Abstract
Background: Intracerebroventricular (ICV) experimental route is highly promising due to immediate approach of a "therapy" to the cerebrospinal compartment. Ischemic edema causes structural dislocations and stereotaxia alterations after temporary Middle Cerebral Artery Occlusion (t-MCAO), while there is no similar study for intracerebroventricular (ICV) invasion after permanent MCAO (p-MCAO).
Methods: Male Wistar rats were subjected to right p-MCAO and clinically evaluated 6 and 18 hours post-occlusion, using the modified Neurological Stroke Scale (mNSS) and modified Bederson's Scale (mBS). Infarction volume, hemispheric edema, middle line dislocation and stereotaxia of the lateral ventricles were studied at the same time-points.
Results: P-MCAO induced mild but significant changes in the stereotaxia of the infarcted (ipsilateral) lateral ventricle on 18- (P<0.05), though not 6-hours (P>0.05) post-occlusion. These changes correlated with the mNSS and mBS scores (P<0.01) and allowed the expression of linear mathematical equations (stereotaxic coordinate = b0 + b1*mNSS; calculated by regression analysis) predicting the new ventricular position in each individual animal. The contralateral ventricular system was structurally unaffected on both time-points. Verification experiments indicated that the new coordinates were necessary on 18-hours post-occlusion for successful ICV invasion in all p-MCAO rats (Number Needed to Treat 2.28), compared to 56.25% success when using the classical coordinates for normal rats.
Conclusion: P-MCAO causes relatively late but predictable stereotaxia shifts for ICV invasion, which are different compared to t-MCAO.
Keywords: permanent middle cerebral artery occlusion, stroke, edema, transplantation, intraventricular stereotaxic coordinates, lateral ventricles
Temporary and permanent middle cerebral artery occlusion (t-MCAO and p-MCAO, respectively) models are considered to be the most commonly used ones for ischemic stroke1-4. However, their choice and application is usually important for the final outcomes5 when studying the effect of a new treatment. Permanent ischemia lacks the reperfusion injury of the prolonged (> 60 minutes) temporary ischemia6 and this induces significant differences in ischemic tissue damage and edema formation6,7 between the 2 models.
Since each experimental design should meet the STAIR criteria with practical and feasible administration routes for human clinical conditions8, the intravenous (IV), per os and intracisternal or intracerebroventricular (ICV) routes of administration can be used in experimental design for new treatments. Among them, the stereotaxic ICV route, although is not a usual approach for clinical development so far1, still remains the closest practical experimental procedure in rodents that mimics the route of cerebrospinal fluid (CSF) administration in humans. The advantage of experimental ICV infusion is that it delivers high "treatment" concentrations directly to the CSF compartment, with minimum systemic side-effects9, 10.
However, we have recently shown that post-ischemic edema renders ICV invasion in the acute phase of t-MCAO merely impossible due to structural dislocations, unless new coordinates are calculated11, whereas no similar study in the p-MCAO model has ever been performed.
In the present study, we applied the recently described methodology valid for the t-MCAO11 on the p-MCAO model in Wistar rats. We correlated the stereotaxic ventricular shifts with the clinical scores of the animals on 6 and 18 hours post-occlusion and draw out linear mathematical equations for successful stereotaxia. Moreover, using the results from the t-MCAO model11 for indirect comparisons, we discuss the possible significance of our proposed tools for future experimental studies on treatment administration to the CSF compartment, through ICV invasion in Wistar rats.
Methods
Animal handling and groups
Male adult Wistar rats (n=42) of various age (4-6 months) and weight (305-441 gr) were purchased from the Hellenic Pasteur Institute (Athens, Greece) and housed in the P3 animal facility of our Department. All experimental procedures were conducted according to Institutional guidelines, in compliance with Greek Regulations and the European Communities Council Directive of November 24 1986 (86/609/EEC).
Permanent focal cerebral ischemia was induced using the p-MCAO model3, 4. Animals with p-MCAO were randomly sacrificed at 2 time-points: group A (n=19) at 6 hours and group B (n=21) at 18 hours post-ischemia. Most of the animals in each group (subgroups Ac, n=14, and Bc, n=16) were used for calculations and some (subgroups Av, n=5, and Bv, n=5) for verification experiments. Additionally, 2 naïve Wistar rats (group N) were used to demonstrate the stereotaxic ICV invasions in normal animals, applying the coordinates described in paragraph "Stereotaxic injections – Marking of the lateral ventricles"
Experimental procedures for p-MCAO.
