Abstract
Aim:This clinical study was planned to assess pulmonary microvascular permeability in patients with Syndrome X (SX) by using a functional imaging tool, technetium-99m-diethyltriaminepentaaceticacid (99mTc-DTPA) lung clearance scintigraphy, and the pulmonary functions test, which includes diffusion capacity of the lung for carbon monoxide (DLCO).
Methods:The study population consisted of 22 non-smoker subjects divided into two groups. First group comprised 12 patients (4 male, 8 female, mean age: 48±4 years, range 36 to 65) with SX. Ten healthy subject (4 men, 6 female, mean age: 45±3 years, range 34 to 58) were served as control group. Volumetric pulmonary functions, including DLCO were also performed before lung scintigraphy. Alveolar epithelial permeability was assessed by measuring the pulmonary clearance of an inhaled 99mTc-DTPA using a gamma camera.
Results: Spirometric data was comparable in both groups. Although volumetric pulmonary measurements were similar, DLCO values of SX patients were lower than those in control (20.9±1.7 ml/min/mmHg vs. 27.8±1.3 ml/min/mmHg, p=0.002). The mean clearance rate of 99mTc-DTPA in control subjects was 106±6 min, and this value was lower than patients with SX (179±19 min; p=0.0001).
Conclusion: We conclude that lung is a target organ for SX. The pulmonary gas exchange and microvascular permeability, which is measured by 99mTc-DTPA scintigraphy, are restricted without change of volumetric pulmonary functions in patients with SX.
Keywords: 99mTc-DTPA, syndrome X, lung, carbon monoxide diffusing capacity, microvascular
Kemp1 introduced the term Syndrome X (SX) to describe a group of patients with angina pectoris and positive exercise electrocardigraphic test with normal coronary angiograms. Some of these patients had regional myocardial perfusion defect and/or wall motion abnormalities2-4. The exact pathophysiological mechanisms underlying this condition are not well understood, and many mechanisms for the chest pain have been suggested. Since the epicardial coronary arteries are defined angiographically normal, attention has been focused on dysfunction of the coronary microcirculation5. In some studies, microvascular dysfunction has been accused to cause angina6-8. In addition to exertional chest pain, all these patients had complain of breathlessness, with no evidence of airway obstruction or resting left ventricular dysfunction9. Cannon et al10 reported that airway hyper responsiveness is frequently demonstrable in patients with microvascular angina; moreover they hypothesized that this syndrome may represent a more generalized abnormality of vascular and nonvascular smooth muscle function. Recent studies reported that patients with SX had endothelial dysfunction11 and generalized arterial distensibility12. There are a few studies about the microvascular angiopathy of SX on the other organ system13-15. As the other microangiopathic disease, the best known is diabetes mellitus16. Besides the others, the most famillier microangiopathic disease is DM in which the lung could be a target organ for microvascular pathologies in patients with SX. Thereby, this clinical study was planned to assess pulmonary microvascular permeability in patients with SX by using a functional imaging tool, technetium-99m-diethyltriaminepentaaceticacid (99mTc-DTPA) lung clearance scintigraphy, and the pulmonary functions test, which includes diffusion capacity of the lung for carbon monoxide (DLCO).
Material and methods
The study population consisted of 22 non-smoker subjects divided into two groups. First group comprised 12 patients (4 men, mean age: 48±4 years, range 36 to 65) with SX, which was defined as a combination of chest pain, positive treadmill exercise test, negative ergonovine and hyperventilation test, and angiographically proven normal coronary arteries, and no existence of diabetes mellitus, hypertension and left ventricular hypertrophy. Left ventricular hypertrophy was defined echocardiographically as a left ventricular mass index greater than 134 g/m2 in men and greater than 110 g/m2 in women. Ten healthy subject (4 men, mean age: 45±3 years, range 34 to 58) without coronary artery disease, pulmonary disease and diabetes mellitus served as control group.
