Abstract
Background: Aim of our study was to evaluate degree of genetic homozygosity in male and female gender of spina bifida (SB) occulta and SB aperta patients.
Patients and Methods: We evaluated 95 patients with SB occulta and 51 with SB aperta. Degree of genetic homozygosity was evaluated by direct observation of 15 homozygously recessive characteristics (HRC) by HRC-test separately for SB occulta and SB aperta participants. Additionally 370 individuals without SB from Serbia were randomly selected and evaluated as control group. Male and female gender was separately evaluated for assessing degree of genetic homozygosity.
Results: There was no significant difference in mean values of HRC between male and female gender in control group (male gender –3.9±1.2, female gender -4.0±1.4, z=0.39; p>0.05), SB occulta (male gender –4.1±1.5, female gender -4.7±1.4, z=1.87, p>0.05) and SB aperta patients (male gender –4.3±1.6, female gender -4.5±1.4, z=0.66, p>0.05), while there was significantly increased recessive homozygosity in female SB occulta group versus control female group (Females: SB occulta –4.7±1.4, Control group –4.0±1.4, z=3.16, p<0.01) and female SB aperta group versus control female group (Females: SB aperta –4.5±1.4, Control group –4.0±1.4, z=2.05, p<0.05).
Conclusion: There is increased recessive homozygosity in tested female SB occulta and female SB aperta individuals versus SB male participants and significantly increased recessive homozygosity in female groups of SB patients versus control female group. These findings could lead to the possible assumption that different genes in different degree might be expressed in SB occulta and SB aperta patients.
Keywords: spina bifida occulta, spina bifida aperta, male gender, female gender, homozygously recessive characteristics
One of the most common defects in humans are neural tube defects (NTD) including spina bifida (SB)1. Even though etiology of SB is still unknown, due to the complex influence of genetic factors during neurulation processes2, multifactorial polygenic or oligogenic etiology was previously suggested, pointing out to the importance of gene-gene and gene-environment interactions for the development of SB1. Familial inheritance of certain forms of SB was previously reported suggesting the possible role of genetic component3. It is stated as well that neural tube formation is based on the balance between cell proliferation and cell death, therefore, all potential factors that might lead to the disbalance between these processes could be responsible for the defects in the neural tube formation and onset of SB4. Present cutaneous marks as specific traits in spina bifida occulta (SBO) (hyperpigmentation, hypertrichosis, fovea sacralis, dermal sinuses and others)5, led some authors6 to analyze degree of genetic homozygosity in patients with SB. They have noticed increased degree of recessive homozygosity and decreased variability in patients with SB6.
Genetic inheritance1,7 and gender differences8, governed us to establish hypothesis that the increased genetic homozygosity in the samples of different genders of patients with SBO and/or spina bifida aperta (SBA) could be population-genetic parameters for the prediction of this illness.
Therefore, the aim of our study was to analyze and compare population-genetic structure and to evaluate degree of genetic homozygosity in male and female gender of patients with diagnosed SBO and SBA versus healthy control individuals.
Material and Methods
Study group
In prospective study we have evaluated 95 patients with diagnosed SBO and 51 with SBA in lumbosacral region from 2005-2010 years. The study was conducted according to the Declaration of Helsinki. To establish and confirm diagnosis of SB, all patients were clinically evaluated by board certified pediatrician, pediatric surgeon, physiatrist and radiologist. Radiographic imaging and in some cases magnetic resonance imaging (MRI) was done to confirm diagnosis, and lesion level and to differentiate SBO from SBA. Prior inclusion in the study, from parents or legal guardians of all participants informed consent was obtained for the inclusion in the study. The patients were grouped in 2 groups regarding the type of spina bifida: group with SBO and group with SBA. For every SB group, male and female gender was separately analyzed.
To compare obtained results, additional 370 participants without SB were included in control group. To exclude the possible SB in participants of control group, all of them were initially screened by board certified pediatrician, physiatrist and radiologist. Therefore, as for the SB participants, prior inclusion in the study, from parents or legal guardians of children from control group informed consent was obtained for the inclusion in the study.
Study methods
For the estimation of the degree of recessive homozygosity we used "HRC-test", as applied methodology for determination of homozygously recessive characteristics (HRC) in humans9. This test includes 15 HRC, where extreme appearance of tested traits were expressed as homozygous states of either individual or group of genes. The HRC-test has been developed to establish the proportion of homozygously recessive clearly expressed characteristics in every individual as markers of chromosomal homozygosities, implicating the degree of genetic homozygosity in humans9,10. It is a highly suitable method for estimating individual homozygosity by direct observation of defined phenotype characteristics.
