Abstract
Little is known about the expression of heme transporters in human placenta and possible associations between these transporters and maternal or neonatal iron status. To address this area of research, relative protein expression of 2 heme transporters, Feline Leukemia Virus, Subgroup C, Receptor 1 (FLVCR1) and Breast Cancer Resistance Protein (BCRP), was assessed using Western-blot analysis in human placental tissue in relation to maternal/neonatal iron status and placental iron concentration. Placental FLVCR1 (n = 71) and BCRP (n = 83) expression were assessed at term (36.6–41.7 wk gestation) in a cohort of pregnant adolescents (13–18 y of age) at high-risk of iron deficiency. Both FLVCR1 and BCRP were detected in all placental samples assayed. Placental FLVCR1 expression was positively related to placental BCRP expression (n = 69; R2 = 0.104; P < 0.05). Adolescents that were anemic at delivery had lower placental FLVCR1 expression (n = 49; P < 0.05). Placental FLVCR1 expression was positively associated with placental iron concentration at delivery (n = 61; R2 = 0.064; P < 0.05). In contrast, placental BCRP expression was not significantly associated with maternal iron status or placental iron content. Both FLVCR1 and BCRP are highly expressed in human placental tissue, but only FLVCR1 was significantly inversely associated with maternal iron status and placental iron concentration. Further analysis is needed to explore potential functional roles of FLVCR1 in human placental iron transport.
Introduction
Iron is one of the most abundant metals in the human body (1). During pregnancy, substantial amounts of iron are trafficked across the human placenta to endow the neonate with ~300 mg of iron at birth (2). Neonatal body iron content at term (75 mg/kg) is nearly twice that of an adult female (3, 4). To meet the high iron demands of pregnancy, women must absorb sufficient iron from dietary (nonheme and heme iron) and/or supplemental (nonheme) sources. Because there are no physiologically regulatable routes of iron loss from the body, iron transport across the enterocyte is limited even in the face of high iron demands (4). Thus, the increased iron demands of pregnancy are often difficult to meet from diet alone and women often rely on prepregnancy body iron reserves and/or iron supplementation.
Many of the proteins integral to nonheme iron absorption have been identified over the past 10–15 y (5). In contrast, much less is known about the cellular proteins involved in intestinal or placental heme iron trafficking, but heme absorption is known to be less influenced by iron status compared with absorption of nonheme iron (6, 7). Recently, the Feline Leukemia, Subgroup C, Receptor 1 (FLVCR1)7 and the Breast Cancer Resistance Protein (BCRP) have been identified as having roles in cellular heme transport (8, 9).
BCRP is a member of the ATP-binding cassette efflux transporter family (9) and is responsible for multidrug resistance in cancer cells (10, 11). The placenta has consistently been shown to have the highest level of BCRP mRNA (9, 12) and this protein localizes to the apical membrane of the syncytiotrophoblast (13). BCRP knockout mice (Bcrp1−/−) are viable and fertile but exhibit a unique form of protoporphyria (10× greater erythrocyte protoporphyrin IX), suggesting that partial or complete lack of BCRP in humans may affect iron metabolism and lead to porphyrin-related phototoxicities (14).
FLVCR1 functions as a receptor for Feline Leukemia Virus, Subgroup C, a retrovirus that induces pure red-cell aplasia in viremic felines (15). To date, FLCVR1 has been identified in over 15 human tissues and, as observed for BCRP, the highest tissue expression of FLVCR1 is found in the placenta (16). Studies investigating the role of FLVCR1 in erythropoiesis have determined that FLVCR1 exports heme and cell surface expression of this heme export protein decreases as erythropoiesis proceeds (8). These findings suggest that FLVCR1 may control intracellular heme content as heme synthesis increases to support erythrocyte differentiation (17). FLVCR1 may also play a role in the etiology of Diamond Blackfan Anemia (a congenital human pure red-cell aplasia) (18, 19). Recently, FLVCR2 was also identified as a cell surface heme transporter, but at present much less is known about this FLVCR1 homolog (20).
The functions of BCRP and FLVCR1 in the placenta and potential relationships between these cellular heme iron-trafficking proteins and maternal and/or neonatal iron status remain unexplored. The objectives of this study were to determine the relative protein expression of FLVCR1 and BCRP in term placental tissue obtained from pregnant adolescents. Possible associations between these 2 heme transporters and maternal/neonatal iron status and placental iron concentration were explored in a group known to be at high risk for iron deficiency.
Methods
Participants.
Placental tissue utilized in this study was obtained from 2 USDA-funded studies addressing relationships between maternal and neonatal nutritional status in pregnant adolescents. The first study was designed to assess longitudinal changes in maternal and fetal bone health across gestation. The second study was undertaken to explore iron status in archived serum from the first cohort and additional pregnant adolescents were recruited to explore the impact of maternal iron status on neonatal iron status and functional neonatal outcomes at birth. In both studies, adolescents (≤18 y of age) were recruited from the Rochester Adolescent Maternity Program in Rochester, NY. At mid-gestation (25.3 ± 3.4 wk), maternal blood was collected and maternal blood, cord blood, and placental tissue were obtained at delivery (39.8 ± 1.2 wk). Adolescents with HIV infection, eating disorders, malabsorption diseases, diabetes, gestational hypertension, or any other diagnosed medical conditions were excluded from the study. Race and ethnicity of adolescents were self-reported. All procedures were approved by the Institutional Review Boards at Cornell University and the University of Rochester and written informed consent was obtained from all study participants. To date, data on placental transferrin receptor expression in this cohort were previously reported (21).
