Abstract
Background: There is limited data on dermoscopic features of basal cell carcinomas (BCCs). We evaluated the presence of dermoscopic features in superficial (sBCCs), nodular (nBCCs), pigmented and non-pigmented BCCs in order to evaluate the role of dermoscopy in the diagnosis of different subtypes of BCCs.
Patients and Methods: We conducted a retrospective study to evaluate the presence of dermoscopic features in superficial, nodular, pigmented and non – pigmented BCCs. One hundred thirty eight lesions (42 superficial, 96 nodular, 102 pigmented and 36 non-pigmented) were assessed by dermoscopy.
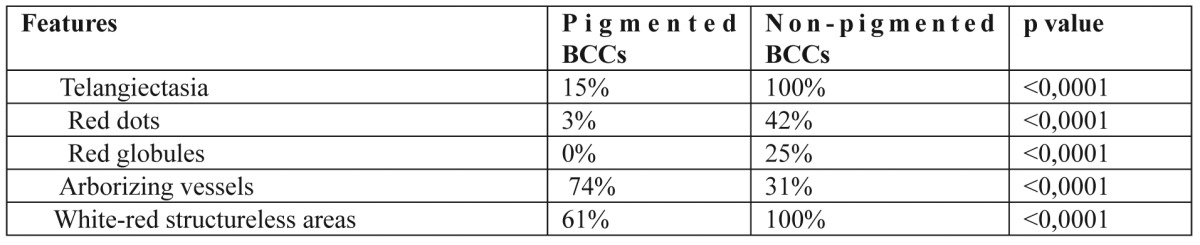
Results: The most significant features in all categories, were a scattered vascular pattern, featureless areas, atypical red vessels, arborizing vessels, comma vessels, background of white-red structureless areas and telangiectasias. Haemorrhage-ulceration, hypopigmented areas and blue-grey ovoid nests were all more likely to be observed in sBCCs, than in nBCCs (p < 0.0001). Arborizing and atypical red vessels in addition to featureless areas, were more frequent in nodular than in sBCCs (p < 0.0001). Telangectasias, white-red structureless areas, red dots and red globules were more common in non- pigmented than in pigmented BCCs (p <0.0001). In addition, a significant difference of arborizing vessels was detected in pigmented lesions in comparison to non-pigmented (p<0.0001).
Conclusions: There is limited data on dermoscopic features of basal cell carcinomas (BCCs). We evaluated the presence of dermoscopic features in superficial (sBCCs), nodular (nBCCs), pigmented and non-pigmented BCCs in order to evaluate the role of dermoscopy in the diagnosis of different subtypes of BCCs.
Keywords: basal cell carcinomas, dermoscopy, non-melanoma skin cancers, pigmented basal cell carcinoma, superficial basal cell carcinoma
Basal cell carcinoma (BCC) is the most common skin cancer. It tends to be locally invasive but rarely metastasizes. In 6.7% to 8.5% of the cases it presents as a pigmented lesion1,2. When clinical diagnosis is not possible, biopsy is used to confirm the diagnosis. Dermoscopy (epiluminescence microscopy, skin surface microscopy, incident light microscopy) is an in vivo, noninvasive diagnostic technique that permits a magnified view of the components of the epidermis and superficial dermis. It is a valuable tool in the evaluation of pigmented lesions and has been shown to greatly enhance clinical diagnosis for nearly all pigmented skin toumors3. Previous studies have described several dermoscopic features of pigmented BCCs4,5. By using dermoscopy, pigmented BCCs are always asymmetric in pattern and relatively hypomelanotic lesions. Two thirds have 50% of their tumor area pigmented and only 7% have 75% of their surface pigmented. Because of their irregularity, the differential diagnosis of pigmented BCCs includes both invasive melanoma and benign pigmented lesions. Taken this into consideration, a dermoscopy model has been previously reported to distinguish pigmented BCCs from both these diagnostic groups6. Dermoscopy can be also useful in non-pigmented skin tumors, for which the clinical diagnosis is often uncertain or implicates a variety of differential diagnoses. In this context, dermoscopic features such as blood vessel morphology and recognition of specific vascular structures may be of primary importance7-9. Currently, only few studies have examined the dermoscopic features, which assist efficiently in the diagnosis of non-pigmented BCCs7,9-11. The aim of this study was to identify the dermoscopic findings of both pigmented and non-pigmented nodular or superficial BCCs, statistically evaluate them and assess their significance.