Animals were anesthetized with 4% halothane in a mixture of 70% N2O and 30% O2, were orally intubated and anesthesia was maintained with 1.5% halothane in the same mixture using mechanical ventilation. Rectal temperature, mean arterial blood pressure (MABP), arterial blood gases, plasma glucose and haematocrit values were monitored as previously described in detail4. The right middle cerebral artery (MCA) was permanently occluded using a 5-0 modified poly-L-lysine-coated Koizumi suture and the methodology previously described in detail4, however without withdrawal of the suture.
Stereotaxic injections – Marking of the lateral ventricles
Just prior to sacrifice, animals (subgroups Ac, Bc and N) were re-anesthetized with halothane, immobilized on a stereotaxic device and stereotaxic marking of the lateral ventricles was performed as previously described in detail11. Briefly, ICV injections of 5μl trypan blue were performed in both lateral ventricles, at 2 sets of coordinates12: for the right or left ventricle, at anteroposterior (AP) = -0.12mm, mediolateral (ML) = 1.6mm, dorsoventricular (DV) = 4.3mm (first set) and at AP = -1.0mm, ML = 2.4mm, DV = 4.0mm (second set). Using this approach we "draw" 2 blue needle traces in each hemispheric side as index marks of the "theoretical" ICV injection sites11, for calculations of the AP displacement as described below (section "Post-ischemic ventricular dislocation – related calculations").
Neurological and Motor testing
All p-MCAO animals were examined at 6 and 18 hours post-occlusion using: 1) the 18-grade modified Neurological Stroke Scale (mNSS)13 and 2) the 7-grade modified Bederson's Scale (mBS)4.
Tissue processing
All animals were humanly sacrificed immediately after stereotaxic injections and their brains were treated for 2% TTC staining of 1mm brain slices as previously described in detail11. The acquired images of the brain slices were analyzed using the ImageJ software 1.34s11.
Calculation of infarction and edema volumes
Infarction volume was calculated as previously described in detail4 and was expressed as a percentage ("% hemispheric infarction volume", %Vinf). Calculation of %Vinf on the 6-hours groups was performed in order to verify the induction of ischemia and have a rough estimate of the infarction. Edema of the infarcted hemisphere was also calculated as previously described in detail4 and was also expressed as a percentage ("% edema of the infarcted hemisphere", %HE).
Post-ischemic ventricular dislocation – related calculations
Post-ischemic ventricular dislocations in animals of subgroups Ac and Bc were studied using a methodology previously described in detail11. Calculations included: 1) dimensions of the lateral ventricles, 2) % middle line dislocation (mlD) and 3) optimal site (S) for stereotaxic invasion in each lateral ventricle (SR and SL for right and left ventricle, respectively).
Ventricular stereotaxia (calculations 1 and 3) and mlD (calculation 2) were studied on TTC slices11. The addition of "zero" or "-1" as subscripts in all abbreviations, where applicable throughout the text, indicates the corresponding AP level according to the Paxinos and Watson atlas12.
Verification of the new post-ischemic stereotaxic coordinates
Verification of the new coordinates was performed in animals of subgroups Av and Bv on 6 and 18 hours post-occlusion respectively. Five μl of trypan blue were stereotaxically injected in the lateral ventricles, using coordinates extracted from subgroups Ac and Bc. For subgroup Av the classical coordinates of the rat brain atlas12 were used (section "Stereotaxic injections – Marking of the lateral ventricles"). On the contrary, for subgroup Bv, new calculated coordinates were used according to Fig 1 (insert tables 1a, 1b, 1c and 1d) and defined as follows: At the level of -0.12mm: - Right ventricle AP = 0 + APR mm, ML = ML-SRO mm and DV = 4.0mm; - Left ventricle AP = 0 + APL mm, ML = ML-SLO mm and DV = 4.0mm. Accordingly, at the level of -1.08mm: - Right ventricle AP = -1 + APR mm, ML = ML-SR-1 mm and DV = 4.0mm; - Left ventricle AP = -1 + APL mm, ML = ML-SL-1 mm and DV = 4.0mm.