Placenta collection and processing.
Placentas were collected shortly after birth. For protein isolations, 4–5 randomly selected placental sections were dissected from multiple cotyledons and maternal and fetal membranes were removed. Each section was divided into quarters, randomly distributed into 10 aliquots, and flash frozen until analysis. Thawed placental tissue was rinsed thoroughly with a 0.9% saline solution containing a protease inhibitor cocktail (Sigma-Aldrich) to remove surface hemoglobin (Hb). Tissue was then homogenized at ~5000 rpm in hypertonic lysis buffer and centrifuged at 14,000 × g for 15–25 min at 4°C. Protein concentrations in the supernatant were determined using a BIO-RAD assay. Lysates were diluted in SDS sample buffer and stored at −20°C until use in Western-blot analyses.
Placental total iron assessment.
Approximately 1.0 g of placental tissue (containing both maternal and fetal membranes) was collected and rinsed thoroughly in sterile deionized water to remove surface Hb. Tissue was then dried to constant weight and digested in Ultrex HNO3 using an Ethos EZ microwave digestion system (Milestone). Acid digests were completely evaporated and resuspended in 0.5% Ultrex HNO3. The total iron concentration was determined using atomic absorption spectrophotometry (Perkin Elmer AAnalyst Model 800, Perkin Elmer Instruments). Data were expressed as μmol iron/g placental dry weight (1 mg iron = 55.8 mmol iron).
Western-blot analysis.
For assessment of FLVCR1, protein isolates were run on SDS-PAGE gels and transferred to polyvinylidene difluoride fluorescence membranes (Millipore). Membranes were blocked in Odyssey blocking buffer (LI-COR Biosciences) for 1 h at room temperature and subsequently probed overnight at 4°C with FLVCR1 antibody diluted 1:5000 in a 1:1 dilution of Odyssey blocking buffer to 1× PBS containing 0.1% Tween. FLVCR1 antibody was provided by Janis Abkowitz (University of Washington, Department of Medicine/Hematology); characteristics of this antibody were previously published (8, 16). Membranes were rinsed and probed for 1 h at room temperature with IRDye 680 conjugated goat (polyclonal) anti-rabbit IgG (LI-COR Biosciences) diluted 1:5000 in 1:1 dilution of Odyssey blocking buffer to 1× PBS with 0.1% Tween and then scanned and quantified using an Odyssey machine (Li-Cor Biosciences). Βeta-Actin (Santa Cruz Biotechnology) was used as a loading control and human liver lysate (Abcam) was used as a membrane control.
Assessment of BCRP expression was undertaken using a commercial BCRP antibody (1:250 dilution of clone BXP-21; Kamiya Biomedical) that was previously found to recognize placental BCRP expression (22). The secondary antibody utilized [IRDye 800 conjugated goat (polyclonal) anti-mouse IgG (LI-COR Biosciences)] was diluted 1:5000. Beta-actin was used as a loading control and human placental homogenate as a membrane control. Methods for determination of placental transferrin receptor expression in this cohort were previously reported (21).
Biochemical assessment.
Maternal and neonatal Hb concentrations were determined in whole blood using the Cell Dyn 4000 system (Abbott Laboratories). Serum ferritin (SF) and transferrin receptor (sTfR) (Ramco Laboratories) and maternal estradiol (ALPCO) were measured using enzyme immunoassays.
Maternal anemia was defined as Hb < 105 g/L (second trimester) or < 110 g/L (3rd trimester) according to CDC guidelines (23). Adolescents with SF < 12 μg/L were classified as having depleted iron stores (24) and those with sTfR > 8.5 mg/L were classified as having tissue iron deficiency (25). Iron depletion was defined if the sTfR:SF ratio was >300 using criteria previously developed among pregnant women (26). Total body iron (mg/kg) was calculated as −{log[sTfR(μg/L)/SF(μg/L)] − 2.8229/0.1207} (27).
Statistical analysis.
All data analyses were conducted using JMP 8.0 (SAS Institute). We used descriptive statistics to analyze general participant characteristics and maternal/neonatal iron status indicators. Post hoc paired t tests were used to compare maternal iron status indicators at mid-gestation and delivery and 2-sided t tests were used to assess differences in neonatal iron status indicators in those born to anemic compared with nonanemic mothers at delivery. A one-way ANOVA was used to evaluate possible differences in placental FLVCR1 expression between anemic compared with nonanemic adolescents at delivery, to evaluate the impact of race on placental BCRP expression, and to assess differences in maternal estradiol concentration between Caucasians and African Americans. A square root transformation of placental BCRP expression was used to normalize the residuals. Values > 3 SD above the mean for placental BCRP or FLVCR1 expression were defined as outliers and were excluded from all analyses. A simple linear regression model was used to evaluate the correlation between placental FLVCR1 and BCRP expression and maternal/neonatal iron status indicators as well as placental iron concentration and maternal estradiol. Values are presented as mean ± SD. Significance was considered for P < 0.05.