Material and Methods
One hundred thirty eight BCCs out of 96 patients (60 males, 36 females, aged from 50 to 78 years) who attended our clinic over an 18-month period were evaluated. The diagnosis was based on clinical, dermoscopical and histopathological findings, and all lesions were divided in two distinctive types, namely superficial, (pigmented and non-pigmented, 42 lesions) and nodular (96 lesions).
Distinction regarding pigmentation was based on a dermoscopical basis and the term non-pigmented was given to the BCCs presented with absence of dermoscopic brown, black, blue, or gray pigmentation. Dermoscopic evaluation was performed with both, a digital dermoscope (Mole Max II, Derma Instruments, Vienna, Austria, 30-fold magnification) and the DermLight II. Three observers performed dermoscopy and dermoscopic features that were agreed on by at least two observers were taken into consideration. In order to avoid the loss of vascular dermoscopic features, during direct contact, the least possible pressure was applied.
For each lesion, a term that best described the global vascular pattern was assigned. These global vascular patterns were either clustered (with similar vessels closely together), scattered (with irregular vessels but diffusely distributed), homogenous (with uniform, symmetrical vessels densely aligned) or avascular (with no vessels seen). After that, local dermoscopic criteria were identified (Figure 1, 2). Background differences among white-red colors, observed within the lesions, were generally defined as white-red structureless areas. Featureless areas and hypopigmented areas were defined as areas that do not meet any known dermoscopic feature, and areas of decreased pigmentation within a homogenously pigmented lesion, respectively.
Figure 1. Schematic global and local dermoscopic features of BCCs.
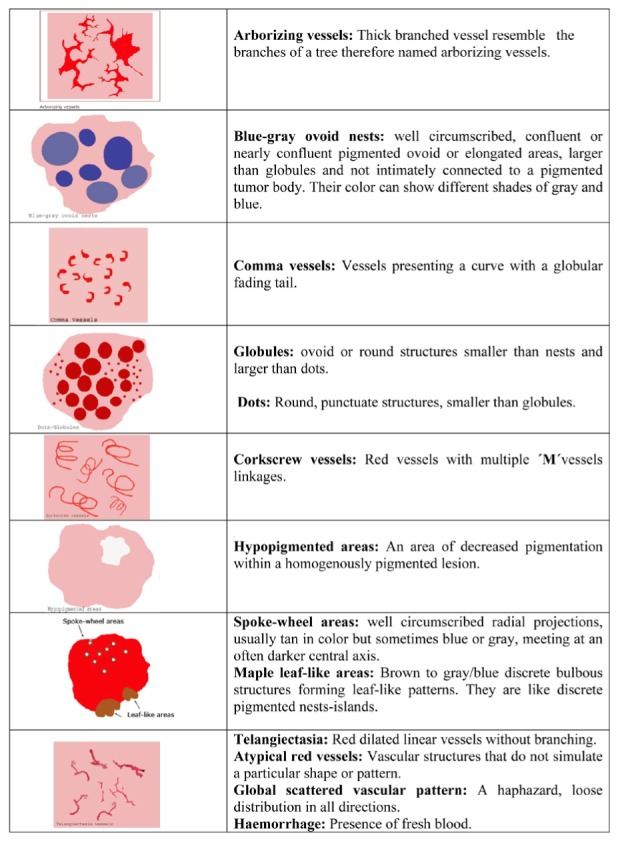
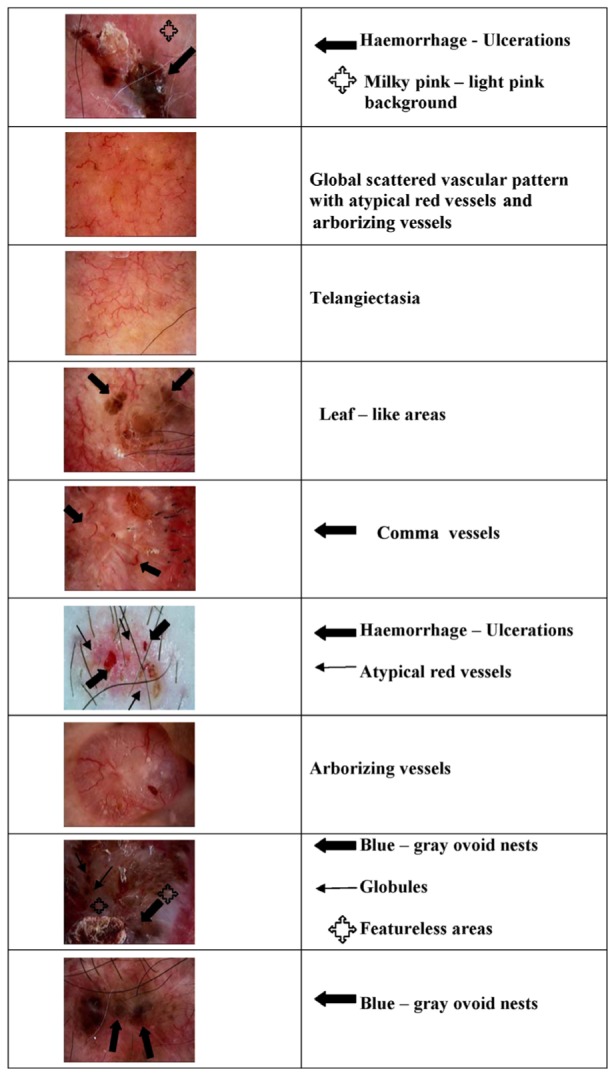
Figure 2. Dermoscopic pictures of BCCs.
All dermoscopic criteria were then analysed depending on the presence or absence of lesion nodularity and pigmentation (Table 1, Table 2). In order to examine whether there was a significant statistical difference between the categories Nodular – Superficial BCCs and Pigmented – Non Pigmented BCCs, the Fisher's exact test was applied. Additionally, the Bonferroni Holm correction method was applied in order to correct for occurrence of false positives.
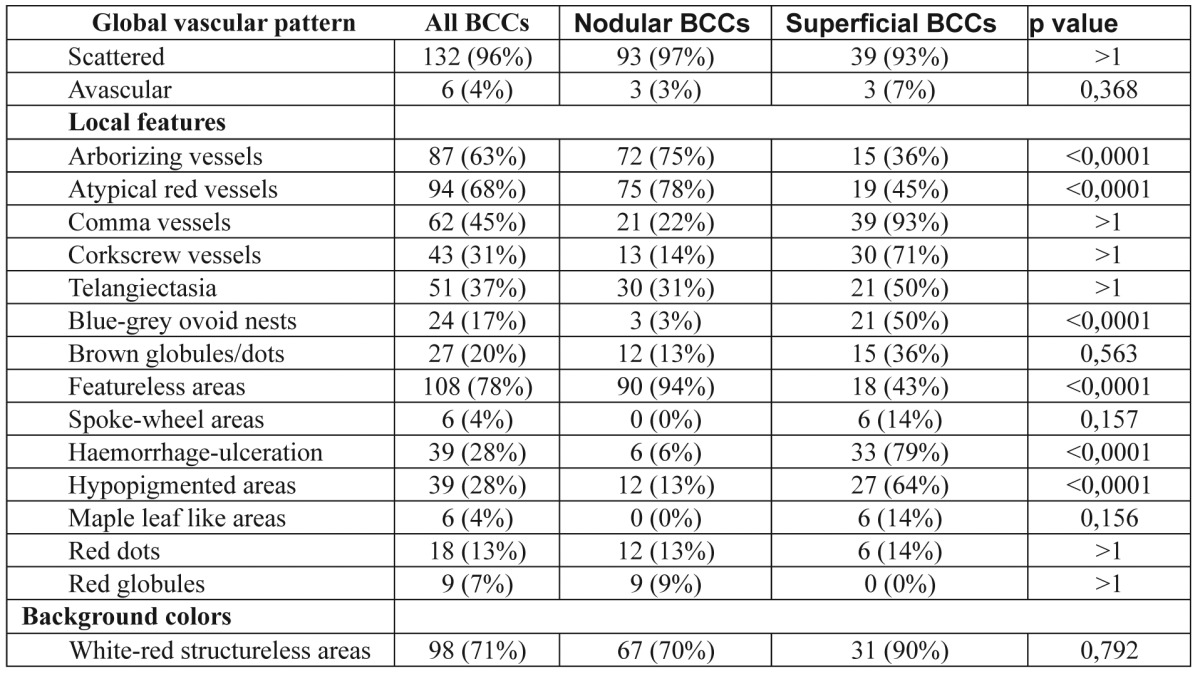
Table 1. Frequency of criteria in nodular and superficial BCC.