Statistical analysis
All data are given as mean ± Standard Deviation (SD). Statistical analysis was performed using the SPSS 11.5 package using the Student's t-test, Mann-Whitney U test and one-way ANOVA. Correlations between neurological scores and ventricular shifts were performed using the Pearson correlation coefficients. Curve estimations and setting of the mathematical equations for calculation of the new ICV coordinates (Figure 1, insert tables and F-scores) were based on regression analysis (Figure 1, scatterplots); data from naïve animals were included in this analysis. Number Needed to Treat (NNT) for a successful invasion was calculated using the formula: NNT=1/|risk reduction|, as previously described14, 11. Minimal statistical significance was set at p < 0.05.
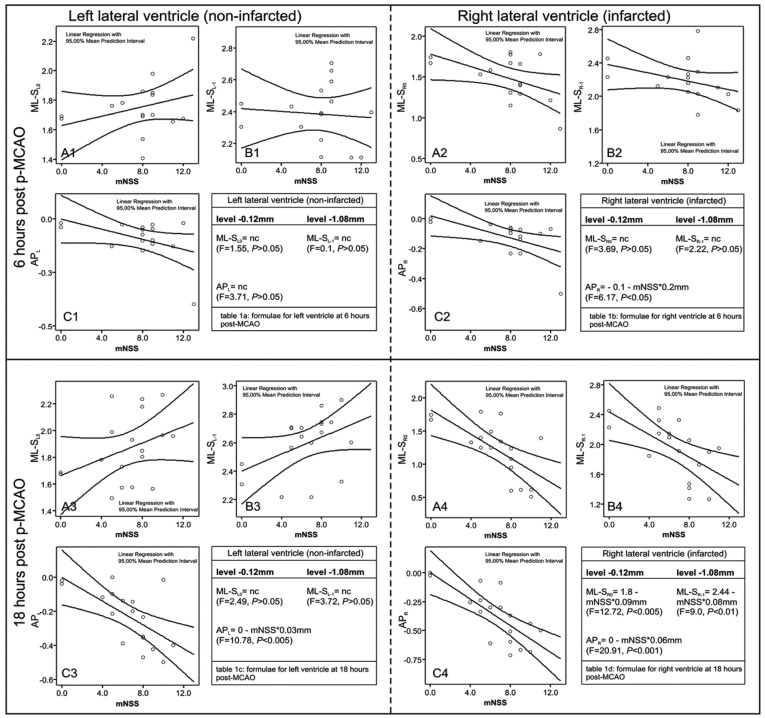
Figure 1. Scatterplots of the mNSS scores to the ML shifts at the levels -0.12 (A1, A2, A3, A4) and -1.08mm (B1, B2, B3, B4) from bregma as well as to the AP shifts (C1, C2, C3, C4), at 6 (upper panel) and 18 hours (lower panel) post MCAO. Insert tables 1a, 1b, 1c, 1d present the corresponding formulae used for calculation of the new stereotaxic coordinates; "nc" indicates no correlation with the mNSS, i.e. no change from the classical coordinates for normal rats [17]. The right ventricle (right panel) is significantly affected at 18-hours and laterally displaced leftwards (A4, B4); the left ventricle (left panel) is displaced milder and not correlated to mNSS (A3, B3). At 6-hours, the entire ventricular system is virtually not affected (A1-C1 and A2-C2).
Results
Physiological Variables and mortality
All measured physiological variables remained within normal limits pre- or post-occlusion of the MCA (p>0.05, data not shown). Mean body weights were 375.8±28.5, 380.2±38.7 and 363.0±30.4 for groups A, B and N respectively (p>0.05). Direct post-ischemia temperature was slightly raised at 37.4°C to 38.1°C in some animals.
No subarachnoid hemorrhage (SAH) was observed in any animal of the study. One animal of subgroup Ac with severe stroke and edema (mNSS =13) died due to heart arrest during the stereotaxic injections at 6 hours; autopsy revealed central herniation. No animals of group B died prior to the predefined time-point of sacrifice.