Results
Participant characteristics.
The mean age at enrollment into the study was 17.0 ± 1.1 y (n = 83) (Table 1). Twenty-three percent (n = 19/83) of the adolescents were overweight (BMI > 25 kg/m2) and 19% (n = 16/83) were obese (BMI > 30 kg/m2) based on self-reported prepregnancy weight at entry into the study. Mean maternal weight gain during pregnancy was 17.1 ± 6.8 kg (n = 83) and 67% (n = 56/83) gained more than the 2009 Institute of Medicine’s recommended weight gain based on prepregnancy BMI (28). Fifty-four percent (n = 45/83) of neonates were boys and 46% (n = 38/83) were girls. Among the adolescents studied, 11% (n = 9/83) reported that they were currently smoking cigarettes.
TABLE 1.
General characteristics of pregnant adolescents1
| Variable | |
| Age at enrollment, y | 17.0 ± 1.1 (13.6–18.7) |
| Parity ≥ 1, % | 16 (0–2) |
| Prepregnancy BMI, kg/m2 | 25.4 ± 5.6 (15.2–41.9) |
| Gestational age at mid-gestation blood draw, wk | 25.3 ± 3.4 (19.7–38.1) |
| Gestational age at delivery, wk | 39.8 ± 1.2 (36.6–41.7) |
| Gynecological age at enrollment, y | 5.3 ± 2.0 (1.6–11.3) |
| Race, % | |
| African American | 64 |
| Caucasian | 35 |
| American Indian | 1 |
| Ethnicity, % | |
| Hispanic | 31 |
| Non-Hispanic | 69 |
| Birth weight, g | 3273 ± 475 (2150–4705) |
| Birth length, cm | 51.1 ± 2.4 (45.5–57.0) |
Values are mean ± SD (range) or percentage (range), = 83.
At delivery, 29% (n = 22/75) of adolescents had low iron stores (SF < 12 μg/L) and 12% (n = 9/75) had tissue iron deficiency (sTfR > 8.5 mg/L) (Table 2). Twenty percent (n = 14/71) of neonates had SF < 76 μg/L, a cutoff previously shown to be associated with subsequent impaired mental and psychomotor function at 5 y of age (29). A positive linear relationship between maternal SF and neonatal SF at delivery approached significance (P = 0.056; R2 = 0.054; n = 68). No significant differences in maternal estradiol at mid-gestation (P = 0.21; n = 67) or at delivery (P = 0.54; n = 74) were observed between African American and Caucasian adolescents.
TABLE 2.
Maternal and neonatal iron status indicators in pregnant adolescents1
| Mothers |
Neonates |
|||||||
| Variable | Mid-gestation | n | Delivery | n | Nonanemic mothers2 | n | Anemic mothers | n |
| Hb, g/L | 113 ± 9.95 (87.0–133) | 41 | 116 ± 15.1 (65.0–146) | 55 | 151 ± 18.8 (113–175) | 16 | 144 ± 11.6 (125–163)# | 9 |
| SF, μg/L | 22.1 ± 17.3 (0.700–76.0) | 68 | 21.3 ± 13.2 (1.80–65.9) | 75 | 132 ± 77.3 (27.8–408) | 37 | 149 ± 73.2 (31.1–329) | 14 |
| sTfR, mg/L | 4.63 ± 1.62 (1.66–11.3) | 68 | 5.46 ± 3.56 (0.125–26.4)* | 75 | 7.76 ± 2.19 (3.91–14.5) | 37 | 7.85 ± 2.94 (4.44–13.4)# | 15 |
| Total body iron,3 mg/kg | 3.24 ± 3.70 (−10.3–9.59) | 68 | 3.24 ± 3.74 (-9.14–14.9) | 74 | 8.27 ± 2.46 (2.51–12.8) | 37 | 8.94 ± 2.52 (4.79–13.1) | 14 |
Values are mean ± SD (range). *Different from mid-gestation, < 0.05; #different from nonanemic mothers, P < 0.05.
Maternal anemia defined as Hb < 110 g/L at delivery.
1 mg iron = 55.8 mmol iron.
Placental expression of FLVCR1.
All placental tissue analyzed had detectable levels of FLVCR1 expression across the range at which deliveries occurred in this cohort (36.6–41.7 wk gestation) (Fig. 1A). Placental FLVCR1 was not significantly associated with maternal age, gestational age at delivery, smoking, prepregnancy BMI, pregnancy weight gain, parity, race, ethnicity, mid-gestation or delivery estradiol, placental weight, or neonatal gender. An inverse trend between placental FLVCR1 expression and neonatal weight at delivery approached significance (P = 0.058; R2 = 0.051; n = 71).
FIGURE 1.
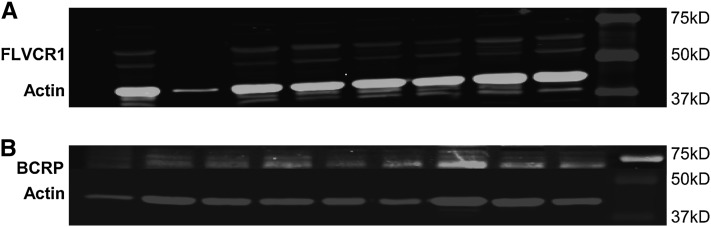
Representative images of FLVCR1 Western blot (A) and BCRP Western blot (B). The mean ± SD for normally distributed placental FLVCR1/Actin was 0.0595 ± 0.0305 (n = 71). The mean ± SD for non-normally distributed placental BCRP/Actin was 0.0708 ± 0.0456, n = 83.
