tissues | Biological functions |
|---|---|
| Vasculature | Vasodilation and hypotension |
| Stimulation of NO synthesis | |
| Inhibition of vascular smooth muscle proliferation | |
| Endothelin secretion | |
| Heart | Inhibition of hypertrophy and fibrosis |
| Kidneys | Diuresis and natriuresis |
| Lungs | Vasodilation |
| Adrenal gland | Inhibition of aldosterone secretion |
| Pancreas | Inhibition of insulin secretion |
| Platelets | cAMP elevation |
Vascular actions
ADM has a potent vasodilatory effect on the systemic circulation and also in specific regions like the cerebral, pulmonary and renal vasculatures, resulting in an increase in blood flow. In both humans and mice, intravenous infusion of ADM decreases total peripheral resistance and blood pressure.8
Multiple mechanisms are responsible for the vasodilatory effect of ADM. As the ADM receptor is coupled with adenyl cyclase, it has been postulated that the vasodilator action is mainly mediated by the production of cAMP. ADM binds to CLR and increases the cAMP level in vascular smooth muscle cells.1 On the other hand, ADM can also bind to receptors on the endothelium and enhance NO synthesis. The vasodilatory effect of ADM can be attenuated by either removing the endothelium or administration of NO synthase inhibitor.9
Apart from the vasodilatory effect, ADM can also regulate the proliferation of cultured vascular smooth muscle cells. ADM inhibits migration and proliferation stimulated by platelet-derived growth factor,10 although it also has a growth-promoting effect in quiescent cells. The cardioprotective effect of ADM has been demonstrated in cultured rat aortic endothelial cells, in which apoptosis was inhibited through a cAMP-independent mechanism.11 An antiapoptotic effect of ADM has also been observed in human umbilical vein endothelial cells.11 These findings suggest that ADM protects the cardiovascular tissues from injury by inhibiting apoptosis and regulating proliferation.
Heart
Systemic administration of ADM increases cardiac output and lowers blood pressure in healthy men and in patients with heart failure.8 The increase in cardiac output can be explained by various mechanisms, including a decrease in systemic vascular resistance and an increase in coronary flow due to coronary vessel dilation. ADM increases cAMP levels and activates protein kinase A, and so augments myocardial contractility. Besides, it can exert a positive inotropic effect on myocardial cells by a cAMP-independent mechanism in which the intracellular calcium level is increased. However, the effect of ADM on contractility is very much dependent on the experimental conditions, and some experiments using human myocardial cells failed to demonstrate either a positive or a negative inotropic effect.12
Antioxidative properties
In heterozygous ADM knockout mice, administration of salt and angiotensin II–generated oxidative stress in the cardiovascular tissues and resulted in perivascular inflammation of coronary arteries.13 ADM appears to play a protective role against oxidative stress and hence prevents cardiovascular damage. Such an antioxidative effect is thought to be mediated by cAMP and the protein kinase A pathway.14
Endocrine system
ADM has various effects on neuroendocrine systems, especially on cardiovascular hormones. Most studies are in agreement that ADM suppresses the renin–angiotensin–aldosterone axis and the action of endothelin.15 Studies have reported that pro-adrenomedullin N-terminal 20 peptide inhibits the release of noradrenaline from the adrenergic nerve endings.16 The interaction between ADM and adrenal hormones is complex, and ADM also regulates body fluid homeostasis by inhibiting water drinking and salt appetite.17
Roles of ADM in cardiovascular diseases
Hypertension
Plasma ADM levels are higher in patients with essential hypertension than in normotensive subjects. Previous studies have shown that plasma ADM increases with the World Health Organization (WHO) stages of hypertension.18 The plasma ADM level correlates with blood pressure and the severity of target organ damage.
The exact relationship between plasma ADM level and blood pressure has yet to be confirmed. Administration of calcium channel blocker and angiotensin-converting enzyme (ACE) inhibitor lowers blood pressure but does not affect plasma ADM level.19 On the other hand, patients with malignant hypertension showed a large increase in plasma ADM level, followed by a drop in blood pressure after antihypertensive treatment.20 Hence, the regulation of blood pressure by ADM deserves further investigation.