Neurological deficits
Groups A and B developed mNSS scores of 8.8±2.1 and 7.3±2.0 and mBS scores of 5.5±1.2 and 4.7±1.6 respectively. No statistically significant differences were observed between the 2 groups (Student t-test, p>0.05).
Infarction volume, edema and mlD
Ischemic brain infarction was detected in all animals. For groups A and B, estimated %Vinf was 12.9±13.2% and 24.6±17.5% respectively (Mann-Whitney U test, P<0.05). The %HE was 6.3±4.3% at 6 hours and was significantly increased at 18 hours (12.0±6.1%) post-occlusion (Mann-Whitney U test, p<0.005). At 6 hours, the mlD at the AP levels of -0.12, -1.08 and -2.04mm was mlD0 = 2.6±2.2%, mlD-1 = 2.4±1.9% and mlD-2 = 2.1±2.9% respectively. At 18 hours, mlD at the same AP levels was 5.7±2.8%, 6.4±2.6% and 5.0±2.3% respectively, indicating a significant increase of the mlD (Mann-Whitney U test, P≤0.001).
Ventricular repositioning - Correlations with neurological scores.
Correlations and scatterplots of the ventricular coordinates with the neurological scores of the animals at the time of sacrifice are shown in the Table and Figure 1, respectively. At 6 hours, coordinates of both lateral ventricle (with exception of APR) were independent (p>0.05) of the neurological scores of the animals (mNSS and mBS scores, Table and Figure 1), indicating no significant structural change or shifting of the ventricular system.
At 18 hours, both ML and AP coordinates of the right ventricle linearly correlated (P<0.01) with mNSS and mBS scores (Table, Figure 1), and higher scores (mNSS>7) caused bigger ventricular shifts. At the same time, the corresponding coordinates of the left ventricle (except for the APL shift) did not practically correlate with the clinical scores (Table, Figure 1). Depths were not affected at either time-points (data not shown).
Stereotaxic verification of coordinates - NNT
Using the AP and ML coordinates for subgroups Av and Bv, described in section "Verification of the new post-ischemic stereotaxic coordinates" and the data from Fig. 1 (linear equations in insert tables), we achieved 100% (5/5) success of ICV invasion in subgroup Av and 100% (5/5) in subgroup Bv. Using the "theoretical" injection sites in the 14 animals of subgroup Ac and the 16 of subgroup Bc, the success of ICV invasion in both ventricles was 92.8% (13/14) (p>0.05 for comparison of subgroups Av and Ac) and 56.25% (9/16) (p<0.05 for comparisons of subgroups Bc and Bv) respectively. The NNT for the proposed new coordinates of the 18-hours time-point (subgroups Bc and Bv) was significantly low (2.28), meaning that in order to successfully achieve ICV invasion 18-hours after p-MCAO in 10 animals, one should use approximately 18 animals using the classical Paxinos coordinates ("theoretical") compared to approximately 10 animals using the proposed corrected coordinates. Representative results for both time-points are shown in Figure 2.
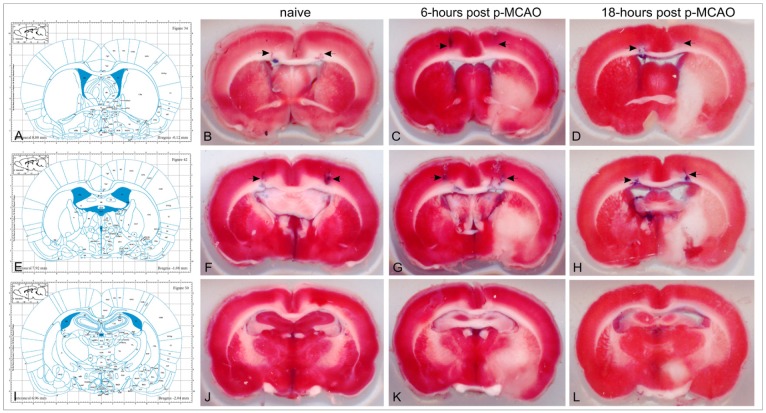
Figure 2. The corresponding Paxinos atlas' plates (with permission from "The rat brain in stereotaxic coordinates", Paxinos G. and Watson C., figures 34, 42 and 50; copyright Elsevier Academic Press 2005) and the representative brain TTC-stained sections at the levels -0.12 (B, C, D), -1.08 (F, G, H) and -2.04mm (J, K, L) from bregma. Blue traces on the TTC sections (arrows) indicate the track and final position of the stereotaxic needle. Naïve animals (group N) did not need any correction of the Paxinos coordinates (B, F). On 6-hours, the classical coordinates (uncorrected) were sufficient enough for invasion in the lateral ventricles of p-MCAO animals (subgroup Av) (C, G). On 18-hours (subgroup Bv), the new (corrected) coordinates were needed to successfully reach the targeted ventricle in p-MCAO animals (D, H).
