Abstract
Background and aim: Induction with anti-thymocyte globulin (ATG) during solid organ transplantation is associated with an improved clinical course and leads to prolonged lymphopenia. This study aims to investigate whether prolonged lymphopenia, caused by ATG induction, has an impact on patient and graft survival following liver and kidney transplantation.
Patients and Methods: This was a single-center, retrospective study. A total of 292 liver and 417 kidney transplants were performed with ATG induction (6 mg/kgr, divided into four doses), and the transplant recipients were followed for at least three months. The average lymphocyte count for the first 30 days after the operation was calculated, and the cut-off value for defining lymphopenia was arbitrarily set to ≤ 500 cells/mm3.
Results: There were 210 liver transplant recipients (71.9%) who achieved prolonged lymphopenia, whereas the remaining 82 recipients (28.1%) did not. The mean survival time of these patient groups was 10.27 and 12.71 years, respectively (p = 0.1217), and the mean graft survival time was 8.98 and 12.25 years, respectively (p = 0.0147). Of the kidney transplant patients, 330 (79.1%) recipients achieved prolonged lymphopenia, whereas the remaining 87 (20.9%) did not. The mean survival time of these patient groups was 13.94 and 14.59 years, respectively, (p = 0.4490), and the mean graft survival time was 11.84 and 11.54 years, respectively (p = 0.7410).
Conclusion: The efficacy and safety of ATG induction partially depend on decreased total lymphocyte counts. Following ATG induction in liver transplant recipients, a reasonable average lymphocyte count during the first postoperative month would be above 500 cells/mm3.
Keywords: lymphodepletion, lymphopenia, lymphocytes, anti-thymocyte globulin, induction, transplantation, liver, kidney, outcomes, survival
Induction with anti-thymocyte globulin (ATG) in liver transplant recipients is associated with an improved clinical course in cases with a rejection episode, and this treatment also has beneficial effects on renal function1,2. Moreover, ATG has been used in liver transplantation as a "prope" tolerogenic regimen3. In addition, ATG induction is associated with a reduced incidence of delayed (DGF) or slow (SGF) graft function4 and a higher rate of graft survival following renal transplantation5.
Induction with ATG leads to prolonged lymphopenia that exists beyond the typical post-operative period and may last for two or even three years6. Moreover, the efficacy of ATG in renal transplantation is reported to stem from this period of prolonged lymphopenia7, and early (post-operative) lymphopenia following liver transplantation is associated with fewer episodes of acute cellular rejection (ACR)8.
Lymphopenia has long been implicated in the development of post-transplant malignancies and opportunistic infections9. However, recent studies have not reported increases in either post-transplant lympho-proliferative disease or cytomegalovirus (CMV) infection following the use of ATG1,7.
This study aims to investigate the extent to which lymphopenia is achieved following induction with ATG in cases of solid organ transplantation and whether prolonged lymphopenia has an impact on patient and graft survival following liver and kidney transplantation.
Patients and methods
This was a single-center, retrospective study of prospectively collected data. Between 1990 and 2006, 292 primary adult liver and 417 adult kidney transplants were performed with ATG induction (6 mg/kgr, divided into 4 doses that were administered within 14 days). These cases were followed for at least 3 months. The treatment regimen for maintenance immunosuppression included the use of tacrolimus, cyclosporine, mofetil mucophenolate, azathioprine, mammalian target of rapamycin inhibitors and steroids (Table 1). Re-transplants and multi-organ recipients were excluded from the study. The average lymphocyte count for the 30 days following the operation was calculated, and the cut-off value for defining prolonged lymphopenia was arbitrarily set to ≤ 500 lymphocytes/mm3. For each type of transplanted organ, two cohorts were investigated: cohort A', which included recipients with prolonged lymphopenia, and cohort B', which included recipients without prolonged lymphopenia. Graft survival (censored for patient death), patient survival, ACR (incidence/patient), the occurrence of infections (incidence/patient), the recurrence of primary disease (for liver transplants only) and the glomerular filtration rate (calculated using the Nankivell formula at 1 year post-transplant, for renal transplants only) were analyzed. SGF was defined as a < 20% daily decrease in serum creatinine levels following kidney transplant, whereas DGF was defined as the need for hemodialysis during the early post-operative (30-day) period.
