Abstract
Background: The influence of factors such as age, sex, life style and smoking on oxidative stress status of the organism remains unclear. There is evidence that dietary intake of antioxidants is thought to enforce the organism ability to counteract free radicals. Administration of synthetic antioxidants as dietary supplements does not seem to have the same beneficial effect as consumption of the same antioxidants as part of food ingredients. This work focuses on the investigation of age and diet effects on oxidative stress and examines the hypotheses of their significant influence.
Methods: Blood samples of 146 volunteers, were collected and allocated in three age groups. All volunteers completed a questionnaire concerning home and working environmental conditions, special habits and dietary preferences. We implemented a thirty days diet rich in antioxidants in 55 volunteers. Antioxidant activity was estimated before and after the special diet by measuring the influence of serum in oxidation of ABTS by the ferryl myoglobinhydrogen peroxide system.
Results: Our findings showed unexpected lower serum total antioxidant capacity (TAC) in younger people (ages 18–35 yrs) 79%, compared to middle aged and elderly individuals and a large increase 62% in serum TAC of all age-groups after the one-month special diet.
Conclusion: These results imply that a diet rich in antioxidants based on antioxidant rich food consumption and not on single antioxidants administration, can increase the antioxidant status of the organism and offer better health. The total serum antioxidant status increases with age and this fact should be taken into account when TAC is measured in different diseases.
Keywords: antioxidants, serum, age, diet, cortisol
Reactive Oxygen Species (ROS) are produced in human organism during the normal aerobic metabolism or due to the action of the macrophages as part of the immune response1. They are playing a dual role as both deleterious and beneficial species2. ROS are normally generated by tightly regulated enzymes, such as Nitric oxide synthase (NOS) and Nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase isoforms. It is known that oxidative stress induces or promotes various diseases3 such as atherosclerosis and heart diseases4-6, cancer7, neurodegenerative diseases (Alzheimer disease, Parkinson’s disease), hypertension8,9, diabetes mellitus10 and ageing11-15. It is also argued that oxidative stress constitutes one of the main factors responsible for ischemia/reperfusion injury.
Human organism has developed an antioxidant system16 to counteract ROS and reduce the damage they cause. The antioxidant system includes various endogenous molecules17 such as reduced glutathione (GSH), uric acid, billirubin, ceruloplasmin, ferritin, vitamins such as ascorbic acid, and tocopherol18 and a lot of enzymes such as superoxide dismutase, catalase, glutathioneperoxidase19-21. Food derived antioxidants are added in the total antioxidant activity of human organism. In these antioxidants we can include polyphenols such as quercetin, tannins and flavonoids present in olive oil, tomatoes, berries, grapes, red wine and beer, coffee, tea and coccoa, lycopene found in red and orange fruits such as tomatoes, carrots, watermelon and antioxidant vitamins found in all fruits and vegetables22. The ten top food sources of antioxidants are coffee, tea, banana, dry beans, corn, red wine, beer, apple, tomato, and potato. Administration of synthetic antioxidants as dietary supplements does not seem to have the same beneficial effect as the consumption of the same antioxidants as part of food ingredients. Moreover, the kind of antioxidants in food, as well as, food preparation procedure and food mixtures used, influences their effectiveness.
The cooperation of all antioxidant components can provide more protection against ROS than any single compound alone. So, a balanced natural antioxidant rich diet is preferred than a diet rich in a single antioxidant. High concentration of blood antioxidants seems to increase organism’s defense against certain diseases and treatment with antioxidants is often proposed as part of the therapy23,24. On the other hand, antioxidant levels may be used as a diagnosis or prognosis indicator in some cases. Measurement of the whole blood antioxidant capacity of the organism may give us valuable biological information about the status of the organism.
At present, there are no consistent perspectives about age-associated change of antioxidant capacity. Certain researchers studied the presentation of catalase, peroxide oxidase, some antioxidant vitamins, lipid peroxidation or protein and DNA oxidation and found increase or decrease of different factors with age25-27. In most cases a serum Total Antioxidant Capacity (TAC) increase with age was observed. However, different measurement methods lead to different results. For example, measurement with FRAP method which cannot determine antioxidants with sulfydril groups28, showed decrease in TAC with age29. In other studies an increase in TAC with age was found as a secondary observation in patients suffering from cancer disease24.