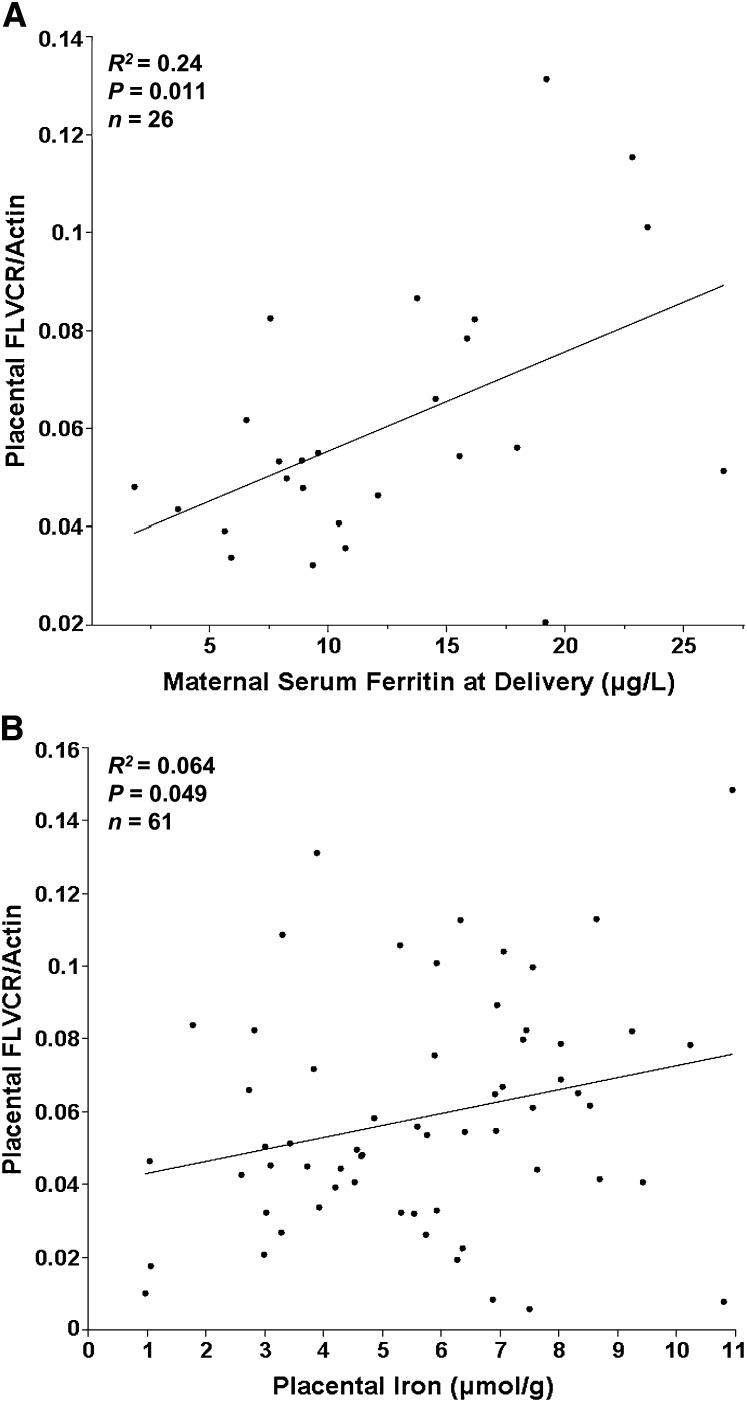
Adolescents that were anemic at delivery [29% (n = 16/55); Hb < 110 g/L] had significantly lower placental FLVCR1 expression (P = 0.034; n = 49). Similarly, in iron-depleted adolescents [42% (n = 31/74); sTfR: SF > 300], maternal delivery SF was positively related to placental FLVCR1 expression (P = 0.011; R2 = 0.24; n = 26) (Fig. 2A). This relationship was not evident in adolescents with sufficient iron stores (sTfR:SF < 300). Mean placental iron concentration was 5.72 ± 2.4 μmol iron/g placental dry weight (n = 72) and was directly related to placental FLVCR1 (Fig. 2B; P = 0.049; R2 = 0.064; n = 61). Placental FLVCR1 expression was not significantly associated with neonatal SF, total body iron, or maternal or neonatal sTfR.
FIGURE 2.
Associations of placental FLVCR1 protein expression with maternal SF in adolescents with depleted iron (sTfR:SF > 300) (A) and with iron concentration of placental tissue (μmol iron/g dry weight of placental tissue) (B).
Placental expression of BCRP.
