Abstract
Background: Understanding the full effect of chronic low-dose folic acid is important in interpreting the effect of the mandatory folic acid fortification program in North America.
Objective: We aimed to describe the rate of attainment and steady state (plateau) of red blood cell (RBC) folate in response to long-term intake of 140 μg (designed to mimic fortification) and 400 μg (recommended dose for the primary prevention of neural tube defects) folic acid/d in reproductive-aged women living in a country with minimal fortification.
Design: On the basis of pharmacokinetics principles, it was recently proposed that a steady state should be reached after 40 wk. Thus, 144 women aged 18–40 y were randomly assigned to receive a daily folic acid supplement of 140 (n = 49) or 400 (n = 48) μg or placebo (n = 47) for 40 wk. RBC folate was measured at baseline and at 6, 12, 29, and 40 wk.
Results: After 40 wk, RBC folate did not reach a plateau in either treatment group. Kinetic modeling of the data indicated that RBC folate would approximately double from 779 to 1356 nmol/L in response to 140 μg folic acid/d with only ≈50% of model-estimated steady state conditions achieved at 40 wk. An average RBC folate concentration of 1068 nmol/L after 12 wk of supplementation with 400 μg folic acid/d was readily achieved at 36 wk after continuous intake of 140 μg/d.
Conclusion: Our model shows the considerable length of time required to attain the full effect of low-dose folic acid, which suggests that 140 μg folic acid/d could be as effective as 400 μg folic acid/d taken during the periconceptional period if given sufficient time. This trial is registered at www.anzctr.org.au as ACTRN12609000215224.
INTRODUCTION
The well-established role of periconceptional folic acid supplementation in the prevention of neural tube defects (NTDs) (1–3) has led to public health efforts targeted at increasing the folate status of all reproductive-aged women. Folate status can be raised either by supplementation or fortification or by increasing consumption of folate-rich foods. Understanding the cumulative effect of low-dose folic acid given chronically and the knowledge of the time required to reach steady state conditions has implications for the design and interpretation of intervention trials and for the understanding of whether a dose lower than the official recommendation of 400 μg folic acid/d would be efficacious in the primary prevention of NTDs. This is particularly important in light of recent data reported from the US National Birth Defects Prevention Study (1998–2003), which showed that periconceptional use of folic acid supplements in the postfortification era no longer appears to further reduce NTD risk (4). One potential explanation for the lack of association is that fortification may have provided the necessary level of folic acid needed to prevent most folate-sensitive NTDs (4, 5).
Recent estimates in the United States and Canada show that folic acid fortification of the food supply has provided an additional intake of 100 to 150 μg/d to reproductive-aged women (6, 7). This is approximately one-quarter of the recommended dose associated with a reduction in NTDs in most epidemiologic studies (8–12). However, because food fortification provides life-long exposure, the cumulative effect of this lower dose over time could result in protective RBC folate concentrations similar to the efficacious response of 400 μg during the periconceptional period. Whereas the time to reach steady state serum or plasma folate concentrations in response to varying folate intakes is well described (13), the effect of folic acid supplementation on red blood cell (RBC) folate status is less well understood. RBC folate has a slower response to changes in folate intake than do serum and plasma folate concentrations as a result of the incorporation of folate during erythropoiesis and the average 120-d life span of a normal erythrocyte (14). In many folate-supplementation trials with doses varying from 100 to 4000 μg/d, RBC folate concentrations did not reach a steady state within the 12–24-wk study periods (15–17). To date, no study has described the amount of time needed for RBC folate to become saturated after supplementation of low-to-moderate folic acid intakes.
A recently proposed, and partly confirmed, working model based on pharmacokinetic principles and an estimated 8-wk biological half-life of RBC folate has predicted that steady state RBC folate (≈97% of the plateau) would be achieved after 5 half-life periods, or ≈40 wk after commencement of supplementation (18). Thus, the aim of our study was to evaluate the long-term effect of daily supplementation with 140 μg (dose designed to mimic fortification) and 400 μg (dose recommended for primary prevention of NTDs) folate on the appearance of RBC folate over 40 wk in a group of healthy reproductive-aged women. This study was conducted in a population that has a moderately low intake of synthetic folic acid and no mandated fortification policy.