Factors such as sex, stress, physical activity29-31 smoking32 and diet 33-37 also affect total antioxidant capacity. It has been reported that habits such as smoking and bad diet incline toward increase of oxidative stress, while dietary antioxidant intake can increase total antioxidant capacity38-42.
Another important factor, which is associated with antioxidant levels, is cortisol, usually referred to as the “stress hormone”. Cortisol is involved in organism’s response to stress and anxiety. It triggers all metabolic mechanisms leading to production of compounds used as energy sources in emergency conditions. Increased cortisol43 concentrations are measured in blood serum of people under biological or emotional stress, depression, sleep deprivation, fever, hypoglycemia, anorexia nervosa and after surgery44. Moreover, increase in cortisol concentration has been associated with decrease in total antioxidant levels45. The level of some tissue antioxidants is inversely related to cortisol tissue levels46. Antioxidant depletion may result as a consequence of the various pathways activated by cortisol or may be the effect of free radical production during cortisol metabolism. Antidepressant therapy resulting in decrease of cortisol levels also results in the increase of the antioxidant concentration.
The effects of age and diet on antioxidant capacity have not investigated sufficiently up to date in Greece. The present study deals with examining the hypotheses of the statistically significant effects of age and diet in Total Antioxidant Capacity using the 2,2’-azino-bis(3-ethylbenzthiazoline)-6-sulphonic acid (ABTS) method to random samples of Thessaloniki residents. The correlation between cortisol and TAC levels is also examined.
Material and methods
Blood samples
Total blood antioxidants were measured in serum of 146 healthy individuals, 55 male and 91 female, living in the same region and were classified in three groups based on their age; A: 16–35 years (n=65), B: 36–60 years (n=33) and C: 61–90 years (n=48). All individuals were volunteers, residents of Thessaloniki, Greece. The participants were fully informed about the purpose and content of the experiment and their informed consent of participation were obtained. They completed a questionnaire concerning place and type of work, life style, special habits, smoking, diet preferences, medicines and chronic diseases. All subjects had to meet the following criteria: they were no athletes, no pregnant and they had not a metabolic disease
Sample collection
Blood samples were collected from the cubitus vein, at rest, at 08.00 a.m. After the first blood collection, 55 volunteers of all age groups followed a diet rich in antioxidants (fresh fruits and vegetables, coffee, chocolate, olive oil, honey, red wine, tea, tomatoes) for one month. At the end of the month a second blood sample was collected (2nd blood collection). After one week a third blood sample (3d blood collection) was collected. During this week the volunteers were let to select their diet freely. Detailed dietary records were maintained before the study, during the thirty-day period of antioxidant-rich diet and during the week after the second blood collection. The special diet was rich in different antioxidants, such as vitamin C, E, lycopene flavonoids tannins, carrotenoids. All samples were kept at –70°C for maximum one month until measurement.
Measurements of total antioxidant capacity
After studying different methods47,48 TEAC (trolox equivalent antioxidant capacity) methodology was used to measure the serum total antioxidant capacity. Antioxidant activity was estimated by the influence of 10 μl of serum in oxidation of 2,2’-azino-bis(3-ethylbenthiazoline)-6-sulfonic acid (ABTS) by the ferryl myoglobin–H2O2 system, using the antioxidant assay kit of Sigma. Trolox, the water soluble analogue of vitamin E, obtained from the same company, was used as control. Antioxidant concentration was expressed as trolox equivalents.
Measurement of cortisol
Serum cortisol was measured using the enzyme-linked immunosorbent assay (ELISA) kit of HUMAN. The method is based on competitive interaction of serum cortisol and a cortisol-enzyme conjugated provided by HUMAN for a limited number of monoclonal anti-cortisol antibodies bound to the wells of a microtitre ELISA plate49. The amount of the bound hormone-enzyme conjugate is inversely proportional to the concentration of cortisol in the specimen. After removal of unbound conjugate by washing, a substrate solution is added and a blue color develops, which changes to yellow by the addition of stop solution. The absorbance is measured at 450 nm using a mictotitre plate reader. Concentration of unknown specimens is interpolated from a dose response curve generated by utilizing serum calibrators of known cortisol concentration.