Heart failure
Plasma ADM increases with the severity of heart failure. The level is raised in both diastolic and systolic heart failure.21 Intravenous administration of ADM reduces pulmonary wedge pressure and increases cardiac output, accompanied by an increase in urine volume and urinary sodium excretion. In animal studies, chronic ADM administration attenuates transition of hypertrophy to heart failure and improves survival in rats with heart failure.22
The plasma ADM levels in different parts of the circulation in patients with ischaemic heart disease have been examined. A significant rise in plasma ADM levels was found between femoral artery and vein and between aortic root and coronary sinus.23 In vitro findings showed that cultured cardiac cells and vascular smooth muscle or endothelial tissues actively secrete ADM into the culture medium.24 Taken together, these suggest that part of the increased circulating ADM comes from the heart as well as the vasculature.
The mechanisms accounting for the elevation of plasma ADM levels in heart failure patients are yet to be understood. Humoral or mechanical factors are likely to be involved. In vitro and in vivo studies have shown that ADM production could be stimulated by different hormones and cytokines in the myocardium and the vasculature.25 Mechanical stress is another factor that increases ADM production in cardiac tissues. It seems that ADM functions as an autocrine or paracrine hormone that acts against the progression of heart failure systematically and locally.
Acute myocardial infarction
Plasma ADM level is elevated in the early phase of acute myocardial infarction.3 ADM may have compensatory and protective effects by causing coronary vasodilation and increasing coronary blood flow. Besides, ADM administration after myocardial infarction could inhibit progression of heart failure and improve survival in rats.26 Short-term infusion of ADM reduces the infarct size and attenuates myocardial ischaemia or reperfusion injury in rats via its antioxidant and antiapoptotic properties.27
Atherosclerosis
As ADM is produced and secreted in endothelial and vascular smooth muscle cells, there may be a relation between plasma ADM levels and endothelial damage. Indeed, plasma ADM levels can reflect the degree of endothelial injury in patients with atherosclerosis.3
ADM inhibits apoptosis of cultured endothelial cells and suppresses the agonist-induced hyperpermeability of human vascular smooth muscle cells.28 The aorta of apo-lipoprotein E (apoE)-knockout mice with ADM gene overexpression had significantly less fatty streak formation than those without ADM over-expression.29 These studies suggest a vasculo-protective role of ADM against progression of atherosclerosis.
Other cardiovascular diseases
ADM is associated with renal dysfunction; its plasma level increases with the severity of renal impairment.18 The decreased clearance of ADM due to renal dysfunction may account for the higher plasma levels. ADM is also implicated in septic, haemorrhagic or cardiogenic shock. Plasma ADM level is correlated with the cardiac index and inversely related to diastolic pressure. Other disease conditions like pulmonary hypertension, stroke and diabetes are also associated with elevated plasma ADM levels.
Clinical applications of ADM
ADM has a role to play in various pathological conditions due to its potent biological properties, including vasodilation, cardiac inotrophy and antiproliferative effect in vascular smooth muscle cells. An increase in plasma ADM level may reflect a counter-regulatory or compensatory mechanism in cardiovascular diseases.
Hypertension
Chronic infusion of ADM has renoprotective effects in a rat model of malignant hypertension.30 It significantly reduces plasma renin concentration, plasma aldosterone level, intrarenal angiotensin II level and the gene expression of ACE. Increased endogenous ADM may play a compensatory role in chronic hypertensive renal failure. Chronic infusion of ADM has a hypotensive effect in both hypertensive and normotensive rats.1 All of these findings suggest that chronic ADM infusion and endogenous ADM could have beneficial effects in hypertension and organ protection.