Table 1. Immunosuppression among liver transplant recipients. Immunosuppression status one year after liver transplantation.
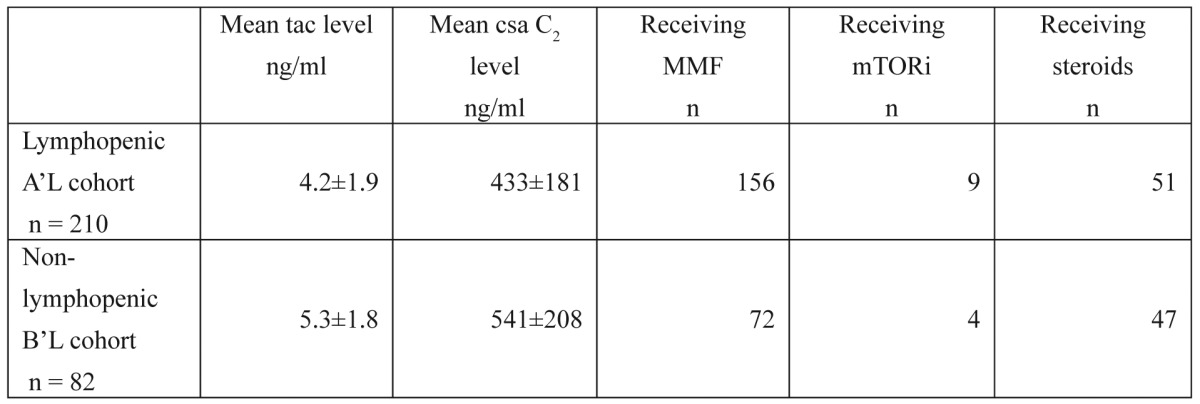
tac: tacrolimus, csa: cyclosporine, MMF: mofetil mucophenolate, mTORi: mammalian target of rapamycin inhibitor
Results Liver
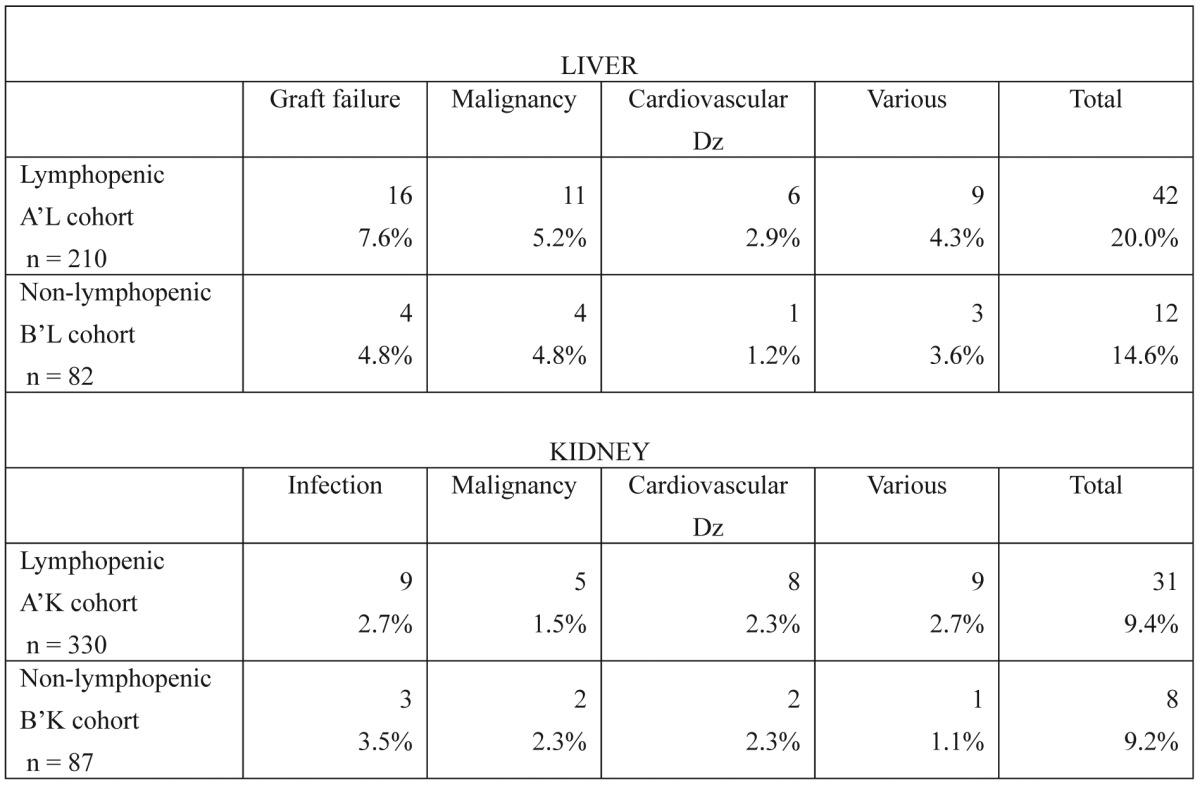
There were 210 liver transplant recipients (71.9%) who achieved prolonged lymphopenia (cohort A'L), whereas the remaining 82 recipients (28.1%) did not (cohort B'L). Mean patient survival time was 10.27 (95% CI: 9.39, 11.15) and 12.71 (95% CI: 10.98, 14.43) years for the two cohorts, respectively (p = 0.1217). The main causes of transplant recipient death in both cohorts included graft failure, malignancy and cardiovascular disease (Table 2).