SUBJECTS AND METHODS
Study participants and study design
The study used data from a folate-supplementation trial that investigated the long-term effect of 140 μg folic acid/d (dose designed to mimic average folic acid intake provided by fortification) and 400 μg folic acid/d (dose recommended for primary prevention of NTDs) on blood folate status and estimated NTD risk in reproductive-aged women over a 40-wk intervention period (19). Healthy women aged 18–40 y (inclusive) were recruited in July 2008 from the staff and student population at the University of Otago, Dunedin, New Zealand, and from the local community through newspaper advertisements. Women were excluded from the study if they were pregnant, lactating, or planning a pregnancy in the next 12 mo. Additional exclusion criteria for the study included regular use of folic acid–containing supplements in the previous 6 mo; self-reported history of cardiovascular, gastrointestinal, hepatic, renal, or hematologic disease; and use of medications that are known to interfere with folate metabolism (eg, methotrexate, sulfasalazine, or anticonvulsants). Ethical approval was obtained from the Human Ethics Committee of the University of Otago, New Zealand, and all participants gave written informed consent.
The intervention was a 40-wk double-blinded, placebo-controlled trial. At baseline, sociodemographic and general health data were collected by using a self-administered questionnaire. Ethnicity data were collected by using the same questions used in the Statistics New Zealand census, which allows participants to select up to 3 ethnic groups to which they feel they belong. Participants are then categorized into a prioritized single ethnic group by using the following Statistics New Zealand algorithm: Maori first, then Pacific peoples, then Asian, then other groups (except New Zealand European), and last New Zealand European (20). The height and weight of each participant were measured according to standardized procedures (21) measured to the nearest 0.1 kg and 0.1 cm, respectively. Body mass index (BMI; in kg/m2) was calculated. Participants were then randomly assigned to receive 140 μg folic acid/d, 400 μg folic acid/d, or placebo. For the purpose of the long-term kinetic analysis and intent to mimic the inevitable exposure of mandatory folic acid fortification, only participants with ≥70% compliance were included herein.
Supplements
Supplements were provided in tablet form with instructions to be taken once per day. The supplements were manufactured in a single batch by New Zealand Nutritionals Ltd (Christchurch, New Zealand) as hard tablets, each containing microcrystalline cellulose as a filler and either 140 μg (317 nmol) folic acid or 400 μg (907 nmol) folic acid. Supplements were coded by a third party, and all investigators remained blinded to the treatment groups until all statistical analyses were completed. The folate content of the tablets was measured by microbiological assay at the beginning of the study. The actual amounts in the tablets aimed to provide 140 and 400 μg were 133 and 359 μg, respectively. Folate was undetectable in the placebo tablet. The subjects were provided with a sufficient supply of their randomly assigned tablets, and compliance was assessed by returned pill counts at 12, 29, and 40 wk.
Blood sampling and laboratory analysis
Fasting blood samples were collected, after the subjects had fasted overnight for ≥12 h, by venipuncture at baseline and 6, 12, 29, and 40 wk. After the measurement of hematocrit, RBC hemolysates were prepared [dilution (1:10) of whole blood with 1% ascorbic acid and incubated at 37°C for 30 min] before storage at −80°C. The remaining whole blood was centrifuged (1500 × g for 15 min at 4°C) to separate the plasma, and aliquots were stored immediately at −80°C. Plasma and whole-blood folate concentrations were measured by microbiological assay (22) by using the test organism chloramphenicol-resistant Lactobacillus rhamnosus (ATCC 27773; American Type Culture Collection, Manassas, VA). Erythrocyte folate concentrations were calculated from whole-blood values by using individual packed cell volumes and after correction for plasma folate concentration. To avoid between-run variation, all study visit samples from each participant were measured in one batch. Accuracy and interassay variability were monitored with the use of 2 external quality controls: whole-blood folate standard (National Institute for Biological Standards and Control, Hertfordshire, United Kingdom) with a certified value of 29.5 nmol/L (mean ± SD: 27.3 ± 4.6; CV: 17%) and a human serum folate standard reference material (SRM 1955; National Institute of Standards and Technology, Gaithersburg, MD) with a total folate information value as determined by microbiological assay of 44 nmol/L (mean ± SD: 42.5 ± 8.7; CV: 20%).
RBC folate modeling and statistical analysis
Baseline characteristics (age, weight, height, BMI, and baseline RBC folate concentrations) are presented as geometric means (± geometric SDs). For the current project, the analysis was restricted to the participants with ≥70% known treatment compliance and had at least one postbaseline blood folate measurement.