Discussion
In the present study we measured for the first time the ventricular shifts after p-MCAO in Wistar rats and we evaluated the success and reliability of stereotaxic ICV invasion. Our data indicate that post-ischemic edema kinetics in this model resulted in mild ventricular shifts within the first 6 hours (that were not correlated with the clinical scores of the animals), whereas18 hours post-occlusion the ventricular shifts were more pronounced (linearly correlated with the clinical scores). Accordingly, applied stereotaxic coordinates for successful ICV invasion at 6 and 18 hours post-infarction, were significantly different but highly predictable.
The first issue raised by our data, was the kinetics of edema and ventricular shifts using the model of p-MCAO, which were different when compared with the corresponding ones of the t-MCAO model11. The use of the same suture and general MCAO method, the same animal strain and provider, as well as the same tissue evaluation techniques allowed for such an indirect comparison of the 2 models. The accumulated edema and the secondary middle line shifts increased slowly within the first hours post p-MCAO, leading to relatively low pressure phenomena (structural dislocations) up to 6 hours. These data were partially verified by a recent in vivo MRI study of permanent focal ischemia, though in different model and rat strain15. Later on, edema gradually increased causing significant middle line dislocations and pressure-effect phenomena. This absence of reperfusion and blood re-supply, which are present in t-MCAO11, are probably a cause for delayed edema development in the p-MCAO and may explain the absence of herniation and acute mortality up to 18 hours16. In addition, it determined significantly different stereotaxic coordinates for ICV invasion at the 2 different time-points.
When the STAIR criteria are considered, the application of an ICV experimental infusion in a practically reasonable clinical therapeutic window after initiation of ischemia (i.e. 3-24 hours post-occlusion) is highly desired8. Given the fact that human stroke is not always followed by reperfusion of the ischemic area, both p-MCAO and t-MCAO models should be used during the experimental study of a new treatment8. However, as in the case for the t-MCAO models11, only one study22 to our knowledge has applied so far the ICV route of administration within 3 hours post-occlusion (using a pre-implanted cannula for successful administration), while most of the studies performed an ICV infusion either prior18-22, immediately after18, 23, 24 or shortly after the MCA occlusion19, thus avoiding the ischemic edema. Our data indicated that permanent occlusion did not significantly alter stereotaxia up to 6 hours post-p-MCAO and rendered the use of Paxinos and Watson coordinates12 safe for successful ICV invasion. On the contrary, on 18 hours post-occlusion, ventricular shifting was significant and correlated well with both neurological scores (mNSS and mBS), rendering the use of our new stereotaxic coordinates almost obligatory. However, the mNSS score was finally selected for mathematical calculations, as it is more widely accepted in the literature13, and its application was both time- and cost-effective with significantly low NNT score (2.28).
According to our opinion, the study of ventricular stereotaxia in p- and 2-hour t-MCAO opens up the scientific field of ICV infusions in the clinically feasible window of 6 and 18 hours post-occlusion. ICV route is highly desirable because it avoids systemic circulation and side effects and it allows for direct CNS administration of drugs in lower doses, "bypassing" the BBB9. Pre-implantable pumps or cannula that "keep" the route to the lateral ventricles open, irrespective of their post-ischemic dislocations, is a possible solution with relatively few attempts of ICV administration after cerebral ischemia in such time-points though25. We believe that our proposed methodology is simple, reproducible and cost-effective rendering a single ICV administration in MCAO models highly applicable for experimental "therapies" directed into the CSF compartment.