Tested determinants
From 15 HRC, 10 of them were in the region of human head: unattached ear lobe (OMIM number 128900), continuous frontal hair line (OMIM number 194000), blue eyes (gene location 19p13.1-q13.11, OMIM number 227240), straight, soft and blond hair (OMIM numbers 139450 and 210750), double hair whorl, opposite hair whorl orientation (OMIM number 139400), color blindness (gene location Xq28, OMIM number 303800) and ear without Darwinian notch. Other 5 HRC were from the human arms region: distal or proximal hyperextensibility of the thumb, index finger longer than the ring finger (OMIM number 136100), left-handedness (gene location 2p12-q22, OMIM number 139900), right thumb over left thumb (hand clasping) (OMIM number 139800) and top joint of the thumb > 45° 11.
Known locations of genes with suggestive linkage to neural tube defects and SB include: 17q11.2-q12, 1p13, 6q27, 14q24, 1p36.3, 5p15.3-p15.2, 1q43 (OMIM numbers 601634, 182940) and there is as well X linked suggested SB inheritance (OMIM number 301410)11-13.
To ensure equal criteria in estimation of certain phenotype presence, one person did the testing of individuals from control and diseased groups.
Statistical analysis
Data in the study are presented as mean values with standard deviation. Comparison of mean HRC values between genders in control group, the group of SBO and in the group of SBA patients was done by non-parametric Mann-Whitney U test. Comparison of mean HRC values for male gender between control and SBO groups; control and SBA groups; and SBO and SBA groups, as well for female gender between control and SBO groups; control and SBA groups; and SBO and SBA groups was done by non-parametric Mann-Whitney U test. Statistical significance was set on p<0.05.
Results
Patients and control group characteristics
From 95 (65.1%) patients with SBO there were 42 (44.2%) male patients and 53 (55.8%) female patients. From 51 (34.9%) patients with SBA there were 23 (45.1%) male patients and 28 (54.9%) female patients. All participants within patient groups were from Serbian population age between 3 to 18 years of life.
From 370 participants of control group, there were 170 (45.9%) male individuals and 200 (54.1%) female individuals. The control group included individuals from same locality (Serbian population), of similar age (4-18 years) and with similar socioeconomic status as participants from the SBO and SBA groups.
Variability of HRC between genders in control, SBO and SBA group of individuals
From the data presented (Figure 1), it was shown that mean values of HRC between male and female gender in control group did not significantly differ (male gender – 3.9±1.2, female gender - 4.0±1.4, z=0.39, p>0.05). In control group of individuals the most frequent average number of HRC was 3 (20.0%) both for male and female gender.
Figure 1. Distribution and variability of homozygous recessive characteristics (HRC) in control group.
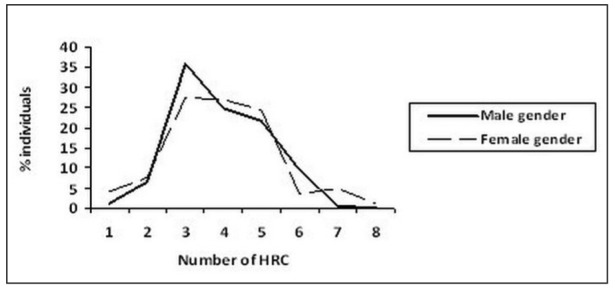
Male gender N=170 χ HRC/15 = 3.9± 1.2
Female gender N=200 χHRC/15= 4.0 ±1.4 (z=0.39; p>0.05)
For the affected SB individuals that were tested, comparing mean values of HRC between male and female gender in SBO group (Figure 2), it is pointed out that there is no significant difference in the presence of HRC between two genders (male gender – 4.1±1.5, female gender - 4.7±1.4, z=1.87, p>0.05). In tested individuals from SBO group the most frequent average number of HRC was 3 (20.0%) for male gender and between 4 (26.7%) and 6 (40.0%) for female gender, stressing out higher recessive homozygosity of female SBO patients. Comparing mean values of HRC between male and female gender in tested individuals from SBA group (Figure 3), it is shown that there is no significant difference in the presence of HRC between two genders (male gender – 4.3±1.6, female gender - 4.5±1.4, z=0.66, p>0.05). In SBA group the most frequent average number of HRC was 5 (33.3%) for male gender and 6 (40.0%) for female gender. These findings point out that female gender from SBA group of patients has no significant increase of recessive homozygosity.