Results
All superficial BCCs were located on the body (six on the lower extremities, three on the upper extremities and thirty three on the trunk). Thirty of them were pigmented and 12 non-pigmented. Fifty four of the nodular BCCs were found on the head and neck and 42 on the trunk occurring as a firm "pearly" papule or nodule commonly covered by telangiectasias. Seventy two of them were pigmented and 24 non-pigmented.
In total seventeen dermoscopic features were scored for BCC. The most consistent global pattern of vessels in all categories of BCCs was scattered (96%). Regarding the local dermoscopic criteria and background colors, featureless areas (78%), white-red structureless background (71%), atypical red vessels (68%), arborizing vessels (63%), comma vessels (45%) and telangiectactic vessels (37%) were all more likely to be observed in all BCCs (Table 1, Table 2). The majority of nodular BCCs presented featureless areas (94%), atypical red vessels (78%) and arborizing vessels (69%). Superficial BCCs mostly presented with comma vessels (93%), white-red structureless areas background (90%),hypopigmented areas (64%), whereas only half of them (50%) revealed telangiectatic vessels and blue-grey ovoid nests in our study. A further important hint in the diagnosis of superficial BCC might be found in the presence of small ulcerations, which appear on dermoscopy as red to brown homogenous structureless areas, observed in 79% of our lesions. Elongated arborizing vessels were found in 15 out of our 42 cases of superficial BCCs. Spoke-wheel areas and leaf-like areas, although rare in our study (14% each), are considered in general as an exclusive feature seen in superficial BCCs.
Table 2. Frequency of criteria in pigmented and non-pigmented BCCs.
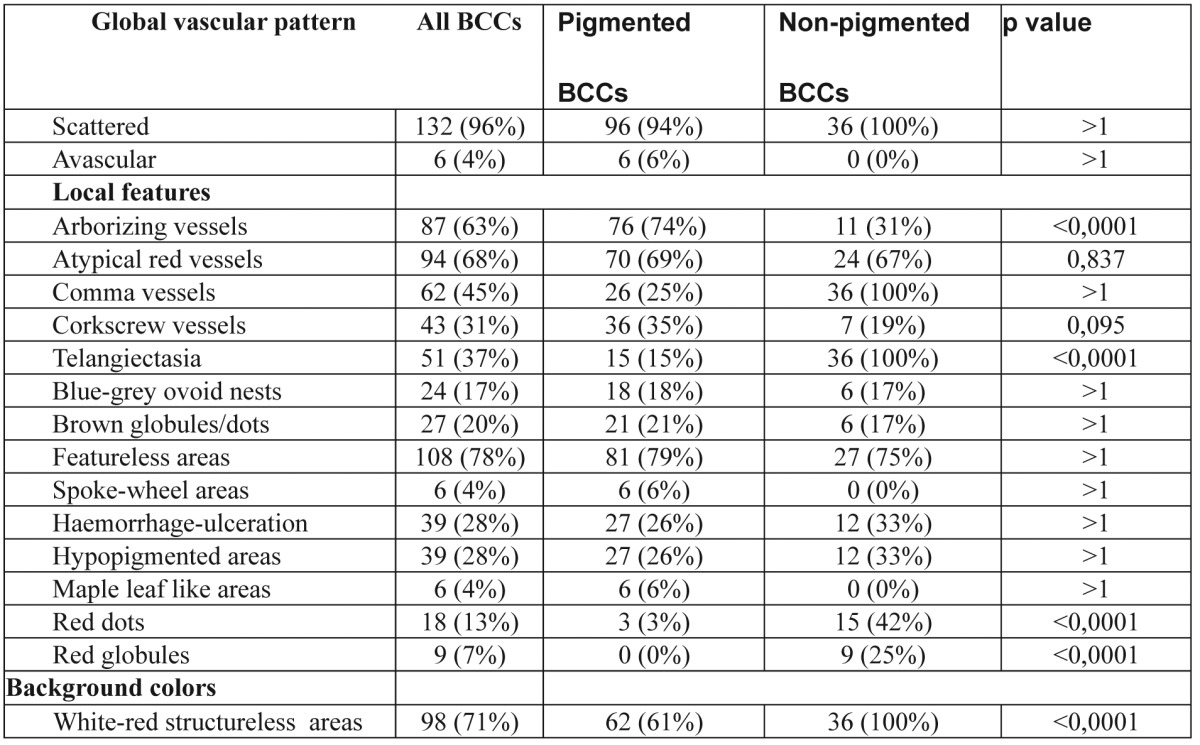