Conclusions
The classical stereotaxic coordinates12 are well applicable for ICV invasion in the lateral ventricles 6-hours after p-MCAO in Wistar rats. However, on 18-hours post p-MCAO, the observed ventricular shifts preclude successful ICV invasion in the infarcted ventricle unless new, calculated, ML and AP coordinates are applied. Combination of our present data with the recently published ones for t-MCAO11 provides, to our opinion, a practical methodology for ICV infusions in acute phase after focal cerebral ischemia in 2 basic MCAO models in Wistar rats. In this way, our data render the ICV route of administration more accessible for future experimental interventions that target the CSF compartment.
Acknowledgements
The authors would like to thank Prof. Nikolaos Grigoriadis for critical reviewing of the manuscript. The present Project was funded by the European Union – European Social Fund and National Resources under a "Regional District Fund of Central Macedonia" Grant. Equipment used in the present study was kindly donated by "Empeirikion" and "Bodosakis" Foundations, Athens, Greece.
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study.
Table 1. Correlations between ventricular shifts and neurological scores at 6 and 18 hours (hrs) post-occlusion.
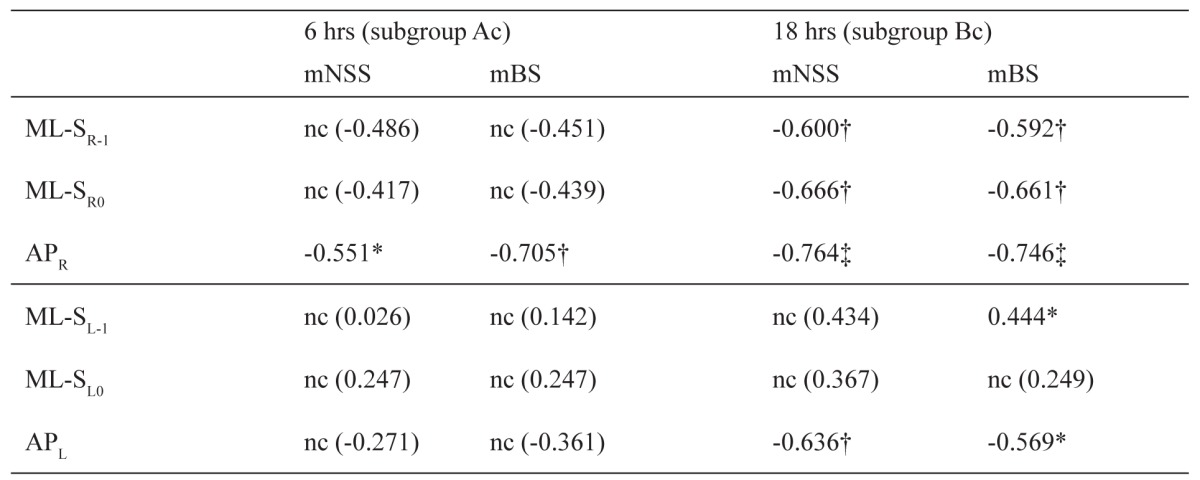
Values display Pearson's r score indicating positive or negative significance (* for p≤0.05; † for p≤0.01; ‡ for p≤0.001). "nc": no correlation between the 2 values. ML-SR0, ML-SR-1 and APR are the final stereotaxic mediolateral (ML) and anteroposterior (AP) shift for the right ventricle (sites SR0 and R-1). Accordingly, ML-SL0, ML-SL-1 and APL are the final shift for the left ventricle (sites SL0 and L-1).