Figure 2. Distribution and variability of homozygous recessive characteristics (HRC) in spina bifida occulta (SBO) patients Male gender N=42 χ HRC/15 = 4.1± 1.5 Female gender N=53 χHRC/15= 4.7 ±1.4 (z=1.87; p>0.05).
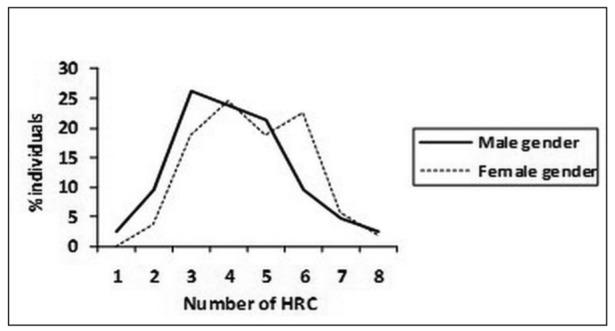
Figure 3. Distribution and variability of homozygous recessive characteristics (HRC) in spina bifida aperta (SBA) patients.
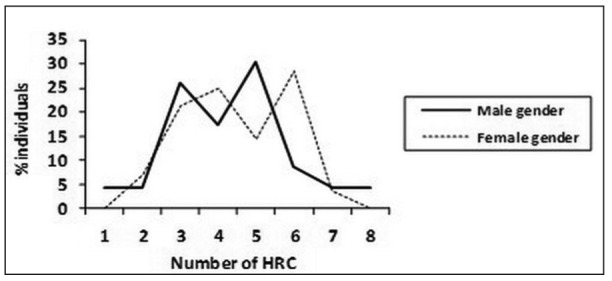
Male gender N=23 χ HRC/15 = 4.3± 1.6
Female gender N=28 χHRC/15= 4.5 ±1.4 (z=0.66; p>0.05)
Variability of HRC between different groups of same gender individuals
Regarding the distribution of HRC frequency for male gender, it was shown that mean values of HRC between male control group and male SBO group of patients, did not significantly differ (Control groupmale gender – 3.9±1.2, SBOmale gender – 4.1±1.5, z=0.71, p>0.05). The most frequent number of average HRC in both male control group and male SBO group was 3 (20.0%). Male individuals of SBA group had increased degree of recessive homozygosity but not significant, when compared to male individuals of control group, (Control groupmale gender – 3.9±1.2, SBAmale gender – 4.3±1.6, z=1.18, p>0.05). The most frequent number of average HRC in male control group was 3 (20.0%), while in male SBA group was 5 (33.3%), stressing out increased recessive homozygosity. Comparing mean values of HRC between male SBO group and male SBA group of individuals, it was shown that there was no significant difference in the presence of HRC between two tested groups (SBOmale gender – 4.3±1.6, SBAmale gender – 4.6±1.5, z=0.50, p>0.05).
Regarding the distribution of HRC frequency for female gender, it was shown that mean values of HRC between female control group and female SBO group, significantly differed (Control groupfemale gender – 4.0±1.4, SBOfemale gender – 4.7±1.4, z=3.16, p<0.01). The most frequent number of average HRC in female control group was 3 (20.0%), while for female SBO group it was between 4 (26.7%) and 6 (40.0%), stressing out increased recessive homozygosity and possible genetic predisposition.
Comparing mean values of HRC between female control group and female SBA group of individuals, it is shown that there is significant difference in the presence of HRC between two groups (Control groupfemale gender – 4.0±1.4, SBAfemale gender – 4.5±1.4, z=2.05, p<0.05). The most frequent number of average HRC in female control group was 3 (20.0%), while for female SBA group was 6 (40.0%), stressing out increased recessive genetic homozygosity and possible genetic predisposition. Comparing mean values of HRC between tested female individuals from SBO group and SBA group, it is shown that there is no significant difference in the presence of HRC between these two groups (SBOfemale gender – 4.7±1.4, SBAfemale gender – 4.5±1.4, z=0.32, p>0.05).
Discussion
Even though tremendous efforts were generated to explore and find out single gene that is responsible for the development of SB, it is more certain that many genes are engaged and have an influence in the processes that govern formation of the SB. Controversial opinions for the gender role in the formation of SB were discussed as well12,13.