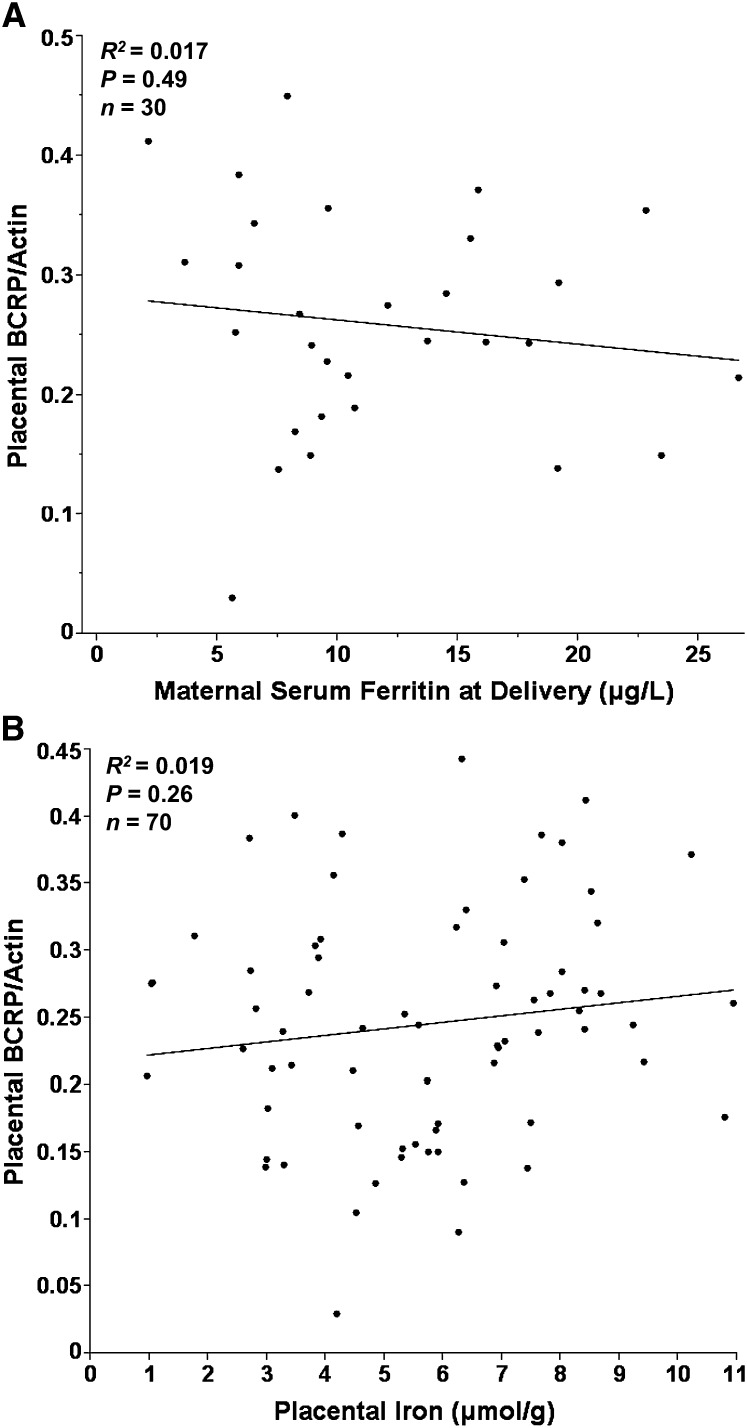
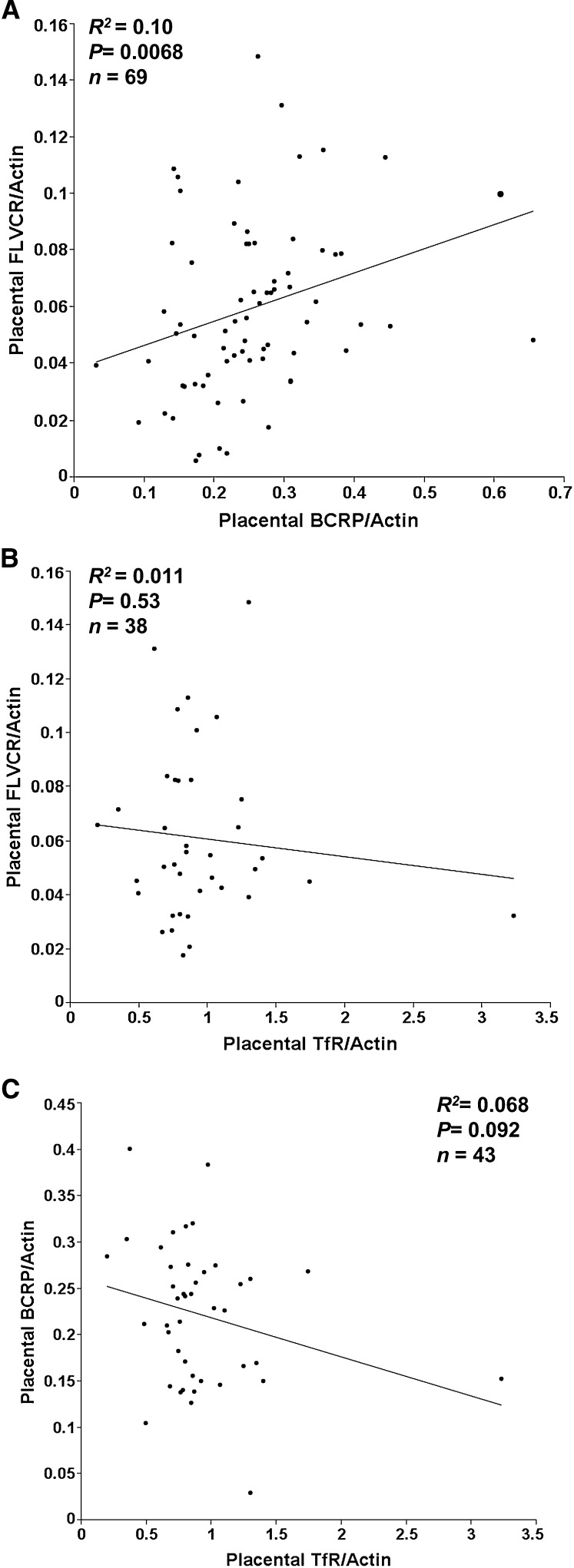
As observed for FLVCR1, all placentas analyzed (36.6–41.7 wk gestation) had detectable levels of BCRP expression (Fig. 1B). Two BCRP values were >3 SD above the mean and were excluded from all subsequent analyses. Placental BCRP expression was not associated with maternal or neonatal SF or placental iron concentration (Fig. 3A,B), nor was it associated with other iron status indicators measured. Furthermore, no significant associations were evident between BCRP and maternal age, gestational age at delivery, maternal smoking, prepregnancy BMI, pregnancy weight gain, parity, ethnicity, placental weight, neonatal weight, or neonatal gender. In this population, African American adolescents (n = 51) had higher levels of placental BCRP expression (P = 0.034) compared with Caucasians (n = 29). A direct relationship was observed between placental BCRP expression and maternal estradiol (P = 0.022; R2 = 0.079; n = 66) at mid-gestation but not at delivery. A direct relationship was observed between relative placental FLVCR1 expression and relative placental BCRP expression (Fig. 4A; P = 0.007; R2 = 0.10; n = 69). No significant relationships were evident between either of the heme transporters and placental expression of TfR (a cellular receptor for nonheme iron) (Fig. 4B,C).
FIGURE 3.
Associations of placental BCRP protein expression with maternal SF in adolescents with depleted iron (sTfR:SF > 300) (A) and iron concentration of placental tissue (μmol/g dry weight of placental tissue) (B).
FIGURE 4.
Associations of placental FLVCR1 and BCRP (A), FLVCR1 and transferrin receptor (B), and BCRP and transferrin receptor (C).
Discussion
We found that both heme transport proteins examined, FLVCR1 and BCRP, were highly expressed in all placental tissue analyzed. The function of these 2 heme transport proteins in the human placenta is unknown and may be multifactorial. Heme is known to play a regulatory role through its ability to affect gene transcription and translation (30). Heme is also an integral component of many hemoproteins, including those involved in oxygen transport and storage (Hb and myoglobin), electron transfer, drug and steroid metabolism (cytochromes), and signal transduction (nitric oxide synthases, guanylate cyclase) (1).
FLVCR1 has recently been reported to be present at sites of high-heme flux, including the placenta, liver, duodenum, macrophages, and liver (16). In nonerthryoid cells, FLVCR1 functions in heme iron trafficking, and in erythroid cells, FLVCR1 may prevent heme toxicity by exporting excess heme that is not needed for erythrocyte formation. Hemopexin, a systemic protein that transports free heme, has recently been found to be necessary for FLVCR1 function and both proteins are necessary for heme iron trafficking and maintenance of systemic iron balance (31). Hemopexin itself has been shown to be capable of downregulating the expression of the human placental transferrin receptor (32).