References
- 1.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 2.Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema. 1. A new experimental model of cerebral embolism in rats in which recirculation can be introduced in ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- 3.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 4.Lourbopoulos A, Karacostas D, Artemis N, Milonas I, Grigoriadis N. Effectiveness of a new modified intraluminal suture for temporary middle cerebral artery occlusion in rats of various weight. J Neurosci Methods. 2008;173:225–234. doi: 10.1016/j.jneumeth.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87:179–197. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Memezawa H, Smith ML, Siesjo BK. Penumbral tissues salvaged by reperfusion following middle cerebral artery occlusion in rats. Stroke. 1992;23:552–559. doi: 10.1161/01.str.23.4.552. [DOI] [PubMed] [Google Scholar]
- 7.Walberer M, Stolz E, Muller C, Friedrich C, Rottger C, Blaes F, et al. Experimental stroke: ischaemic lesion volume and oedema formation differ among rat strains (a comparison between Wistar and Sprague-Dawley rats using MRI) Lab Anim. 2006;40:1–8. doi: 10.1258/002367706775404426. [DOI] [PubMed] [Google Scholar]
- 8.Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 9.Barcia JA, Gallego JM. Intraventricular and intracerebral delivery of anti-epileptic drugs in the kindling model. Neurotherapeutics. 2009;6:337–343. doi: 10.1016/j.nurt.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sipos EP, Brem H. New delivery systems for brain tumor therapy. Neurol Clin. 1995;13:813–825. [PubMed] [Google Scholar]
- 11.Lourbopoulos A, Grigoriadis N, Karacostas D, Spandou E, Artemis N, Milonas I, et al. Predictable ventricular shift after focal cerebral ischaemia in rats: practical considerations for intraventricular therapeutic interventions. Lab Anim. 2010;44:71–78. doi: 10.1258/la.2009.009043. [DOI] [PubMed] [Google Scholar]
- 12.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, ed 5th edition. Elsevier Academic Press. 2005 [Google Scholar]
- 13.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 14.Kirkwood BR, Sterne JAC. Essential Medical Statistics, ed 2nd edition. Oxford: Blackwell Science. 2003;pp:455–456. [Google Scholar]
- 15.Gerriets T, Walberer M, Ritschel N, Tschernatsch M, Mueller C, Bachmann G, et al. Edema formation in the hyperacute phase of ischemic stroke. Laboratory investigation. J Neurosurg. 2009;111:1036–1042. doi: 10.3171/2009.3.JNS081040. [DOI] [PubMed] [Google Scholar]
- 16.Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH, et al. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke. 2000;31:1965–1972. doi: 10.1161/01.str.31.8.1965. [DOI] [PubMed] [Google Scholar]
- 17.Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- 18.Chan KM, Lam DT, Pong K, Widmer HR, Hefti F. Neurotrophin-4/5 treatment reduces infarct size in rats with middle cerebral artery occlusion. Neurochem Res. 1996;21:763–767. doi: 10.1007/BF02532298. [DOI] [PubMed] [Google Scholar]
- 19.Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Reglodi D, Tamas A, Somogyvari-Vigh A, Szanto Z, Kertes E, Lenard L, et al. Effects of pretreatment with PACAP on the infarct size and functional outcome in rat permanent focal cerebral ischemia. Peptides. 2002;23:2227–2234. doi: 10.1016/s0196-9781(02)00262-0. [DOI] [PubMed] [Google Scholar]
- 21.Tokime T, Nozaki K, Sugino T, Kikuchi H, Hashimoto N, Ueda K. Enhanced poly(ADP-ribosyl)ation after focal ischemia in rat brain. J Cereb Blood Flow Metab. 1998;18:991–997. doi: 10.1097/00004647-199809000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Xu XH, Hua YN, Zhang HL, Wu JC, Miao YZ, Han R, et al. Greater stress protein expression enhanced by combined prostaglandin A1 and lithium in a rat model of focal ischemia. Acta Pharmacol Sin. 2007;28:1097–1104. doi: 10.1111/j.1745-7254.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- 23.Rauca C, Schafer K, Hollt V. Effects of somatostatin, octreotide and cortistatin on ischaemic neuronal damage following permanent middle cerebral artery occlusion in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:633–638. doi: 10.1007/s002109900136. [DOI] [PubMed] [Google Scholar]
- 24.Zhang HL, Gu ZL, Savitz SI, Han F, Fukunaga K, Qin ZH. Neuroprotective effects of prostaglandin A(1) in rat models of permanent focal cerebral ischemia are associated with nuclear factor-kappaB inhibition and peroxisome proliferator-activated receptor-gamma up-regulation. J Neurosci Res. 2008;86:1132–1141. doi: 10.1002/jnr.21569. [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Zhang Y, Shi J. Effects of intracerebroventricular infusion of angiotensin-(1-7) on bradykinin formation and the kinin receptor expression after focal cerebral ischemia-reperfusion in rats. Brain Res. 2008;1219:127–135. doi: 10.1016/j.brainres.2008.04.057. [DOI] [PubMed] [Google Scholar]