Nonlinear regression was used to model the changes in RBC folate over all available time points (each participant's observed days since baseline for each measurement occasion) by using the following equation with interactions where variables were allowed to vary between groups:
where Ctj is the predicted RBC folate concentration at time t in the intervention group j, C0j is the observed initial RBC concentration at the start of the study (t = 0) for group j, CSSj is the plateau (asymptote for the steady state) for group j, k is the first-order rate constant representing the proportion of the total mass of RBC folate used (or metabolized) per day, and t is the time in d. Ctj, C0j, and CSSj are all expressed in nmol/L.
Each treatment group had its own mean baseline concentration of RBC folate, which was based on observed data, and the plateau was estimated from the data separately for the groups. Robust SEs were used to accommodate the repeated measures. The rate constant (k) was assumed to be the same for both groups. This yielded estimates of Css (asymptotes) for each intervention group j and a single estimate for the k parameter (the optimal rate constant shared over both groups). An additional model was used to test for a difference in the rate constant between treatment groups. All statistical analyses were performed by using Stata (version 11; StataCorp, College Station, TX). A 2-sided P value ≤0.05 was considered significant.
Evaluation of the kinetic model in comparison with a folic acid intervention trial
With the use of baseline RBC folate measurements from an independent published randomized folate-intervention trial (16) and our model-estimated parameters, we calculated the predicted appearance and estimated steady state of RBC folate in a group of reproductive-aged women (n = 171) in response to supplementation with 400 μg folic acid/d for a 6-mo period. In the published trial, RBC folate concentrations were measured at the start of the study and 1, 3, and 6 mo thereafter. On average, women in this trial took >95% of all pills during the 6-mo trial period, and an intention-to-treat analysis was conducted. This study was a large, randomized trial designed to evaluate the effect of folic acid dosing regimens on blood folate status. The participants were also similar in reproductive age to those enrolled in our study. To assess the validity of the model, the predicted and measured increases in RBC folate concentrations would need to be similar.
RESULTS
One hundred and forty-four women were recruited for the study; 49 women were allocated to receive 140 μg folic acid/d, 48 women to receive 400 μg folic acid/d, and the remainder to receive placebo (n = 47). Of these women, 22 (16%) were excluded from the final analysis (n = 10 for unknown compliance; n = 12 for compliance <70%), which resulted in a total of 122 (85%) women participating in the intervention trial with pill compliance ≥70% (140 μg folic acid/d, n = 45; 400 μg folic acid/d, n = 40; placebo, n = 37). In this group of participants with ≥70% compliance, the distribution of the data showed 21 (17%) participants with 70–79% compliance, 45 (37%) with 80–89% compliance, 51 (42%) with 90–99% compliance, and 5 (4%) with 100% compliance. The overall mean pill compliance of the group included in the final analysis was 88%. The geometric mean (± geometric SD) age of the 122 participants was 23.2 ± 1.2 y, 89% (108 of 122) of the participants had at least some postsecondary education, 80% (98 of 122) of the participants identified as New Zealand European per prioritized ethnicity, and the geometric mean (± geometric SD) BMI of the participants was 23.5 ± 1.2. No statistically significant differences in age (P = 0.277), BMI (P = 0.339), education (P = 0.725), or baseline RBC folate concentrations (P = 0.118) were found between those with known compliance ≥70% and those who were excluded. However, differences in ethnicity were found (P = 0.005): 80% of participants who met the compliance requirements identified as New Zealand Europeans compared with only 50% of those who were excluded.