Pulmonary function tests: Spirometric parameters, forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), total lung capacity (TLC), diffusing capacity of the lung for carbon monoxide (DLCO) and alveolar volume (VA) were measured with a water-sealed spirometer (Sensor Medics 2400, Bilthoven, The Netherlands). Values were expressed as percentages of the predicted values calculated according to sex, weight, height and age 17. Spirometry was performed in the standard sitting position at least three times in accordance with American Thoracic Society recommendations 18. The best values of FVC and FEV1 were selected for analysis. TLC was estimated with the helium dilution method. DLCO was measured in the sitting position with the single-breath method 19. DLCO values were corrected for the hemoglobin concentration of the patient. For this correction, we used the following equation: Corrected DLCO = Measured DLCO* [1.00 + (%COHb/100)]. Transfer coefficient (KCO) was calculated from DLCO /VA.
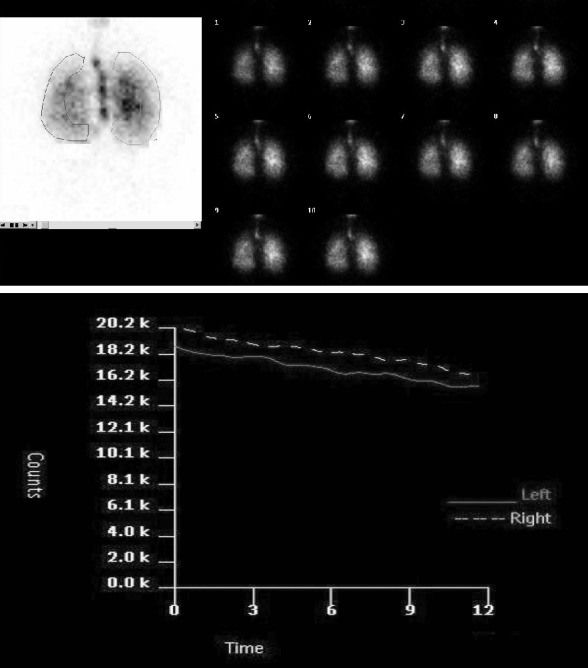
99mTc-DTPA aerosol inhalation scintigraphy: Tc-99m DTPA (CIS, France) was chelated by introducing 1480MBq of sodium 99mTcO4 - into 5 ml of normal saline. Tc-99m DTPA was placed in the nebulizer reservoir of a commercially available system (Venticis II, CIS, France). Aerosols with a mass median diameter of 0.8 µ were produced with oxygen in flow of 10-12 L/min. Patients inhaled for 3 min in the sited position. Approximately 10% total activity was administered to patients during the 3-min inhalation. The subjects were placed over a gamma camera (Orbiter; Siemens Corp., Iselin, NJ, USA) with low- energy; all-purpose collimator and lung fields were imaged in posterior projection. One-minute frames were acquired in a 64x64 matrix for 30 min. Regions of interest (ROIs) were drawn around the periphery of the lungs and on the major airways on the first-minute image (Figure 1). To obtain a pure alveolar ROI and to exclude the entire bronchial activity, the outer third of each lung was used as the peripheral lung region. The inner two-thirds of the lung was defined as the central lung region. The brightness of the image was increased to visualize body background and the lung periphery, thereby permitting correct definition of the peripheral ROIs. Time-activity curves were generated and curves were corrected for 99mTc decay. T1/2 was calculated by placing a mono-exponential fit on the curves. T1/2 of whole lung was calculated as the mean of the T1/2 of the left lung and the right lung. Penetration index (PI) was also calculated by dividing the peripheral total counts by the sum of the peripheral and central total counts on the first-minute image, in order to quantify the distribution of the inhaled aerosol [PI = peripheral total counts/(peripheral total counts + central total counts)].
Figure 1. A: Serial images of 99mTc-DTPA aerosol inhalation scintigraphy and ROI selection on the first-minute image. B:99mTc-DTPA clearance curve.