Table 2. Causes of death in liver and kidney transplant recipients. No statistically significant differences were found between the lymphopenic and non-lymphopenic cohorts regarding any of the parameters studied for either organ (p < 0.05).
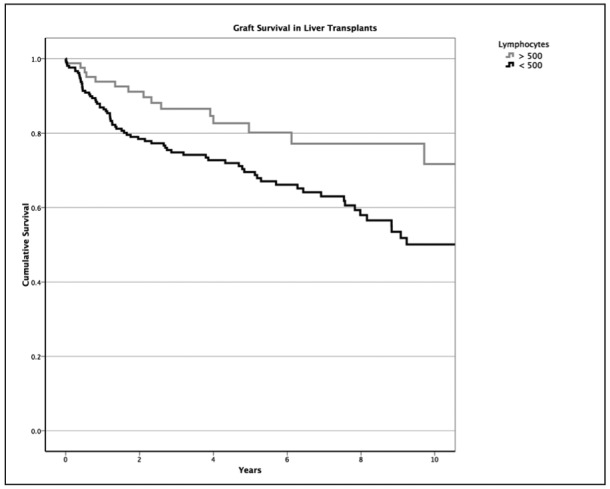
Also for these liver recipient cohorts, the mean graft survival periods were 8.98 (95% CI: 8.07, 9.88) and 12.25 (95% CI: 10.48, 14.02) years, respectively (Figure 1), and this difference between the groups was statistically significant (p = 0.0147). However, there was no impact of prolonged lymphopenia on graft survival when a subgroup analysis according to primary patient disease was performed (data not shown).
Figure 1. The impact of lymphopenia on liver graft survival is depicted. Early post-transplant deaths (first 90 post-operative days), secondary mainly to technical complications, were excluded because these recipients could not be followed for a minimum of three months.