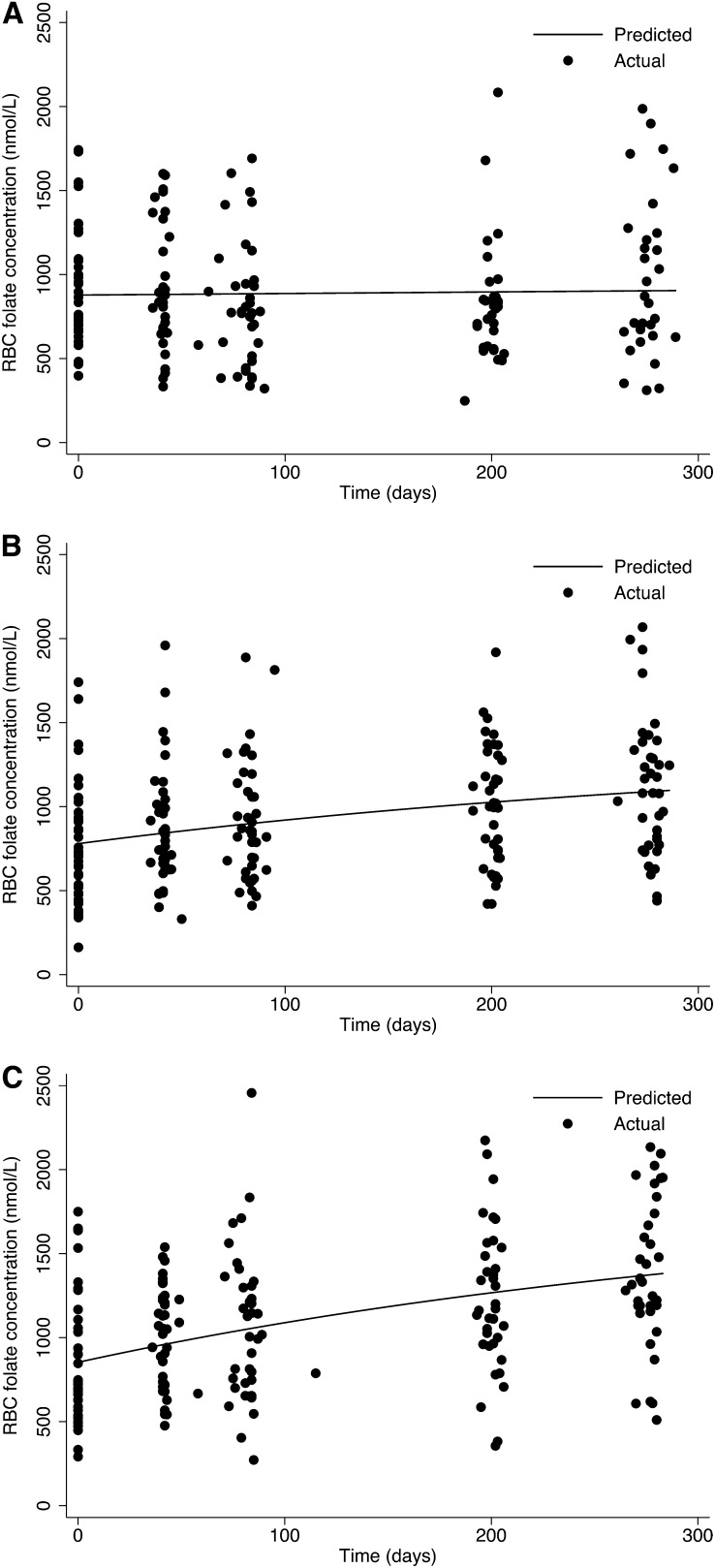
Time course data of the relation of RBC folate concentration as a function of continuing intake (140 μg, 400 μg/d, and placebo) are shown in Figure 1. Both the actual individual values at each time point and the curves fitted to the means are shown. No evidence of a change in RBC folate concentrations over time was observed in the placebo group; however, RBC folate increased in both folic acid dosage groups throughout the 40-wk period, and no evidence that RBC folate concentrations plateaued in either group was found. The average rate constant estimate (k) was 0.00278 d−1; thus, 0.28% of the maximum saturation of RBC folate was achieved per day. No evidence indicated that the rate constant estimate (k) differed between folic acid treatment groups (estimated difference = 0.001; 95% CI: −0.011, 0.013; P = 0.863). The rate constant (k) can be further interpreted to mean that ≈15% of the estimated relative maximum saturation of RBCs (steady state) was achieved after 60 d of supplementation [e-kt = e–(0.00278)(60 d) = 0.8461].
FIGURE 1.
Plot of observed red blood cell (RBC) folate concentrations over time and model-estimated lines that were fitted to the data with the use of nonlinear regression, allowing for separate plateaus for each intervention group but with the assumption of the same rate constant (k) for both treatment groups. Placebo (A), 140-μg folic acid/d (B), and 400-μg folic acid/d (C) groups.
The observed and model-derived group means for RBC folate, calculated at each time point, are shown in Table 1. On the basis of our model estimating the best fitting plateau over all available time points, we calculated average steady state values of 1356 and 1822 nmol/L for those participants consuming 140 and 400 μg folic acid/d, respectively. We further estimated that 90% of the estimated steady state would be achieved at 74 and 86 wk after continuing daily supplementation of 140 and 400 μg folic acid/d, respectively.
TABLE 1.