Statistical analysis:
Values are expressed as mean ± SEM. Statistical comparisons of the data between the groups were carried out using Mann Whitney U tests. Relation between scintigraphic data and pulmonary function test variables were assessed with Pearson correlation an r coefficient. A p value < 0.05 was set as statistical significance.
Results
Demographic variables and hemoglobin values were similar in SX and control group (Table 1). Spirometric data was comparable in both groups. When comparison to volumetric lung function parameters no difference was found in SX and control groups (Table 2). Hovewer, DLCO and DLCOc values of SX patients were lower than those in control (Table 3). 99mTc-DTPA aerosol was distributed uniformly throughout the lungs of all subjects without central deposition. SX patients had lower PI values than control subjects, but did not reach significant level. The mean PI values of SX and control groups were 52±2% and 59±3%, respectively. The mean clearance rate of 99mTc-DTPA in control subjects was 106±6 min. The highest T ½ value was noted in SX patients (179±19 min; p=0.0001) (Figure 2). Scintigraphic index were summarized in Table 3.
Table 1. Comparison of demographic variables and hemoglobin values in patients with syndrome X and control subjects.
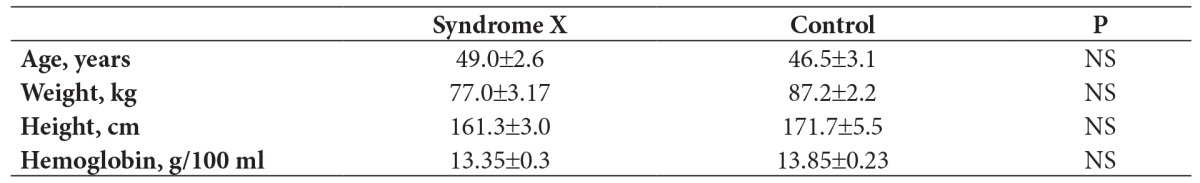
NS: Non-significant. Values are expressed as mean±SE.
Table 2. Volumetric lung function values in patients with syndrome X and control subjects.

FEV1: Forced expiratory volume in 1 s, FVC: Forced vital capacity, TLC: Total lung capacity, VC: Vital capacity, NS: Non-significant. *: Values are expressed as percent predicted. Values are expressed as mean±SE.
Table 3. DLCO, DLCOc, KCO values and scintigraphic index in patients with syndrome X and control subjects.

DLCO: Diffusing capacity of the lung for carbon monoxide, DLCOc: Corrected diffusing capacity of the lung for carbon monoxide, KCO: Transfer coefficient, PI: Penetration index, CR: 99mTc-DTPA clearance rate (t ½ values), NS: Non-significant. *: Values are expressed as percent predicted. Values are expressed as mean±SE.
Figure 2. The mean t1/2 values of study groups. There are significant differences between syndrome X and control subjects (p=0001).

Discussion
The most important contribution of presented study is to show pulmonary gas exchanges including DLCO (transfer factor) for carbon monoxide and microvascular permeability measuring 99mTc-DTPA scintigraphy, which are restricted without change of volumetric pulmonary function in patients with SX.
DLCO as a lung function test can provide information about the transport of gas from alveolar air to haemoglobin. Decreased DLCO in patients with SX can explain microvascular angiopathy of lung alveolar capillaries. Cannon et al10 are hypothesized that this syndrome may represent a more generalized abnormality of vascular and nonvascular smooth muscle function. Recently, Pai et al13 findings were supported this systemic microvascular abnormalities in patients with SX, and reported that SX was a systemic vascular disorder with a high incidence of hypoperfusion lesions of the brain using 99m Tc-ECD brain SPECT. Our previous two scintigraphic studies, including brain15 and myocardial perfusion4, have also been suggested the systemic microvascular abnormalities in patients with SX. Besides Bund et al14 also showed that there were morphological abnormalities in small arteries obtained from biopsies of skin and subcutaneous fat.