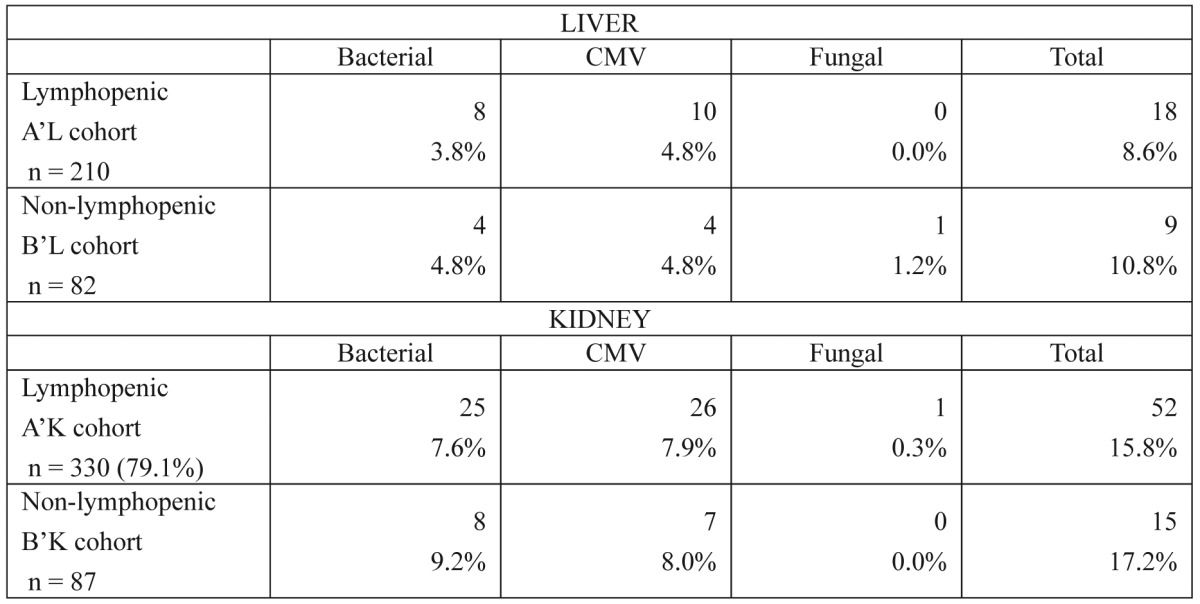
Sixty-three patients from the first group (30%) and 36 patients from the second group (43.9%) experienced at least one biopsy-proven episode of ACR (Table 3) (p = 0.028). In addition, 10 patients from the A'L group (4.8%) and 4 patients from the B'L group (4.8%) were diagnosed with CMV infections (Table 4) (p = 0.989).
Table 3. Episodes of acute rejection in liver transplant recipients. The values listed represent patients who experienced at least one episode of acute rejection, and some recipients experienced more than one rejection episode. Of note is the higher rate of late (more than one year) and steroid-resistant rejection episodes among non-lymphopenic recipients.
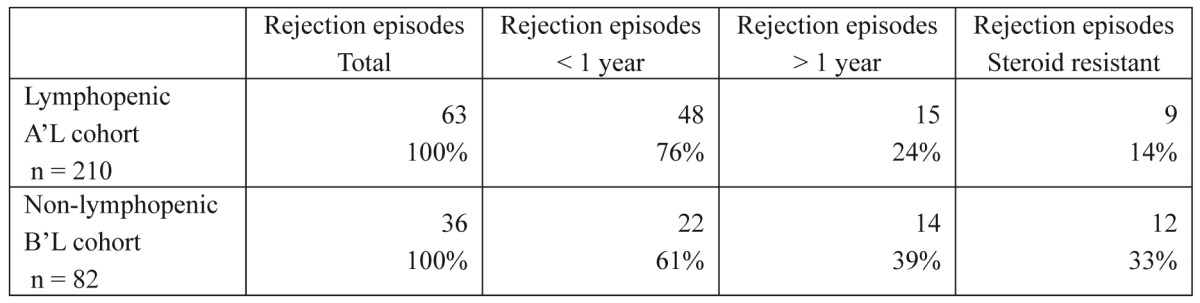
Table 4. Episodes of infection in liver and kidney transplant recipients. Only episodes of bacterial infections that required hospitalization were included. No significant differences were identified between the lymphopenic and non-lymphopenic cohorts regarding any of the parameters studied for either organ (p < 0.05).