Observed and modeled appearance of red blood cell (RBC) folate over 40 wk and estimated steady state in response to 2 doses of folic acid intake in reproductive-aged women
| Intervention |
||||||
| Placebo (n = 37) |
140 μg Folic acid/d (n = 45) |
400 μg Folic acid/d (n = 40) |
||||
| Study visit | Average number of study days | Observed RBC folate1 | Observed RBC folate1 | Model-derived RBC folate2 | Observed RBC folate1 | Model-derived RBC folate2 |
| nmol/L | ||||||
| 1 (Baseline) | 0 | 918 ± 342 | 779 ± 346 | — | 853 ± 395 | — |
| 2 | 41 | 909 ± 360 | 858 ± 329 | 842 | 997 ± 296 | 961 |
| 3 | 83 | 819 ± 371 | 902 ± 350 | 898 | 1056 ± 441 | 1052 |
| 4 | 200 | 826 ± 352 | 1006 ± 356 | 1026 | 1225 ± 431 | 1267 |
| 5 | 277 | 976 ± 474 | 1103 ± 404 | 1089 | 1400 ± 465 | 1373 |
| Estimated RBC folate plateau3 | 1356 (424, 2228) | 1822 (302, 3341) | ||||
Values are arithmetic means ± SDs.
The predicted appearance of folate in RBCs at each time point as calculated by the following equation: Ctj = C0j + (CSSj – C0j)(1 – e-kt), where Ctj is the predicted RBC folate concentration at time t in the intervention group j, C0j is the observed initial RBC concentration at the start of the study (t = 0) for group j, CSSj is the plateau (asymptote at the theoretical new steady state) for group j, k is the rate constant representing the proportion of the total mass of RBC folate used (or metabolized) per day = 0.00278 d−1, and t is the time in days. Ctj, C0j, and CSSj are all expressed in nmol/L.
Values are estimated means; 95% CIs in parentheses.
For application of the model, we calculated that RBC folate concentration would increase by 215 nmol/L reaching an average RBC folate concentration of 1068 nmol/L after 12 wk (90 d) of continuing intake of 400 μg folic acid/d (typical period of time recommended for periconceptional use of folic acid for to prevent first occurrence NTDs). For the participants who consumed 140 μg folic acid/d, an equivalent RBC folate concentration of 1069 nmol/L was achieved after 36 wk (252 d) of continuous intake.
In addition, with the use of our model and the baseline RBC folate concentration reported in a previously published, well-controlled randomized intervention (16), we calculated the estimated RBC folate concentration at each reported time point and estimated RBC folate steady state (Table 2). The results indicate that the predicted and published RBC folate concentrations are highly comparable. On the basis of our estimated average RBC plateau concentration (1822 nmol/L), the average reported RBC folate concentration of 1033 nmol/L in a previously published study (16) after 6 mo of daily supplementation reached only ≈36% of the estimated steady state conditions for this level of folate intake.
TABLE 2.
Observed red blood cell (RBC) folate after supplementation with 400 μg folic acid/d measured in an independent intention-to-treat intervention trial in young Chinese women by Hao et al (16) as compared with estimated RBC folate appearance and steady state calculated by our kinetic model
| Study visit | Average number of study days | RBC folate from Hao et al (n = 171)1 | Model-derived RBC folate2 |
| nmol/L | nmol/L | ||
| Baseline | 0 | 626 | — |
| Month 1 | 30 | 729 | 719 |
| Month 3 | 90 | 912 | 885 |
| Month 6 | 180 | 1033 | 1088 |
| Estimated RBC folate plateau | — | — | 1822 |
Values are geometric means.
The predicted appearance of folate in RBCs as calculated by the following equation: Ctj = C0j + (CSSj – C0j)(1 – e-kt), where Ctj is the predicted RBC folate concentration at time t in the intervention group j, C0j is the observed initial RBC concentration at the start of the study (t = 0) of 626 nmol/L for group j, CSSj is the plateau (asymptote at the theoretical new steady state) of 1822 nmol/L for group j as calculated from our model, k is the rate constant representing the proportion of the total mass of RBC folate used (or metabolized) per day = 0.00278 d−1, and t is the time in d. Ctj, C0j, and CSSj are all expressed in nmol/L.