Although many advances have been made in explaining the role of FLVCR1 in systemic iron homeostasis and erythropoiesis, little is understood about the role of this protein within the placenta. FLVCR1 in the human placenta may function as it does in the erythroid cell to prevent accumulation of free heme that may be presented to the placenta bound to hemopexin from intravascular RBC catabolism. Approximately 10% of RBC catabolism is thought to occur intravascularly and the heme released is transported in the circulation bound to hemopexin (33). Macrophages may aid in heme iron recycling as they release heme to hemopexin during phagocytosis of senescent RBC. Tissue utilization of scavenged heme from intravascular Hb breakdown is known to occur in other body tissues, such as the proximal tubules of the kidney (34).
FLVCR1 may serve other placental iron-trafficking functions to support the 3–8 mg/d of iron that are transferred to the fetus during the 3rd trimester of pregnancy (35). Consistent with this possibility, a significant relationship was observed between placental FLVCR1 expression and maternal iron status. Maternal anemia was associated with a significantly lower expression of placental FLVCR1, and in adolescents with depleted iron stores, placental FLVCR1 expression was directly correlated with maternal iron status at delivery. Approximately 24% of the variability in placental FLVCR1 expression in these adolescents could be explained by maternal iron reserves (SF) at delivery. Moreover, higher placental iron concentration was associated with higher expression of FLVCR1. The positive association we observed between FLVCR1 and placental iron concentration supports our finding of lower levels of placental FLVCR1 expression in anemic compared with nonanemic adolescents. Few normative data on placental iron concentration have been published and existing data have been obtained using relatively small sample sizes. The mean placental iron concentration we found, 319.7 ± 132.6 μg/g (n = 72), is considerably higher than that reported by Langini et al. [170 ± 56 μg/g (n = 38) (36)] but considerably lower than that reported by Osada et al. [~720 μg/g (n = 30) (37)]. However, our sampling method (representative sampling of the entire placenta with homogenization of collected samples) differed from that used in prior studies (a single placental sample obtained only from the immediate umbilical insertion area) (36, 37).