Kidney
There were 330 renal transplant recipients (79.1%) who achieved prolonged lymphopenia (cohort A'K), whereas the remaining 87 recipients (20.9%) did not (cohort B'K). Mean patient survival time was 13.94 (95% CI: 13.33, 14.55) and 14.59 (95% CI: 13.19, 15.99) years for the A'K and B'K cohorts, respectively (p= 0.4490). The main causes of recipient death in both cohorts included infection, malignancy and cardiovascular disease (Table 2).
Also for these renal recipient cohorts, the mean graft survival periods were 11.84 (95% CI: 11.08, 12.59) and 11.54 (95% CI: 9.67, 13.41) years, respectively (p= 0.4553). A subgroup analysis revealed that there was no impact of lymphopenia on patient or graft survival, when the analysis was performed according to graft extended criteria donor (ECD) status or human leukocyte antigen (HLA) mismatches (data not shown). In addition, the calculated GFR one year post-transplant was 65.85 ± 20.81 and 60.49 ± 19.77 ml/sec/m2 for the A'K and B'K cohorts, respectively (p= 0.038).
Forty-one patients from the A'K group (14.2%) and 13 patients from the B'K group (17.6%) experienced at least one biopsy-proven episode of ACR (p= 0.590). Additionally, 26 patients from the A'K group (8.7%) and 7 patients from the B'K group (9.5%) were diagnosed with CMV infections (Table 4) (p= 0.822).
Discussion
Lymphopenia has been shown to persist for two weeks when equine ATG is used for induction following renal transplantation10. In contrast, the use of rabbit ATG typically leads to long-term lymphopenia that can last for more than two years7. In these studies, lymphopenia was defined as the persistence of an absolute lymphocyte count below the baseline level. For instance, the average lymphocyte count five years post-kidney transplant for recipients considered lymphopenic was 1,017 ± 200 cells/mm3,7. In the present study, which assessed the use of rabbit ATG, the arbitrary definition of prolonged lymphopenia used the much lower lymphocyte count of 500 cells/mm3. This may explain the discrepancy between studies regarding the number of lymphopenic patients identified (values in this study ranged from 71.9% to 79.1%, as opposed to the 100% value reported in previous studies)7,10. Indeed, in the present analysis, if the threshold for lymphopenia had been raised to 1000 cells/mm3, the percentage of lymphopenic patients would have been 100% at 30 days and greater than 60% at one year post-transplant.
The total ATG dose of 6 mg/kgr is considered an average to low dose, especially when compared to the doses administered for kidney transplantation that can exceed 8 mg/kgr11. However, in certain tolerogenic studies on liver transplantation12, the ATG doses used were even smaller. For example, a study by Eason et al. 13 used a 3 mg/kgr dose of ATG. However, this particular dosing scheme (which was in combination with a regimen of maintenance immunosuppression) resulted in an unacceptably high rate of acute rejection. Therefore, a total ATG dose below 6 mg/kgr may not be effective, and the use of lower doses has fallen out of favor in the most recent tolerogenic studies14.
Graft function is significantly impaired in liver transplant recipients who display prolonged lymphopenia. This may seem to be an odd result, as the rejection rate for liver transplant recipients with prolonged lymphopenia is significantly lower than that of recipients without lymphopenia. However, upon a more in-depth analysis of the rejection episodes, as depicted in Table 3, liver transplant recipients with prolonged lymphopenia were found to suffer from additional late, steroid-resistant acute rejection episodes, and this may explain the inferior graft outcome in this cohort. These severe rejection episodes may have resulted from either the use of less potent maintenance immunosuppression in lymphopenic liver transplant recipients (as shown in Table 1) or the greater number of effector memory CD8+ T cells that develop six months after transplantation with ATG induction (as increased lymphopenia is associated with increased CD8+ T-cell expansion)14. In addition, it is tempting to speculate that minor rejection episodes in non-lymphopenic liver transplant recipients promote chimerism15, induce tolerance at a later stage and ultimately lead to improved graft survival.