DISCUSSION
This 40-wk intervention study was designed to investigate the appearance and steady state conditions of RBC folate after initiation of chronic low-to-moderate intakes of folic acid. To our knowledge, this was the longest trial conducted that measured the appearance of folate and its rate of saturation in RBCs. Despite the prolonged duration, we found no evidence that RBC folate concentrations had reached steady state in either treatment group over the 40-wk intervention period. Kinetic modeling of the time course data indicated that RBC folate concentrations would approximately double with an increased intake of 140 μg folic acid/d (≥70% known compliance), with only ≈ 50% of model-estimated steady state conditions achieved at 40 wk. Thus, the time required to reach steady state is considerably greater than the pharmacokinetic model proposed by Pietrzik et al (18), and the prolonged nature and design of our study allowed us to present a model depicting the response of RBC folate with a greater degree of certainty than in previous investigations. With the knowledge that nearly half of the increase in RBC folate would potentially occur after 40 wk, these findings demonstrate that shorter-term intervention trials underestimate the full effect of this vitamin. Moreover, the potential effect of the chronic intake of low-dose folic acid as delivered by means of the North American mandatory fortification program was likely underappreciated.
This study was not designed to present an intention-to-treat analysis of supplement use; rather, the focus was motivated by the desire to investigate the long-term effects of low-dose folic acid intake similar to the level of intake provided from mandatory fortification. As such, participants with low reported compliance (who do not accurately represent a fortified population, which by its very nature leads to high compliance) were not of interest. The treatment compliance threshold of 70% was selected before data modeling. To avoid biases potentially arising from a comparison of compliant with noncompliant participants, the same criteria were used for the placebo group. An inspection of the compliance data showed that increasing the level to 80% would have reduced the sample size available for analysis by another 21 participants, which would have appreciably decreased the statistical power of the tests and the precision of the estimates. However, our inclusion of less-compliant participants (ie, <95% compliance) introduced a potential source of error in the analysis with regard to the response of RBC folate to varying doses, and caution should be taken when interpreting and applying the absolute quantitative results of this model. Nonetheless, whole-body folate turnover is highly variable (23–25), and a substantial amount of data would have been required to precisely estimate the given parameters. Determination of the exact plateau or the exact time required to achieve steady state was not the objective of this study; rather, we aimed to yield an approximation of the rate of RBC folate appearance.
Another limitation of our analysis was that we assumed that the changes in RBC folate would behave exponentially in a first-order fashion, which we recognize is an oversimplification. If the body's total folate truly behaved kinetically in a first-order process, the mean residence time of folate (ie, 1/k), which is influenced primarily by the rate of large, slow-turnover tissue pools (23), would be neither affected by the intake of folate nor the initial concentration of RBC folate. However, evidence derived from studies using folate tracers indicate that the turnover of folate pools is greatly accelerated with both increased folate intake and higher folate status (25–27). For example, kinetic evaluation of folate metabolism with the use of folates labeled with stable isotopes in nonpregnant women fed diets that provided intakes of 200, 300, or 400 μg of folate/d for 10 wk found that mean residence times for total-body folate were 212, 169, and 124 d, respectively (25). Thus, the use of a single exponential term for both treatment groups in our model is a further simplification, although there was no statistical evidence that this term varied between treatment groups. Finally, the concept of steady state assumes that all variables of the system are constant, including dietary folate intake. In New Zealand, there is no mandatory folic acid fortification of a staple food. Since 1996, legislation in New Zealand has permitted voluntary fortification of certain food products with folic acid, including breakfast cereals, breads, pasta, and fruit and vegetable juices. With the exception of breakfast cereals, there has not been any significant uptake by the food industry of voluntary fortification. Food Standards Australia New Zealand estimates a mean intake of folic acid from voluntary fortified foods among reproductive women to be 62 μg/d (28). We did not attempt to estimate dietary folate or folic acid intakes from fortified foods because the food-composition database is incomplete. However, plasma (data not shown) and RBC folate concentrations were similar in all groups at baseline, which suggests that dietary intakes did not differ markedly. Likewise, no evidence of a change in blood folate concentrations over time was found in the placebo group. Therefore, the differences in RBC folate concentrations between groups reflect the effects of the study supplementation.