As far as the existence or absence of lesion pigmentation was concerned, atypical red vessels (69% in pigmented, 67% in non-pigmented BCCs) and featureless areas (79% in pigmented, 75% in non-pigmented BCCs) were seen in both pigmented and non-pigmented BCCs in a high frequency, indicating a dermoscopic hallmark of great interest. Moreover, a common presence of telangiectasias (100%), comma vessels (100%), white-red structureless areas (100%) and featureless areas (75%) were observed in non-pigmented BCCs. Arborizing vessels were observed in 74% of pigmented BCCs with a significant difference (p<0.0001) vs. 31% of non-pigmented BCCs. The analysis of dermoscopic criteria regarding the presence or absence of lesion nodularity and pigmentation revealed that certain features were statistically, significantly more common in specific BCC-categories. Overall, three features, haemorrhage-ulceration, hypopigmented areas and blue-grey ovoid nests were all more likely to be observed in sBCCs, than nBCCs (p<0.0001). In addition, another three features, featureless areas, arborizing and atypical red vessels were found to be most valuable in differentiating nodular from superficial BCCs (Table 3). Four dermoscopic criteria were identified more frequently in non-pigmented rather than pigmented BCCs and these included telangiectasia, white-red structureless background colour, red dots and globules. Furthermore, arborizing vessels were found to be statistically more common in pigmented lesions (p<0.0001) (Table 4).
Table 3. Most valuable dermoscopic criteria for the differential diagnosis of nodular and superficial BCCs.
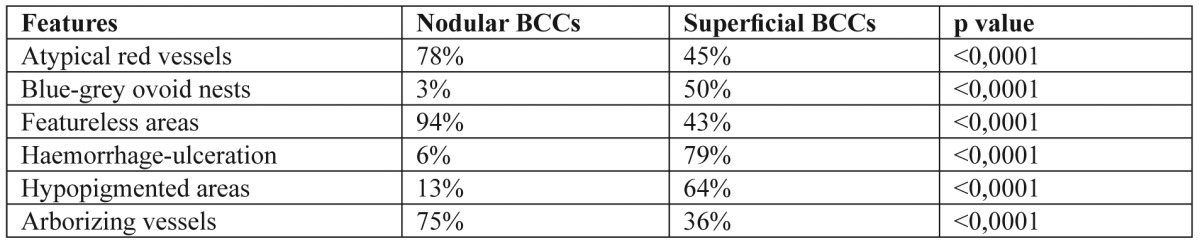
Table 4. Most valuable dermoscopic criteria for the differential diagnosis of pigmented and non-pigmented BCCs.