Heart failure
Systemic administration of ADM shows promising therapeutic effects in cardiovascular disorders. The cardiovascular and renal effects of intravenous infusion of ADM in rats with heart failure have been examined.31 A low dose of ADM increased urine flow and urine sodium excretion, while a high dose of ADM slightly lowered mean arterial blood pressure but increased cardiac output significantly in both normal rats and those with heart failure. Also, the heart failure rats showed a significant decrease in right ventricular pressure and atrial pressure, an increase in renal plasma flow and other effects similar to low dose treatment. These results implied a role for ADM in regulating pressure and volume in heart failure, and such a role is also shown in clinical studies.8
Since a proportion of ADM is metabolized by neutral endopeptidase, the combination of ADM and endopeptidase inhibitor has been studied in sheep with heart failure.32 In that study, co-administration of ADM and endopeptidase inhibitor produced larger increases in plasma ADM and cAMP levels, and greater hemodynamic effects than ADM administration alone. Moreover, there was improvement in renal function. This suggests that combined treatment with ADM and endopeptidase inhibitor may have beneficial renal and haemodynamic effects in heart failure, and raises the possibility of a new approach in treating heart failure.
A recent study has tried to co-admininster ADM and human atrial natriuretic peptide (hANP) in patients with acute decompensated heart failure, and a significant improvement in hemodynamics was observed.33 Although the results are preliminary, they suggest that intravenous infusion of ADM and hANP could be used for acute heart failure.
Myocardial infarction
A recent clinical pilot study has demonstrated that intravenous administration of ADM can be an adjunct to percutaneous coronary intervention.34 Wall motion and infarct size appeared to be improved, but further studies are needed to confirm these benefits.
ADM as a biomarker
There is accumulating evidence that ADM is an excellent biomarker for cardiovascular disease. A study that compared the predictive power of plasma ADM with adiponectin and high sensitive C-reactive protein (hsCRP) for future cardiovascular events showed that it was superior to both.35 Mid-regional pro-adrenomedullin (MR-proADM) is more stable than ADM and is therefore more suitable for clinical use as a biomarker. One recent study showed that MR-proADM had good prognostic value in patients with heart failure after an acute myocardial infarction.36
Conclusion
Although ADM was only discovered 20 years ago, extensive studies have provided us with a large amount of information on the structure, distribution and functions of this peptide hormone. The actions of ADM include vasodilation, natriuresis, stimulation of NO production and inhibition of apoptosis. ADM also plays a significant role in various pathological conditions including hypertension, myocardial infarction and heart failure. Hence, therapy based on increased activation of the ADM receptor may be considered as one therapeutic possibility in treating these cardiovascular diseases.
The angiogenic and cytoprotective properties of ADM may provide a promising opportunity in the development of regeneration therapy for injured organs, particularly in myocardial infarction and arteriosclerosis. Since most of the therapeutic applications of ADM were studied in experimental models, clinical trials should be carried out to confirm the therapeutic benefits in various cardiovascular diseases.
The most immediate clinical application of ADM is to measure it, or MR-proADM, in the venous blood samples of patients. Recent studies have shown that it is as good as, if not better than, hsCRP as an index of prognosis. It is now time to develop a user-friendly and economical assay for clinical use.
DECLARATIONS
Competing interests
The authors declare that there is no conflict of interest
Funding
HKW and BMYC receive support from the University of Hong Kong Seed Funding Programme for Basic Research
Guarantor
BMYC
Contributorship
HKW and BMYC drafted the manuscript. TTC revised the manuscript.