These results are timely given that the introduction of mandatory folic acid fortification, and the optimal level of fortification, is still under debate in many countries. To reduce NTD risk, most public health authorities worldwide recommend the consumption of 400 μg folic acid/d 12 wk before pregnancy until the end of the first trimester. This dose is based mainly on the amount of folic acid observed to be associated with a reduction in NTDs in most epidemiologic studies (8–12). Although the minimum effective blood folate concentration for the maximum NTD protection is unknown, Daly et al (29) documented an inverse relation between NTD risk and RBC folate concentrations based on retrospective measurements of folate status at a median of 15 wk gestation. After 12 wk of continuous intake of 400 μg folic acid/d, RBC folate concentrations in our folate-replete study population increased by ≈200 nmol/L, reaching an estimated concentration of 1068 nmol/L. Although the time required was greater, a similar concentration of RBC folate was achieved after 36 wk in the participants who consumed a daily intake of 140 μg folic acid/d. Moreover, RBC folate concentrations were estimated to increase by an additional 300 nmol/L after continuous intake. Our model hypothetically showed that, given sufficient time to achieve steady state conditions, an extra 140 μg folic acid/d (a typical amount delivered to women of reproductive age by means of food fortification) (6, 7) could be as protective as the current recommended periconceptional dose of 400 μg folic acid/d. This is of particular interest given the recent evidence that fortified foods in the United States may be providing a sufficient level of folic acid to prevent most folate-related NTDs (4, 5).
Estimating the elimination rate of folate in RBCs to that of a new lower steady state after the discontinuation of folic acid intake would further the application of this working model. It is unlikely that the appearance rate of RBC folate is equal to the rate of elimination because whole-body folate turnover would be altered (ie, decreased) because of the loss of dietary folate intake. The proposal by Pietrzik et al (18) to add folic acid to oral contraceptive agents as a means to increase blood folate concentrations in reproductive-aged women highlights the importance of understanding the elimination rate for the purposes of approximating the length of time required to maintain optimal folate status after the discontinuation of oral contraceptive agents.
In conclusion, the major objective in modeling of this type is to yield an approximation of the rate of attainment of the new steady state after initiation of a dietary treatment. Despite the complexity of whole-body folate physiology and metabolism, our findings provide a working model for estimating the appearance behavior of RBC folate under nutritionally relevant conditions. In doing so, we showed the relative, and likely underappreciated, potency of low-dose folic acid after chronic exposure to low-to-moderate intakes of folic acid. Additional long-term supplementation studies are needed to confirm the appearance behavior of RBC folate in response to various doses and forms of folic acid and to investigate the rate of elimination after dosage termination.
Acknowledgments
We thank Ivy Salihs and Holiday Wilson for their assistance in carrying out the microbiological assay and research nurse Margaret Waldron for blood collection.
The authors’ responsibilities were as follows—LAH: designed the research; MCR, JCM, and NAH: conducted the research; ARG: analyzed the data; LAH, ARG, and JFG: wrote the manuscript; and LAH: had primary responsibility for the final content. All authors read and approved the final manuscript. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.MRC Vitamin Study Research Group Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 1991;338:131–7 [PubMed] [Google Scholar]
- 2.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832–5 [DOI] [PubMed] [Google Scholar]
- 3.Berry RJ, Li Z, Erickson JD, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med 1999;341:1485–90 [DOI] [PubMed] [Google Scholar]
- 4.Mosley BS, Cleves MA, Siega-Riz AM, et al. Neural tube defects and maternal folate intake among pregnancies conceived after folic acid fortification in the United States. Am J Epidemiol 2009;169:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mills JL, Carter TC. Invited commentary: preventing neural tube defects and more via food fortification? Am J Epidemiol 2009;169:18–21, discussion 22–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey RL, Dodd KW, Gahche JJ, et al. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003-2006. Am J Clin Nutr 2010;91:231–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shakur YA, Garriguet D, Corey P, O'Connor DL. Folic acid fortification above mandated levels results in a low prevalence of folate inadequacy among Canadians. Am J Clin Nutr 2010;92:818–25 [DOI] [PubMed] [Google Scholar]