Discussion
Dermoscopy is a non-invasive technique that allows in vivo examination of the skin and is especially used for the differential diagnosis of pigmented skin lesions 3, 12. Most of the dermoscopic literature regarding BCCs to date has primarily concerned pigmented variants4-6,13,14. In the Consensus meeting via the internet in 2000, ulceration, maple leaf-like areas, large grey-blue ovoid nests, multiple grey-blue globules, spoke wheel areas and arborizing telangiectasia were found to be the dermoscopic criteria significant for the diagnosis of pigmented BCCs 4. Moreover, irregular depigmentation, black dots, irregular border and surface, whitish veil that are all found in melanoma and milia like cysts seen in non-melanocytic skin lesions can also be observed during the dermoscopic examination of BCCs15, 16. Menzies et al6 analysed dermoscopic features of 142 pigmented BCCs and concluded that some of these features were found in a relatively high frequency in pigmented BCCs compared with melanoma and benign pigmented skin lesions. At the end of the study they proposed a dermoscopic model for the diagnosis of pigmented BCCs based on the absence of pigment network (negative feature) and the presence of at least one of the dermoscopic features found (positive feature). Dermoscopy can be also useful in the diagnosis of non-pigmented skin tumors, as it permits, besides the pigmented structures, the recognition of specific vascular structures7-9. However, only few studies have examined the dermoscopic features, which assist efficiently in the diagnosis of non-pigmented BCCs7,9-11. One study of the dermoscopic features of superficial BCCs evaluated 24 cases and found the dermoscopic hallmarks to be a shiny white to red appearance (100%) and the presence of short, fine telangiectasias (92%)10. In our study we evaluated dermoscopically and histopathologically 138 BCCs. All lesions were divided into pigmented or non-pigmented, nodular or superficial. In total 17 criteria regarding the global vascular pattern, local features and background colours were identified by dermoscopy in all subtypes. The most consistent global pattern of vessels was scattered (96%) and this is in keeping with the existing literature11. Atypical vessels were the type of vessels most commonly seen and the one of the most frequent local dermoscopic feature (68%), followed by arborizing vessels (63%), when all lesions were taken into account. Correspondingly, four dermoscopic criteria, regarding blood vessel morphology, were identified as the most valuable in the differentiation between non-pigmented and pigmented BCCs. These include telangiectasias, arborizing vessels, red dots and red globules and are generally considered to be of primary importance7-9. Statistical analysis of our results revealed that arborizing vessels were more often in pigmented rather than non-pigmented BCCs, which means that this dermoscopic criterion might represent a valuable tool in differential diagnosis between them. The latter observation has been recently reported in the literature. Micantonio et al concluded that arborizing vessels were detected in a highly significant difference in superficial pigmented vs. superficial non-pigmented BCCs17. Contrary to other investigators, Altamura et al18, reported a high percentage of arborizing in non-pigmented BCCs. This discrepancy might be the result of the subdivision of the lesions into several different groups, based on the degree of dermoscopic pigmentation. Our findings, regarding telangiectactic vessels and scattered vessels, are in accordance with the currently available literature. Investigators usually report a high percentage of comma vessels, haemorrhage, small ulcerations, hypopigmented areas, telangiectasias and blue-gray ovoid nests in superficial BCCs. consistent with our observations, is the usual presence of white-red structureless areas in the background of superficial BCCs9,11,18,19. However, there are some minor differences concerning the rates of vessels morphology. Interestingly, opposed to the other studies6,18,20,21, atypical vessels were more commonly seen in all categories of BCCs in our patients. Furthermore, telangiectasias were less frequent in our lesions compared to the high percentage, reported by Micantonio et al17. The authors of the latter article subdivided telangiectatic vessels into arborizing telangiectasias and short fine telangiectasias. Our rate (37%) is almost equal with theirs (33.1%), when only short fine telangiectasias are taking into account. Based on our results, we suggest that atypical and arborizing vessels in a scattered distribution, as well as featureless areas and white-red structureless areas, might represent dermoscopic hallmarks of great interest regarding all categories of BCCs. In addition, superficial BCCs are characterized by the presence of comma vessels, haemorrhage, small ulcerations, hypopigmented areas, telangiectasias and blue-gray ovoid nests, whereas nodular BCCs are also characterized by the presence of arborizing vessels. Furthermore, atypical and arborizing vessels, as well as featureless areas are statistically more frequent in nBCCs than in sBCCs. Establishment of specific dermoscopic criteria is essential for early and accurate diagnosis of different BCC subtypes22-24. Further studies are needed to evaluate specificity and sensitivity of these dermoscopic features.