In contrast to our observed FLVCR1 findings, placental BCRP was not associated with any of the maternal iron status indicators assessed in this study. Placental BCRP was, however, significantly correlated with maternal estradiol at mid-gestation but not at delivery. The observed relationship at mid-gestation is consistent with the finding of an estrogen response element in the promoter of the BCRP gene (ABCG2) (38) and with the previous report that placental BCRP expression is highest at mid-gestation (39). The variable estrogen concentrations that can occur during the labor process (40) may explain why we found no significant relationship between placental BCRP expression and estradiol concentrations at parturition.
African American adolescents in our study had significantly higher BCRP expression compared with the Caucasian adolescents. This did not appear to be due to estrogen, because no significant differences in estrogen were evident between the African American and Caucasian adolescents studied. Previous work on the frequency of BCRP SNPs in various racially diverse populations (n = 222) found that 25.9% of Caucasians and 0% of African Americans had the C421A SNP in exon 5 of the BCRP gene (41). This SNP has been shown to be associated with lower levels of BCRP and therefore these individuals may express lower levels of the BCRP protein (22).
To date, little information is known concerning the determinants and mechanisms of iron transport in the placenta. These data confirm the expression of 2 heme transport proteins in the human placenta and suggest that the expression of the placental heme iron transporter FLVCR1 is associated with maternal iron status and placental tissue iron concentration. Given the growing body of literature highlighting the importance of neonatal iron status at birth on subsequent cognitive and behavioral outcomes, more work on mechanisms of placental heme iron transport and the role of heme transporters in relation to fetal iron status is needed.
Acknowledgments
K.O., M.Y., T.M., E.C., E.P., A.M., M.O., and R.G. assisted with participant recruitment and all clinical aspects of the study; and K.O. and L.J. prepared the manuscript. All authors read and approved the final manuscript.
Footnotes
Supported by National Research Initiative grants 2008-01857 and 2005-35200 from the USDA National Institute of Food and Agriculture. Additional support was provided by the Howard Hughes Medical Institute.
This trial was registered at clinicaltrials.gov as NCT01019902.
Abbreviations used: BCRP, Breast Cancer Resistance Protein; FLVCR1, Feline Leukemia Virus, Subgroup C, Receptor 1; Hb, hemoglobin; SF, serum ferritin; sTfR, serum transferrin receptor.
Literature Cited
- 1.Lieu PT, Heiskala M, Peterson PA, Yang Y. The roles of iron in health and disease. Mol Aspects Med. 2001;22:1–87 [DOI] [PubMed] [Google Scholar]
- 2.Widdowson EM, Spray CM. Chemical development in utero. Arch Dis Child. 1951;26:205–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widdowson EM. Trace elements in fetal and early postnatal development. Proc Nutr Soc. 1974;33:275–84 [DOI] [PubMed] [Google Scholar]
- 4.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–95 [DOI] [PubMed] [Google Scholar]
- 5.Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook JD. Adaptation in iron metabolism. Am J Clin Nutr. 1990;51:301–8 [DOI] [PubMed] [Google Scholar]
- 7.Hallberg L, Hulten L, Gramatkovski E. Iron absorption from the whole diet in men: how effective is the regulation of iron absorption? Am J Clin Nutr. 1997;66:347–56 [DOI] [PubMed] [Google Scholar]
- 8.Quigley JG, Yang Z, Worthington MT, Phillips JD, Sabo KM, Sabath DE, Berg CL, Sassa S, Wood BL, et al. Identification of a human heme exporter that is essential for erythropoiesis. Cell. 2004;118:757–66 [DOI] [PubMed] [Google Scholar]
- 9.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol. 2006;46:381–410 [DOI] [PubMed] [Google Scholar]
- 11.Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. ABCG2: a perspective. Adv Drug Deliv Rev. 2009;61:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene. 2003;22:7340–58 [DOI] [PubMed] [Google Scholar]
- 13.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–64 [PubMed] [Google Scholar]
- 14.Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci USA. 2002;99:15649–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tailor CS, Willett BJ, Kabat D. A putative cell surface receptor for anemia-inducing feline leukemia virus subgroup C is a member of a transporter superfamily. J Virol. 1999;73:6500–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, Kingsley PD, Domenico ID, Vaughn MB, et al. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–8 [DOI] [PubMed] [Google Scholar]
- 17.Wickrema A, Krantz SB, Winkelmann JC, Bondurant MC. Differentiation and erythropoietin receptor gene expression in human erythroid progenitor cells. Blood. 1992;80:1940–9 [PubMed] [Google Scholar]