References
- 1. Nishikimi T Adrenomedullin in Cardiovascular Disease. New York: SAGE Publications, 2005. [Google Scholar]
- 2. Kato J, Kitamura K, Kangawa K, Eto T Receptors for adrenomedullin in human vascular endothelial cells. Eur J Pharmacol Mol Pharmacol 1995;289:383–5 [DOI] [PubMed] [Google Scholar]
- 3. Bunton DC, Petrie MC, Hillier C, Johnston F, McMurray JJV The clinical relevance of adrenomedullin: a promising profile? Pharmacol Ther 2004;103:179–201 [DOI] [PubMed] [Google Scholar]
- 4. Juaneda C, Dumont Y, Chabot J, Fournier A, Quirion R Adrenomedullin receptor binding sites in rat brain and peripheral tissues. Eur J Pharmacol 2003;474:165–74 [DOI] [PubMed] [Google Scholar]
- 5. Cheung BM, Tang F Adrenomedullin: exciting new horizons. Recent Pat Endocr Metab Immune Drug Discov 2012;6:4–17 [DOI] [PubMed] [Google Scholar]
- 6. Takei Y, Inoue K, Ogoshi M, Kawahara T, Bannai H, Miyano S Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett 2004;556:53–8 [DOI] [PubMed] [Google Scholar]
- 7. Pearson LJ, Yandle TG, Nicholls MG, Evans JJ Intermedin (adrenomedullin-2): a potential protective role in human aortic endothelial cells. Cell Physiol Biochem 2009;23:97–108 [DOI] [PubMed] [Google Scholar]
- 8. Nagaya N, Satoh T, Nishikimi T, et al. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation 2000;101:498–503 [DOI] [PubMed] [Google Scholar]
- 9. Feng CJ, Kang B, Kaye AD, Kadowitz PJ, Nossaman BD L-NAME modulates responses to adrenomedullin in the hindquarters vascular bed of the rat. Life Sci 1994;55:PL433–38 [DOI] [PubMed] [Google Scholar]
- 10. Horio T, Kohno M, Kano H, et al. Adrenomedullin as a novel antimigration factor of vascular smooth muscle cells. Circ Res 1995;77:660–4 [DOI] [PubMed] [Google Scholar]
- 11. Sata M, Kakoki M, Nagata D, et al. Adrenomedullin and nitric oxide inhibit human endothelial cell apoptosis via a cyclic GMP-independent mechanism. Hypertension 2000;36:83–8 [DOI] [PubMed] [Google Scholar]
- 12. Saetrum Opgaard O, Hasbak P, de Vries R, Saxena PR, Edvinsson L Positive inotropy mediated via CGRP receptors in isolated human myocardial trabeculae. Eur J Pharmacol 2000;397:373–82 [DOI] [PubMed] [Google Scholar]
- 13. Shimosawa T, Shibagaki Y, Ishibashi K, et al. Adrenomedullin, an endogenous peptide, counteracts cardiovascular damage. Circulation 2002;105:106–11 [DOI] [PubMed] [Google Scholar]
- 14. Yoshimoto T, Fukai N, Sato R, et al. Antioxidant effect of adrenomedullin on angiotensin II-induced reactive oxygen species generation in vascular smooth muscle cells. Endocrinology 2004;145:3331–7 [DOI] [PubMed] [Google Scholar]
- 15. Ishimitsu T, Ono H, Minami J, Matsuoka H Pathophysiologic and therapeutic implications of adrenomedullin in cardiovascular disorders. Pharmacol Ther 2006;111:909–27 [DOI] [PubMed] [Google Scholar]
- 16. Fujioka H, Okamura T, Toda N Inhibition by adrenomedullin of amine release from adrenergic nerves in dog mesenteric arteries. Eur J Pharmacol 1999;385:155–61 [DOI] [PubMed] [Google Scholar]
- 17. Andreis PG, Neri G, Prayer-Galetti T, et al. Effects of adrenomedullin on the human adrenal glands: an in vitro study. J Clin Endocrinol Metab 1997;82:1167–70 [DOI] [PubMed] [Google Scholar]
- 18. Ishimitsu T, Nishikimi T, Saito Y, et al. Plasma levels of adrenomedullin, a newly identified hypotensive peptide, in patients with hypertension and renal failure. J Clin Invest 1994;94:2158–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kita T, Kitamura K, Kuwasako K, Kawamoto M, Eto T Short-term modulation of the renin-angiotensin system does not alter plasma adrenomedullin concentration in humans. J Hypertens 1998;16:2057–62 [DOI] [PubMed] [Google Scholar]