99mTc- DTPA radioaerosol scintigraphy has been used to detect functional defects of the alveolo-capillary barrier in different interstitial lung disease or lung toxicity20-27. The 99mTc-DTPA complex has a molecular weight of 490 Dalton21. When it is deposited on the pulmonary epithelial surface, it diffuses from the air space to the vascular space and is finally filtered by the kidneys. The overall change in alveolar clearance of the solute is dependents on several factors: the surface area for transfer, the concentration gradient across the alveolar-capillary membrane, and the diffusion distance or epithelial wall thickness10-27. In contrast to gases, which are able to diffuse throughout the whole of the alveolar-capillary surface area, the diffusion of hydrophilic solutes is thought to be restricted to the much more smaller surface area occupied by the intercellular junction. In both diffusion of gas and solute, capillary endothelium is rate limiting part of alveolar-capillary membrane. The finding of present study suggested that lung is a target organ in patients with SX, and microvascular angiopathy of lung capillaries is restricting diffusion, without change of volumetric pulmonary function.
Montorsi et al28 reported a circadian variation of coronary vascular response to exercise in patients with SX by using cardiopulmonary exercise test. Some other studies have suggested a potential etiological role of excessive sympathetic activation29-32. The lungs, capillary endothelium are a major organ for removal of circulating monoamines and play a significant role in the inactivation of circulating norepinephrine33. It might be speculated that if microvascular pathology effects capillary endothelium of lung, this impact also may be responsible for the circadian variation, altering monoamine degradation.
Low DLCO and KCO is a well known pathology of some microvascular damage, e.g. chronic heart failure, primary pulmonary hypertension34. To our best knowledge, there is no data in English literature to assess gas exchange and microvascular permeability by using 99mTc-DTPA lung radioaerosol scintigraphy in patients with SX.
The major study limitation was the small size of the study group. When comparison to SX and control groups we found differences in both DLCO and the mean clearance rate of 99mTc-DTPA measurements in the present study. However futher studies in larger groups could be intensified reability of the results. The present study Additionally, diffusion capacity of the membranes and microvascular blood volume could be calculated separately by Roughton and Forster method19. It should be noted , as a limitation of our study is that the diffusing capacity of the alveolar-capillary membrane and capillary blood volume for available gas exchange were not separately determined; this might be helpful during the clarifying the findings.
We conclude that lung is a target organ for SX. The pulmonary gas exchanges and microvascular permeability, measured by 99mTc-DTPA scintigraphy, are restricted without change of volumetric pulmonary function in patients with SX.
References
- 1.Kemp HG. Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol. 1973;32:375–376. doi: 10.1016/s0002-9149(73)80150-x. [DOI] [PubMed] [Google Scholar]
- 2.Venneker EH, van der Wall EE. Syndrome X: does it exist? Eur J Nucl Med. 1994;21:95–97. doi: 10.1007/BF00175753. [DOI] [PubMed] [Google Scholar]