As lymphopenic liver transplant recipients are not more likely to succumb to post-transplant malignancies or opportunistic infections compared to non-lymphopenic patients, we can assume that the trend of decreased transplant recipient survival in cases with prolonged lymphopenia derives from decreased graft survival. In addition, patients with impaired T-cell reconstitution following ATG induction suffer from an increased rate of atherosclerotic events16. Indeed, in the present study, deaths due to cardiovascular events were more than twice as common among lymphopenic liver transplant recipients. Although this trend was not significant, it may support the evidence for inferior patient survival following hepatic transplantation.
Lymphopenic kidney recipients exhibited rates of patient and graft survival that were similar to those observed among non-lymphopenic patients. This may be easily explained by the fact that these two groups were similar in terms of their rates of ACR, opportunistic infections and post-transplant malignancies (data not shown). Although this observation is a departure from the results of recent studies that have demonstrated decreased survival among lymphopenic kidney recipients11, this discrepancy may be explained by the fact that the value chosen to define lymphopenia in this study was relatively high at 500 cells/mm3. Indeed, if the cut-off value for lymphopenia had been set to 250 cells/mm3, the rate of lymphopenic kidney recipient survival would have been decreased compared to the survival of non-lymphopenic patients (13.62 and 14.95 years, respectively, p= 0.0471). Thus, it is tempting to speculate that one of the many known or unknown immunomodulatory effects of ATG17 may have led to the inferior survival of severely and persistently lymphopenic patients in this study.
It also seems as if the efficacy and safety of ATG treatment may be related to decreased total lymphocyte counts and perhaps even to the elimination of specific lymphocyte subpopulations. A reasonable average lymphocyte count for the first month following surgery and ATG induction in liver transplant recipients would be greater than 500 cells/mm3. Moreover, further investigations that involve immunophenotyping and the use of functional assays should confirm or reject the hypothesis that "molding" or altering the frequencies of specific lymphocyte sub-populations could benefit liver and kidney transplant recipient survival.
References
- 1.Soliman T, Hetz H, Burghuber C, Gyori G, Silberhumer G, Steininger R, et al. Short-term induction therapy with anti-thymocyte globulin and delayed use of calcineurin inhibitors in orthotopic liver transplantation. Liver Transpl. 2007;13:1039–1044. doi: 10.1002/lt.21185. [DOI] [PubMed] [Google Scholar]
- 2.Cantarovich M, Tchervenkov J, Paraskevas S, Ghali P, Wong P, Deschenes M, et al. Early Changes in Kidney Function Predict Long-Term Chronic Kidney Disease and Mortality in Patients After Liver Transplantation. Transplantation. 2011;Nov 7: epub ahead of print doi: 10.1097/TP.0b013e3182384aff. [DOI] [PubMed] [Google Scholar]
- 3.De Ruvo N, Cucchetti A, Lauro A, Masetti M, Cautero N, Di Benedetto F, et al. Preliminary results of a "prope" tolerogenic regimen with thymoglobulin pretreatment and hepatitis C virus recurrence in liver transplantation. Transplantation. 2005;80:8–12. doi: 10.1097/01.tp.0000164349.54297.95. [DOI] [PubMed] [Google Scholar]