- 18.Quigley JG, Gazda H, Yang Z, Ball S, Sieff CA, Abkowitz JL. Investigation of a putative role for FLVCR, a cytoplasmic heme exporter, in Diamond-Blackfan anemia. Blood Cells Mol Dis. 2005;35:189–92 [DOI] [PubMed] [Google Scholar]
- 19.Chiabrando D, Tolosano E. Diamond Blackfan anemia at the crossroad between ribosome biogenesis and heme metabolism. Adv Hematol 2010; 2010:790632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffy SP, Shing J, Saraon P, Berger LC, Eiden MV, Wilde A, Tailor CS. The Fowler syndrome-associated protein FLVCR2 is an importer of heme. Mol Cell Biol. 2010;30:5318–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young MF, Pressman E, Foehr ML, McNanley T, Cooper E, Guillet R, Orlando M, McIntyre AW, Lafond J, et al. Impact of maternal and neonatal iron status on placental transferrin receptor expression in pregnant adolescents. Placenta. 2010;31:1010–4 [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi D, Ieiri I, Hirota T, Takane H, Maegawa S, Kigawa J, Suzuki H, Nanba E, Oshimura M, et al. Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab Dispos. 2005;33:94–101 [DOI] [PubMed] [Google Scholar]
- 23.CDC CDC criteria for anemia in children and childbearing-aged women. MMWR Morb Mortal Wkly Rep. 1989;38:400–4 [PubMed] [Google Scholar]
- 24.Cook JD, Skikne BS, Lynch SR, Reusser ME. Estimates of iron sufficiency in the US population. Blood. 1986;68:726–31 [PubMed] [Google Scholar]
- 25.Akesson A, Bjellerup P, Berglund M, Bremme K, Vahter M. Serum transferrin receptor: a specific marker of iron deficiency in pregnancy. Am J Clin Nutr. 1998;68:1241–6 [DOI] [PubMed] [Google Scholar]
- 26.Cook JD, Dassenko S, Skikne BS. Serum transferrin receptor as an index of iron absorption. Br J Haematol. 1990;75:603–9 [DOI] [PubMed] [Google Scholar]
- 27.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101:3359–64 [DOI] [PubMed] [Google Scholar]
- 28.Institute of Medicine and NRC Weight gain during pregnancy: reexamining the guidelines. Washington, DC: The National Academies Press; 2009 [PubMed] [Google Scholar]
- 29.Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140:165–70 [DOI] [PubMed] [Google Scholar]
- 30.Paoli M, Marles-Wright J, Smith A. Structure-function relationships in heme-proteins. DNA Cell Biol. 2002;21:271–80 [DOI] [PubMed] [Google Scholar]
- 31.Yang Z, Philips JD, Doty RT, Giraudi P, Ostrow JD, Tiribelli C, Smith A, Abkowitz JL. Kinetics and specificity of FLVCR export function and its dependence on hemopexin. J Biol Chem. 2010;285:28874–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taketani S, Kohno H, Sawamura T, Tokunaga R. Hemopexin-dependent down-regulation of expression of the human transferrin receptor. J Biol Chem. 1990;265:13981–5 [PubMed] [Google Scholar]
- 33.Nielsen MJ, Moller HJ, Moestrup SK. Hemoglobin and heme scavenger receptors. Antioxid Redox Signal. 2010;12:261–73 [DOI] [PubMed] [Google Scholar]
- 34.Gburek J, Verroust PJ, Willnow TE, Fyfe JC, Nowacki W, Jacobsen C, Moestrup SK, Christensen EI. Megalin and cubilin are endocytic receptors involved in renal clearance of hemoglobin. J Am Soc Nephrol. 2002;13:423–30 [DOI] [PubMed] [Google Scholar]
- 35.Viteri FE. The consequences of iron deficiency and anemia in pregnancy. : Allen L, King J, Lonnerdahl B, Nutrient regulation during pregnancy, lactation and growth. New York: Plenum Press; 1994 [Google Scholar]
- 36.Langini SH, de Portela ML, Lazzari A, Ortega Soler CR, Lonnerdal B. Do indicators of maternal iron status reflect placental iron status at delivery? J Trace Elem Med Biol. 2006;19:243–9 [DOI] [PubMed] [Google Scholar]
- 37.Osada H, Watanabe Y, Nishimura Y, Yukawa M, Seki K, Sekiya S. Profile of trace element concentrations in the feto-placental unit in relation to fetal growth. Acta Obstet Gynecol Scand. 2002;81:931–7 [DOI] [PubMed] [Google Scholar]
- 38.Ee PL, Kamalakaran S, Tonetti D, He X, Ross DD, Beck WT. Identification of a novel estrogen response element in the breast cancer resistance protein (ABCG2) gene. Cancer Res. 2004;64:1247–51 [DOI] [PubMed] [Google Scholar]
- 39.Meyer zu Schwabedissen HE, Grube M, Dreisbach A, Jedlitschky G, Meissner K, Linnemann K, Fusch C, Ritter CA, Volker U, et al. Epidermal growth factor-mediated activation of the map kinase cascade results in altered expression and function of ABCG2 (BCRP). Drug Metab Dispos. 2006;34:524–33 [DOI] [PubMed] [Google Scholar]
- 40.Smith R, Smith JI, Shen X, Engel PJ, Bowman ME, McGrath SA, Bisits AM, McElduff P, Giles WB, et al. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J Clin Endocrinol Metab. 2009;94:2066–74 [DOI] [PubMed] [Google Scholar]
- 41.Zamber CP, Lamba JK, Yasuda K, Farnum J, Thummel K, Schuetz JD, Schuetz EG. Natural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics. 2003;13:19–28 [DOI] [PubMed] [Google Scholar]