- 8.Mulinare J, Cordero JF, Erickson JD, Berry RJ. Periconceptional use of multivitamins and the occurrence of neural tube defects. JAMA 1988;260:3141–5 [PubMed] [Google Scholar]
- 9.Milunsky A, Jick H, Jick SS, et al. Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA 1989;262:2847–52 [DOI] [PubMed] [Google Scholar]
- 10.Werler MM, Shapiro S, Mitchell AA. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA 1993;269:1257–61 [PubMed] [Google Scholar]
- 11.Shaw GM, Schaffer D, Velie EM, Morland K, Harris JA. Periconceptional vitamin use, dietary folate, and the occurrence of neural tube defects. Epidemiology 1995;6:219–26 [DOI] [PubMed] [Google Scholar]
- 12.Khoury MJ, Shaw GM, Moore CA, Lammer EJ, Mulinare J. Does periconceptional multivitamin use reduce the risk of neural tube defects associated with other birth defects? data from two population-based case-control studies. Am J Med Genet 1996;61:30–6 [DOI] [PubMed] [Google Scholar]
- 13.Quinlivan EP, Gregory JF., III Reassessing folic acid consumption patterns in the United States (1999 2004): potential effect on neural tube defects and overexposure to folate. Am J Clin Nutr 2007;86:1773–9 [DOI] [PubMed] [Google Scholar]
- 14.Shane B. Folate chemistry and metabolism. In: Bailey LB, ed. Folate in health and disease. Boca Raton, FL: CRC Press, 2010:1–24 [Google Scholar]
- 15.Venn BJ, Green TJ, Moser R, McKenzie JE, Skeaff CM, Mann J. Increases in blood folate indices are similar in women of childbearing age supplemented with [6S]-5-methyltetrahydrofolate and folic acid. J Nutr 2002;132:3353–5 [DOI] [PubMed] [Google Scholar]
- 16.Hao L, Yang QH, Li Z, et al. Folate status and homocysteine response to folic acid doses and withdrawal among young Chinese women in a large-scale randomized double-blind trial. Am J Clin Nutr 2008;88:448–57 [DOI] [PubMed] [Google Scholar]
- 17.Lamers Y, Prinz-Langenohl R, Bramswig S, Pietrzik K. Red blood cell folate concentrations increase more after supplementation with [6S]-5-methyltetrahydrofolate than with folic acid in women of childbearing age. Am J Clin Nutr 2006;84:156–61 [DOI] [PubMed] [Google Scholar]
- 18.Pietrzik K, Lamers Y, Bramswig S, Prinz-Langenohl R. Calculation of red blood cell folate steady state conditions and elimination kinetics after daily supplementation with various folate forms and doses in women of childbearing age. Am J Clin Nutr 2007;86:1414–9 [DOI] [PubMed] [Google Scholar]
- 19.Hursthouse NA, Gray AR, Miller JC, Rose MC, Houghton LA. Folate status of reproductive age women and neural tube defect risk: the effect of long-term folic acid supplementation at doses of 140 μg and 400 μg per day. Nutrients 2011;3(1):49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Statistics New Zealand Statistical standard for ethnicity, 2005. Wellington, New Zealand: Statistics New Zealand, 2005 [Google Scholar]
- 21.Lohman TJ. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Publications, 1998 [Google Scholar]
- 22.Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol 1997;281:43–53 [DOI] [PubMed] [Google Scholar]
- 23.Gregory JF, 3rd, Quinlivan EP. In vivo kinetics of folate metabolism. Annu Rev Nutr 2002;22:199–220 [DOI] [PubMed] [Google Scholar]
- 24.Gregory JF, III, da Silva VR, Lamers Y. Kinetics of folate and one-carbon metabolism. In: Bailey LB, ed. Folate in health and disease. Boca Raton, FL: CRC Press, 2010:491–516 [Google Scholar]
- 25.Gregory JF, III, Williamson J, Liao JF, Bailey LB, Toth JP. Kinetic model of folate metabolism in nonpregnant women consuming [2H2]folic acid: isotopic labeling of urinary folate and the catabolite para-acetamidobenzoylglutamate indicates slow, intake-dependent, turnover of folate pools. J Nutr 1998;128:1896–906 [DOI] [PubMed] [Google Scholar]
- 26.O'Keefe CA, Bailey LB, Thomas EA, et al. Controlled dietary folate affects folate status in nonpregnant women. J Nutr 1995;125:2717–25 [DOI] [PubMed] [Google Scholar]
- 27.Wolfe JM, Bailey LB, Herrlinger-Garcia K, Theriaque DW, Gregory JF, III, Kauwell GP. Folate catabolite excretion is responsive to changes in dietary folate intake in elderly women. Am J Clin Nutr 2003;77:919–23 [DOI] [PubMed] [Google Scholar]
- 28.Segal L, Dalziel K, Katz R. A report to FSANZ. Informing a strategy to increase folate levels to prevent neural tube defects: a cost-effectiveness analysis of options. Adelaide, Australia: Centre for Health Economics, Monash University and Division of Health Sciences, University of South Australia, 2007 [Google Scholar]
- 29.Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM. Folate levels and neural tube defects: implications for prevention. JAMA 1995;274:1698–702 [DOI] [PubMed] [Google Scholar]