Estimation of genetic homozygosity in human population is complex and sensitive task, since for a small number of biochemical processes and/or characters, gene locations are presently known. It is established as well that certain morpho-physiological characteristics are expressed as a qualitative and that they are under the control of one or small number of genes14.
Previously it has been assumed that genes which determine expression of certain phenotype traits along with environmental factors could potentially to the certain degree influence the development of certain diseases and conditions either by multifactorial mechanism or through mutations on genes that control expression of homozygous recessive traits15.
From the results of our study on the population-genetic approach it is pointed out that there are differences between genders for the predisposition regarding SBO and SBA, suggesting possible genetic linkage.
We found increased recessive homozygosity in SBO female patients versus SBO male patients and in female SBA patients versus SBA males, pointing out to the certain degree that there could be the possible role of gender for the predisposition for SBO and for SBA, even though there was non significant difference. Significantly increased recessive homozygosity was noticed in female SBO and SBA groups versus control female group, while such distinction was not found for male SB participants versus male control group. In the study of Byrne, it was noticed that maternal relatives (uncles and aunts) of Irish families have birth defects significantly frequent than paternal relatives (uncles and aunts)16. These findings could bring us to the possible assumption that SB is to the certain degree polygenetically determined and that such condition could correlate with other genetically controlled characteristics. Possible oligogenic inheritance was proposed in previous studies as well17.
Our results could point out on a few possible explanations that could explain present difference in the degree of recessive homozygosity between genders of the SBO and SBA participants. Significantly increased recessive homozygosity in female gender (SBO and SBA groups versus control group) could bring such individue in modified genetic-physiologic state and its altered reaction norm that could enable easier expression of SBO or SBA for female gender versus control female group. Further, significantly increased genetic homozygosity in tested female SB individuals versus control female group, could point out the increase of the genetic loads that might decrease body resistance to specific conditions that are responsible for the prevention of SBO and SBA formation in female gender (closure of neural tube during embryogenesis).
These findings are consistent with other studies that have stressed out the correlation of increased (or decreased) degree of recessive homozygosity and onset of certain diseases, implicating that changes in overall and individual genetic homozygosity and heterozygosity could influence in certain degree the changes to the resistance on specific environmental factors or might predispose such individue to the easier expression of specific disease6,18-22.
The presented difference, even though it was not significant, in the degree of genetic homozygosity between genders of groups of individuals with SBO and SBA, and significantly increased degree of recessive homozygosity in female SBO and SBA groups versus female control group could give us an assumption to more specific phenotype existence for the SBO and SBA male and female genders. Therefore, it could be assumed that certain genes determining evaluated phenotype characteristics might differ between genders of SBO and SBA individuals. These impressions suggest that certain preferential phenotypes in male or female gender could have the influence in certain degree on the adaptability potential of the organism with the SBO or SBA to the specific environmental factors that could interfere during morphogenesis.
Our study point out to the conclusion that there is increased degree of recessive homozygosity in female SBO and female SBA groups versus SB males participants and significantly increased degree of recessive homozygosity in female groups of SB patients versus control female group. These findings could lead to the assumption of possibility that different genes in different degree might be expressed in SBO and SBA patients.
Aside a fact that expected phenotype variations in humans are delicate to establish, present differences between evaluated groups in our study were noticed regarding degree of recessive homozygosity.
The results of our study could help to the certain degree better understanding the etiopathology and the possible role of different genders in the SB formation. Such methodology that we applied might be used further as potential early screening method for the conditions in human individuals that could have genetic predispositions with other diagnostic and functional tests.
Acknowledgments
The study was supported by the Ministry of Science and Technological Development of Serbia [175093].
Conflict of interest
There is no conflict of interest.