Conflict of interest:
None declared
References
- 1.Maloney M, Jones D, Sexton F. Pigmented basal cell carcinoma: investigation of 70 cases. J Am Acad Dermatol. 1992;27:74–78. doi: 10.1016/0190-9622(92)70160-h. [DOI] [PubMed] [Google Scholar]
- 2.Betti R, Gualandri L, Cerri A, Inselvini E, Crosti C. Clinical features and histologic pattern analysis of pigmented basal cell carcinomas in an Italian population. J Dermatol. 1998;25:691–694. doi: 10.1111/j.1346-8138.1998.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 3.Kenet RO, Kang S, Kenet BJ, Fitzpatrick TB, Sober AJ, Barnhill RL. Clinical diagnosis of pigmented lesions using digital epiluminescence microscopy. Grading protocol and atlas. Arch Dermatol. 1993;129:157–174. [PubMed] [Google Scholar]
- 4.Argenziano G, Soyer HP, Chimenti S, Talamini R, Corona R, Sera F, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48:679–693. doi: 10.1067/mjd.2003.281. [DOI] [PubMed] [Google Scholar]
- 5.Menzies SW. Dermoscopy of pigmented basal cell carcinoma. Clin Dermatol. 2002;20:268–269. doi: 10.1016/s0738-081x(02)00229-8. [DOI] [PubMed] [Google Scholar]
- 6.Menzies SW, Westerhoff K, Rabinovitz H, Kopf AW, McCarthy WH, Katz B. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012–1016. doi: 10.1001/archderm.136.8.1012. [DOI] [PubMed] [Google Scholar]
- 7.Kreusch JF. Vascular patterns in skin tumors. Clin Dermatol. 2002;20:248–254. doi: 10.1016/s0738-081x(02)00227-4. [DOI] [PubMed] [Google Scholar]
- 8.Vazquez-Lopez F, Kreusch J, Marghoob AA. Dermoscopic semiology: further insights into vascular features by screening a large spectrum of nontumoral skin lesions. Br J Dermatol. 2004;150:226–231. doi: 10.1111/j.1365-2133.2004.05753.x. [DOI] [PubMed] [Google Scholar]
- 9.Argenziano G, Zalaudek I, Corona R, Sera F, Sera L, Petrillo G, et al. Vascular structures in skin tumors: a dermoscopy study. Arch Dermatol. 2004;140:1485–1489. doi: 10.1001/archderm.140.12.1485. [DOI] [PubMed] [Google Scholar]
- 10.Giacomel J, Zalaudek I. Dermoscopy of superficial basal cell carcinoma. Dermatol Surg. 2005;31:1710–1713. doi: 10.2310/6350.2005.31314. [DOI] [PubMed] [Google Scholar]
- 11.Pan Y, Chamberlain AJ, Bailey M, Chong AH, Haskett M, Kelly JW. Dermoscopy aids in the diagnosis of the solitary red scaly patch or plaque-features distinguishing superficial basal cell carcinoma, intraepidermal carcinoma, and psoriasis. J Am Acad Dermatol. 2008;59:268–274. doi: 10.1016/j.jaad.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Soyer HP, Kenet RO, Wolf IH, Kenet BJ, Cerroni L. Clinicopathological correlation of pigmented skin lesions using dermoscopy. Eur J Dermatol. 2000;10:22–28. [PubMed] [Google Scholar]
- 13.Johr R, Soyer HP, Argenziano G, Hofmann-Wellenhof R, Scalvenzi M. Dermoscopy : The Essentials. New York: Mosby. 2004 [Google Scholar]