- 20. Kato J, Kitamura K, Matsui E, et al. Plasma adrenomedullin and natriuretic peptides in patients with essential or malignant hypertension. Hypertens Res 1999;22:61–5 [DOI] [PubMed] [Google Scholar]
- 21. Yu C, Cheung BMY, Leung R, Wang Q, Lai W, Lau C Increase in plasma adrenomedullin in patients with heart failure characterised by diastolic dysfunction. Heart 2001;86:155–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nishikimi T, Yoshihara F, Horinaka S, et al. Chronic administration of adrenomedullin attenuates transition from left ventricular hypertrophy to heart failure in rats. Hypertension 2003;42:1034–41 [DOI] [PubMed] [Google Scholar]
- 23. Hirayama N, Kitamura K, Imamura T, Kato J, Koiwaya Y, Eto T Secretion and clearance of the mature form of adrenomedullin in humans. Life Sci 1999;64:2505–9 [DOI] [PubMed] [Google Scholar]
- 24. Kitamura K, Kangawa K, Eto T Adrenomedullin and PAMP: discovery, structures, and cardiovascular functions. Microsc Res Tech 2002;57:3–13 [DOI] [PubMed] [Google Scholar]
- 25. Kato K, Yin H, Agata J, Yoshida H, Chao L, Chao J Adrenomedullin gene delivery attenuates myocardial infarction and apoptosis after ischemia and reperfusion. Am J Physiol 2003;285:H1506–14 [DOI] [PubMed] [Google Scholar]
- 26. Nakamura R, Kato J, Kitamura K, et al. Adrenomedullin administration immediately after myocardial infarction ameliorates progression of heart failure in rats. Circulation 2005;110:426–31 [DOI] [PubMed] [Google Scholar]
- 27. Okumura H, Nagaya N, Itoh T, et al. Adrenomedullin infusion attenuates myocardial ischemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway. Circulation 2004;109:242–8 [DOI] [PubMed] [Google Scholar]
- 28. Hippenstiel S, Witzenrath M, Schmeck B, et al. Adrenomedullin reduces endothelial hyperpermeability. Circ Res 2002;91:618–25 [DOI] [PubMed] [Google Scholar]
- 29. Imai Y, Shindo T, Maemura K, et al. Resistance to neointimal hyperplasia and fatty streak formation in mice with adrenomedullin overexpression. Arterioscler Thromb Vasc Biol 2002;22:1310–5 [DOI] [PubMed] [Google Scholar]
- 30. Nishikimi T, Mori Y, Kobayashi N, et al. Renoprotective effect of chronic adrenomedullin infusion in Dahl salt-sensitive rats. Hypertension 2002;39:1077–82 [DOI] [PubMed] [Google Scholar]
- 31. Nagaya N, Nishikimi T, Horio T, et al. Cardiovascular and renal effects of adrenomedullin in rats with heart failure. Am J Physiol 1999;276:R213–8 [DOI] [PubMed] [Google Scholar]
- 32. Rademaker MT, Charles CJ, Cooper GJ, et al. Combined endopeptidase inhibition and adrenomedullin in sheep with experimental heart failure. Hypertension 2002;39:93–8 [DOI] [PubMed] [Google Scholar]
- 33. Nishikimi T, Karasawa T, Inaba C, et al. Effects of long-term intravenous administration of adrenomedullin (AM) plus hANP therapy in acute decompensated heart failure: a pilot study. Circ J 2009;73:892–8 [DOI] [PubMed] [Google Scholar]
- 34. Kataoka Y, Miyazaki S, Yasuda S, et al. The first clinical pilot study of intravenous adrenomedullin administration in patients with acute myocardial infarction. J Cardiovasc Pharmacol 2010;56:413–9 [DOI] [PubMed] [Google Scholar]
- 35. Nishida H, Horio T, Suzuki Y, et al. Plasma adrenomedullin as an independent predictor of future cardiovascular events in high-risk patients: comparison with C-reactive protein and adiponectin. Peptides 2008;29:599–605 [DOI] [PubMed] [Google Scholar]
- 36. Klip IT, Voors AA, Anker SD, et al. Prognostic value of mid-regional pro-adrenomedullin in patients with heart failure after an acute myocardial infarction. Heart 2011;97:892–8 [DOI] [PubMed] [Google Scholar]