- 3.Taki J, Nakajima K, Muramori A, Yoshio H, Shimizu M, Hisada K. Left ventricular dysfunction during exercise in patients with angina pectoris and angiographically normal coronary arteries (syndrome X) Eur J Nucl Med. 1994;21:98–102. doi: 10.1007/BF00175754. [DOI] [PubMed] [Google Scholar]
- 4.Sarikaya A, Durmus- Altun G, Altun A, Ozcelik F, Kaya M, Berkarda S. Sendrom X’ li hastalarda semikantitatif Tc-99m MIBI SPECT calısmasının rolu. Turk J Cardiol. 1999;2:8–13. [Google Scholar]
- 5.Vazquez-Rey E, Kaski JC. Cardiovascular syndrome X and endothelial dysfunction. Rev Esp Cardiol. 2003;56:181–192. doi: 10.1016/s0300-8932(03)76843-2. [DOI] [PubMed] [Google Scholar]
- 6.Cannon RO 3rd, Bonow RO, Bacharach SL, Green MV, Rosing DR, Leon MB, et al. Left ventricular dysfunction in patients with angina pectoris, normal epicardial coronary arteries, and abnormal vasodilator reserve. Circulation. 1985;71:218–226. doi: 10.1161/01.cir.71.2.218. [DOI] [PubMed] [Google Scholar]
- 7.Rosen SD, Uren NG, Kaski JC, Tousoulis D, Davies GJ, Camici PG. Coronary vasodilator reserve, pain perception, and sex in patients with syndrome X. Circulation. 1994;90:50–60. doi: 10.1161/01.cir.90.1.50. [DOI] [PubMed] [Google Scholar]
- 8.Lin CP, Lin WT, Leu HB, Wu TC, Chen JW. Differential mononuclear cell activity and endothelial inflammation in coronary artery disease and cardiac syndrome X. Int J Cardiol. 2003;89:53–62. doi: 10.1016/s0167-5273(02)00428-x. [DOI] [PubMed] [Google Scholar]
- 9.Tweddel A, Carter R, Banham SW, Hutton I. Breathlessness in microvascular angina. Respir Med. 1994;88:731–736. doi: 10.1016/s0954-6111(05)80194-5. [DOI] [PubMed] [Google Scholar]
- 10.Cannon RO 3rd, Peden DB, Berkebile C, Schenke WH, Kaliner MA, Epstein SE. Airway hyperresponsiveness in patients with microvascular angina. Evidence for a diffuse disorder of smooth muscle responsiveness. Circulation. 1990;82:2011–2017. doi: 10.1161/01.cir.82.6.2011. [DOI] [PubMed] [Google Scholar]
- 11.Li AH, Lee BC, Chen KC, Weng CS, Chu SH. Brachial artery flow-mediated vasodilation in patients with cardiac syndrome X. Angiology. 2008;59:581–586. doi: 10.1177/0003319707308032. [DOI] [PubMed] [Google Scholar]
- 12.Yildiz M, Altun A, Ozbay G. Assessment of arterial distensibility in patients with cardiac syndrome X. Angiology. 2007;58:458–462. doi: 10.1177/0003319707305064. [DOI] [PubMed] [Google Scholar]
- 13.Pai PY, Liu FY, Kao A, Lin CC, Lee CC. A higher prevalence of abnormal regional cerebral blood flow in patients with syndrome X and abnormal myocardial perfusion. Jpn Heart J. 2003;44:145–152. doi: 10.1536/jhj.44.145. [DOI] [PubMed] [Google Scholar]
- 14.Bund SJ, Tweddel A, Hutton I, Heagerty AM. Small artery structural alterations of patients with microvascular angina (syndrome X) Clin Sci (Lond) 1996;91:739–743. doi: 10.1042/cs0910739. [DOI] [PubMed] [Google Scholar]
- 15.Kaya M, Sarikaya A, Ozcelik F, Durmus-Altun G, Berkarda S. Sendrom X’.li Hastalarda Tc- 99m HMPAO Beyin Perfüzyon SPECT. Nöropsikiatri Arşivi. 1999;36:132–135. [Google Scholar]
- 16.Dalquen P. The lung in diabetes mellitus. Respiration. 1999;66:12–13. doi: 10.1159/000029330. [DOI] [PubMed] [Google Scholar]
- 17.Knudson RJ, Lebowitz MD, Holberg CJ, Burrow B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Resp Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society. Standardization of spiromety 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 19.Roughton FJW, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957;11:290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- 20.Barrowcliffe MP, Jones JG. Solute permeability of the alveolar capillary barrier. Thorax. 1987;42:1–10. doi: 10.1136/thx.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coates G, O’Brodovich H. Measurement of pulmonary epithelial permeability with 99mTc- DTPA aerosol. Semin Nucl Med. 1986;16:275–284. doi: 10.1016/s0001-2998(86)80014-9. [DOI] [PubMed] [Google Scholar]