- 14.Menzies SW, Crotty KA, Ingvar C, McCarthy WH. An atlas of surface microscopy of pigmented skin lesions: dermoscopy. 2nd ed. Sydney, Australia. McGraw-Hill Book Company Australia Pty Ltd. 2003 [Google Scholar]
- 15.Stolz W, Braun-Falco O, Bilek B, Landtheler M, Burgdorf WHC, Cognetta AB. Color Atlas of dermatoscopy. 2nd ed. Berlin. Blackwell Science. 2002 [Google Scholar]
- 16.Menzies SW. A method for the diagnosis of primary cutaneous melanoma using surface microscopy. Dermatol Clin. 2001;19:299–305. doi: 10.1016/s0733-8635(05)70267-9. [DOI] [PubMed] [Google Scholar]
- 17.Micantonio T, Gulia A, Altobelli E, Di Cesare A, Fidanza R, Ritano A, et al. Vascular patterns in basal cell carcinoma. J Eur Acad Dermatol. 2010 Jun 17;[Epub ahead of print] doi: 10.1111/j.1468-3083.2010.03734.x. [DOI] [PubMed] [Google Scholar]
- 18.Altamura D, Menzies SW, Argenziano G, Zalaudek I, Soyer HP, Sera F, et al. Dermoscopy of basal cell carcinoma: Morphologic variability of global and local features and accurancy of diagnosis. J Am Acad Dermatol. 2010;62:67–75. doi: 10.1016/j.jaad.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 19.Zalaudek I, Kreusch J, Giacomel J, Ferrara G, Catricalà C, Argenziano G. How to diagnose nonpigmented skin tumors: a review of vascular structures seen with dermoscopy: part II. Nonmelanocytic skin tumors. J Am Acad Dermatol. 2010;63:377–386. doi: 10.1016/j.jaad.2009.11.697. [DOI] [PubMed] [Google Scholar]
- 20.Puspok-Scwarz M, Steiner M, Binder M, Partsch B, Wolff K, Pehamberger H. Statistical evaluation of epiluminescence microscopy criteria in the differential diagnosis of malignant melanoma and pigmented basal cell carcinoma. Melanom Res. 1997;7:307–311. doi: 10.1097/00008390-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Demirtaşoğlu M, Ilknur T, Lebe B, Kuşku E, Akarsu S, Özkan S. Evaluation of dermoscopic and histopathologic features and their correlations in pigmented basal cell carcinomas. J Eur Acad Dermatol Venereol. 2006;20:916–920. doi: 10.1111/j.1468-3083.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 22.Kyrgidis A, Vahtsevanos K, Tzellos TG, Xirou P, Kitikidou K, Antoniades K, et al. Clinical, histological and demographic predictors for recurrence and second primary tumours of head and neck basal cell carcinoma. A 1062 patient-cohort study from a tertiary cancer referral hospital. Eur J Dermatol. 2010;20:276–282. doi: 10.1684/ejd.2010.0903. [DOI] [PubMed] [Google Scholar]
- 23.Kyrgidis A, Tzellos TG, Kechagias N, Patrikidou A, Xirou P, Kitikidou K, et al. Cutaneous squamous cell carcinoma (SCC) of the head and neck: risk factors of overall and recurrence-free survival. Eur J Cancer. 2010;46:1563–1572. doi: 10.1016/j.ejca.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 24.Chaidemenos G, Stratigos A, Papakonstantinou M, Tsatsou F. Prevention of malignant melanoma. Hippokratia. 2008;12:17–21. [PMC free article] [PubMed] [Google Scholar]