References
- 1.Copp AJ, Greene ND. Genetics and development of neural tube defects. J Pathol. 2010;220:217–230. doi: 10.1002/path.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finnel RH, Junker WM, Wadman LK, Cabrera RM. Gene expression profiling within the developing neural tube. Neurochem Res. 2002;27:1165–1180. doi: 10.1023/a:1020977409850. [DOI] [PubMed] [Google Scholar]
- 3.Kannu P, Furneaux C, Aftimos S. Familial lipomyelomeningocele: a further report. Am J Med Genet. 2005;132A:90–92. doi: 10.1002/ajmg.a.30404. [DOI] [PubMed] [Google Scholar]
- 4.Davidson CM, Northrup H, King TM, Fletcher JM, Townsend I, Tyerman GH, et al. Genes in glucose metabolism and association with spina bifida. Reprod Sci. 2008;15:51–58. doi: 10.1177/1933719107309590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guggisberg D, Hadj-Rabia S, Viney C, Bodemer C, Brunelle F, Zerah M, et al. Skin markers of occult spinal dysraphism in children: a review of 54 cases. Arch Dermatol. 2004;140:1109–1115. doi: 10.1001/archderm.140.9.1109. [DOI] [PubMed] [Google Scholar]
- 6.Nikolic D, Cvjeticanin S, Petronic I, Jekic B, Brdar R, Damnjanovic T, et al. Degree of genetic homozygosity and distribution of ABO blood types among patients with spina bifida occulta and spina bifida aperta. Arch Med Sci. 2010;6:854–859. doi: 10.5114/aoms.2010.19291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell LE, Adzick NS, Melchionne J, Pasquariello PS, Sutton LN, Whitehead AS. Spina bifida. Lancet. 2004;364:1885–1895. doi: 10.1016/S0140-6736(04)17445-X. [DOI] [PubMed] [Google Scholar]
- 8.Van der Put NM, van Straaten HW, Trijbels FJ, Blom HJ. Folate, homocysteine and neural tube defects: an overview. Exp Biol Med (Maywood) 2001;226:243–270. doi: 10.1177/153537020122600402. [DOI] [PubMed] [Google Scholar]
- 9.Marinkovic D, Ilic M, Spremo B. Studies of human population – genetic variation. Comparison of homozygously recessive traits in attendants of special and regular schools in Serbia. Arch Biol Nauka. 1990;42:11–12. [Google Scholar]
- 10.Pesut DP, Marinkovic D. Lung Cancer And Pulmonary Tuberculosis - A Comparative Population-genetic Study. Balkan J Med Genet. 2009;12:45–52. [Google Scholar]
- 11.Online Mendelian inheritance in man (OMIM) http://www.ncbi.nlm.nih.gov.
- 12.Hol FA, Schepens MT, van Beersum SE, Redolfi E, Affer M, Vezzoni P, et al. Identification and characterization of an Xq26-q27 duplication in a family with spina bifida and panhypopituitarism suggests the involvement of two distinct genes. Genomics. 2000;69:174–181. doi: 10.1006/geno.2000.6327. [DOI] [PubMed] [Google Scholar]
- 13.Fryns JP, Devriendt K, Moerman P. Lumbosacral spina bifida and myeloschizis in a female foetus with de novo X/autosomal translocation (t(X;22)(q27;q121)) Genet Counsel. 1996;7:159–160. [PubMed] [Google Scholar]
- 14.McKusick VA. Mendelian inheritance in man: catalogs of autosomal dominant, autosomal recessive and X-linked phenotypes. Baltimore: Johns Hopkins University Press. 1990 [Google Scholar]
- 15.Petricevic B, Cvjeticanin S. Morphogenetic variability and handedness in Montenegro and Serbia. Russ J Genet. 2011;43:406–411. [PubMed] [Google Scholar]
- 16.Byrne J. Birth defects in uncles and aunts from Irish families with neural tube defects. Birth Defects Res A Clin Mol Teratol. 2008;82:8–15. doi: 10.1002/bdra.20406. [DOI] [PubMed] [Google Scholar]
- 17.Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC. Human neural tube defects: developmental biology, epidemiology, and genetics. Neurotoxicol Teratol. 2005;27:515–524. doi: 10.1016/j.ntt.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marinkovic D, Cvjeticanin S. Population genetic study of Balkan endemic nephrophaty in Serbia. Russ J Genet. 2007;43:1134–1138. doi: 10.1134/S1022795407080170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cvjeticanin S, Marinkovic D. Genetic variability in the group of patients with congenital hip dislocation. Russ J Genet. 2005;41:1142–1146. [PubMed] [Google Scholar]
- 20.Marinkovic D, Cvjeticanin S, Stanojevic M. Population genetic analyses of susceptibility to developing alcohol dependence. Addict Res Theory. 2008;16:331–337. [Google Scholar]
- 21.Pesut D. Susceptibility to lung tuberculosis - a population-genetic study. Med Pregl. 2004;57:21–24. [PubMed] [Google Scholar]
- 22.Cvjeticanin S, Marinkovic D. Genetic Variability and Frequencies of ABO Blood Types among Different Samples of Patients from Serbia. Kor J Genet. 2005;27:35–40. [Google Scholar]