- 4.Goggins WC, Pascual MA, Powelson JA, Magee C, Tolkoff-Rubin N, Farrell ML, et al. A prospective, randomized, clinical trial of intraoperative versus postoperative Thymoglobulin in adult cadaveric renal transplant recipients. Transplantation. 2003;76:798–802. doi: 10.1097/01.TP.0000081042.67285.91. [DOI] [PubMed] [Google Scholar]
- 5.Agha IA, Rueda J, Alvarez A, Singer GG, Miller BW, Flavin K, et al. Short course induction immunosuppression with thymoglobulin for renal transplant recipients. Transplantation. 2002;73:473–475. doi: 10.1097/00007890-200202150-00025. [DOI] [PubMed] [Google Scholar]
- 6.Esposito L, Kamar N, Tkaczuk J, Abbal M, Durand D, Rostaing L. Long-term evolution of lymphocytes subsets after induction therapy based on continuous versus discontinuous administration of anti-thymocyte globulins in renal-transplant patients. Transplant Proc. 2005;37:785–787. doi: 10.1016/j.transproceed.2004.12.200. [DOI] [PubMed] [Google Scholar]
- 7.Hardinger KL, Schnitzler MA, Miller B, Lowell JA, Shenoy S, Koch MJ, et al. Five-year follow up of thymoglobulin versus ATGAM induction in adult renal transplantation. Transplantation. 2004;78:136–141. doi: 10.1097/01.tp.0000132329.67611.3f. [DOI] [PubMed] [Google Scholar]
- 8.Roayaie S, Sheiner PA, Emre S, Guy S, Schwartz ME, Boros P, et al. Cytokine profiles in early rejection following OKT3 treatment in liver transplant patients. Mediators Inflamm. 2000;9:141–146. doi: 10.1080/09629350020002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ducloux D, Carron PL, Rebibou JM, Aubin F, Fournier V, Bresson-Vautrin C, et al. CD4 lymphocytopenia as a risk factor for skin cancers in renal transplant recipients. Transplantation. 1998;65:1270–1272. doi: 10.1097/00007890-199805150-00022. [DOI] [PubMed] [Google Scholar]
- 10.Brennan DC, Flavin K, Lowell JA, Howard TK, Shenoy S, Burgess S, et al. A randomized, double-blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999;67:1011–1018. doi: 10.1097/00007890-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 11.Ducloux D, Courivaud C, Bamoulid J, Vivet B, Chabroux A, Deschamps M, et al. Prolonged CD4 T cell lymphopenia increases morbidity and mortality after renal transplantation. J Am Soc Nephrol. 2010;21:868–875. doi: 10.1681/ASN.2009090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Murase N, Abu-Elmagd K, Gray EA, Shapiro R, Eghtesad B, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502–1510. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eason JD, Cohen AJ, Nair S, Alcantera T, Loss GE. Tolerance: is it worth the risk? Transplantation. 2005;79:1157–1159. doi: 10.1097/01.tp.0000162084.46555.10. [DOI] [PubMed] [Google Scholar]
- 14.Benitez CE, Puig-Pey I, Lopez M, Martinez-Llordella M, Lozano JJ, Bohne F, et al. ATG-Fresenius treatment and low-dose tacrolimus: results of a randomized controlled trial in liver transplantation. Am J Transplant. 2010;10:2296–2304. doi: 10.1111/j.1600-6143.2010.03164.x. [DOI] [PubMed] [Google Scholar]
- 15.Bishop GA, McCaughan GW. Immune activation is required for the induction of liver allograft tolerance: implications for immunosuppressive therapy. Liver Transpl. 2001;7:161–172. doi: 10.1053/jlts.2001.22321. [DOI] [PubMed] [Google Scholar]
- 16.Ducloux D, Challier B, Saas P, Tiberghien P, Chalopin JM. CD4 cell lymphopenia and atherosclerosis in renal transplant recipients. J Am Soc Nephrol. 2003;14:767–772. doi: 10.1097/01.asn.0000048718.43419.44. [DOI] [PubMed] [Google Scholar]
- 17.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21:1387–1394. doi: 10.1038/sj.leu.2404683. [DOI] [PubMed] [Google Scholar]