- 22.Durmus-Altun G, Altun A, Salihoglu YS, Altaner S, Berkarda S, Ozbay G. Value of technetium-99m diethyltriamine pentaaceticacid radioaerosol inhalation lung scintigraphy for the stage of amiodarone-induced pulmonary toxicity. Int J Cardiol. 2004;95:193–197. doi: 10.1016/j.ijcard.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Kaya M, Salan A, Tabakoglu E, Aydogdu N, Berkarda S. The bronchoalveolar epithelial permeability in house painters as determined by Tc-99m DTPA aerosol scintigraphy. Ann Nucl Med. 2003;17:305–308. doi: 10.1007/BF02988526. [DOI] [PubMed] [Google Scholar]
- 24.Line BR. Scintigraphic studies of inflammation in diffuse lung disease. Radiol Clin North Am. 1991;29:1095–1114. [PubMed] [Google Scholar]
- 25.Miller RF, O’Doherty MJ. Pulmonary nuclear medicine. Eur J Nucl Med. 1992;19:355–368. doi: 10.1007/BF00177058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uh S, Lee SM, Kim HT, Chung Y, Kim YH, Park CS. The clearance rate of alveolar epithelium using 99mTc-DTPA in patients with diffuse infiltrative lung diseases. Chest. 1994;106:161–165. doi: 10.1378/chest.106.1.161. [DOI] [PubMed] [Google Scholar]
- 27.O’Doherty MJ, Peters AM. Pulmonary technetium-99m diethylene triamine penta-acetic acid aerosol clearance as an index of lung injury. Eur J Nucl Med. 1997;24:81–87. doi: 10.1007/BF01728316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uzal C, Durmus-Altun G, Caloglu M, Ergulen A, Altaner S, Yigitbasi NO. The protective effect of amifostine on radiation-induced acute pulmonary toxicity: Detection by (99m)Tc-DTPA transalveolar clearances. Int J Radiat Oncol Biol Phys. 2004;60:564–569. doi: 10.1016/j.ijrobp.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 29.Montorsi P, Agostoni PG, Annoni L, Vincenzi P, Guazzi MD. Cardiopulmonary exercise testing in syndrome X. Am Heart J. 1993;125:711–717. doi: 10.1016/0002-8703(93)90162-3. [DOI] [PubMed] [Google Scholar]
- 30.Lanza GA, Giordano A, Pristipino C, Calcagni ML, Meduri G, Trani C, et al. Relationship between myocardial 123I-metaiodobenzylguanidine scintigraphic uptake and heart rate variability in patients with syndrome X. Ital Heart J. 2000;1:221–225. [PubMed] [Google Scholar]
- 31.Lee WL, Chen JW, Lin SJ, Hsu NW, Chang MS, Ting CT. Parasympathetic withdrawal antedates dynamic myocardial ischemia in patients with syndrome X. Int J Cardiol. 1998;66:253–260. doi: 10.1016/s0167-5273(98)00223-x. [DOI] [PubMed] [Google Scholar]
- 32.Altun A, Erdogan O, Tatli E, Ugur-Altun B, Durmus-Altun G, Ozbay G. Increased P wave dispersion: a new finding in patients with syndrome X. Can J Cardiol. 2002;18:1207–1210. [PubMed] [Google Scholar]
- 33.Ponikowski P, Rosano GM, Amadi AA, Collins P, Coats AJ, Poole-Wilson PA, Kaski JC. Transient autonomic dysfunction precedes ST-segment depression in patients with syndrome X. Am J Cardiol. 1996;77:942–947. doi: 10.1016/s0002-9149(96)00007-0. [DOI] [PubMed] [Google Scholar]
- 34.Sole MJ, Drobac M, Schwartz L, Hussain MN, Vaughan-Neil EF. The extraction of circulating catecholamines by the lungs in normal man and in patients with pulmonary hypertension. Circulation. 1979;60:160–163. doi: 10.1161/01.cir.60.1.160. [DOI] [PubMed] [Google Scholar]