Statistical Analyses
Data were analyzed using the Statistical Package for the Social Sciences (SPSS). Normality of continuous data was determined by Kolmogorov-Smirnov or Shapiro-Wilk normality test. All continuous data following normal distribution are presented as mean ± standard deviation (SD). Independent-samples t-test was used to compare the blood samples of different age groups while paired-samples t-test was used to determine the effect of extra diet in TAC. A two tailed P value less than 0.05 was considered statistically significant for all comparisons.
Results
Experiment 1: Correlation between TAC and age
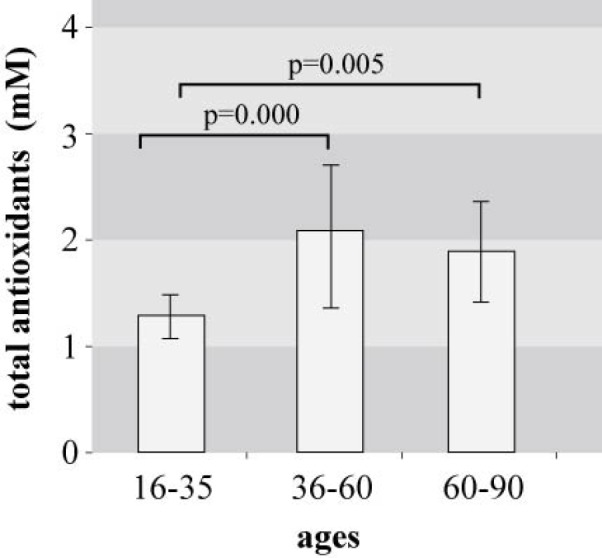
Factors such as smoking and physical activity which can influence the total antioxidant capacity were similar in all subjects. No significant difference was observed in serum TAC between male and female individuals. Evaluation of the blood antioxidant capacity of the subjects in relation to age revealed a significant difference among young people 16-35 years old and the middle-aged and elderly individuals. (Table 1, Figure 1). The mean values of antioxidant capacity were lower in group A (by 79%) than in the other groups.
Table 1. Percentage differentiation of total serum antioxidant Capacity (TAC) in different age groups compared to the young group of 16-35 yrs.
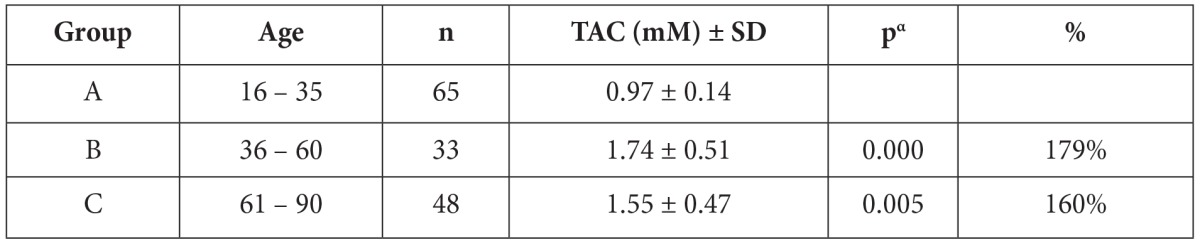
Data are means ± standard deviation (SD). The Student’s t test has been used for all samples. The results of all age groups were compared with the results of age group 16-35. pa is the significance level. % increase of TAC compared to the results of age group 16-35.
Figure 1. Total antioxidant capacity in blood serum of three age groups. Significance according to Student’s t-test: between age groups 16-35 yrs and 36-60 yrs: p=0.000; between age groups 16-35 yrs and 60-90 yrs: p=0.005.
Experiment 2: Correlation between TAC and cortisol levels
After the results of the first experiment we tried to understand the lower antioxidant level in younger people. Thus, we measured in this group the serum cortisol levels and compared it with random population. As shown in Figure 2 the antioxidant potential and the cortisol levels were lower in the first age group.
Experiment 3: Correlation between TAC and diet
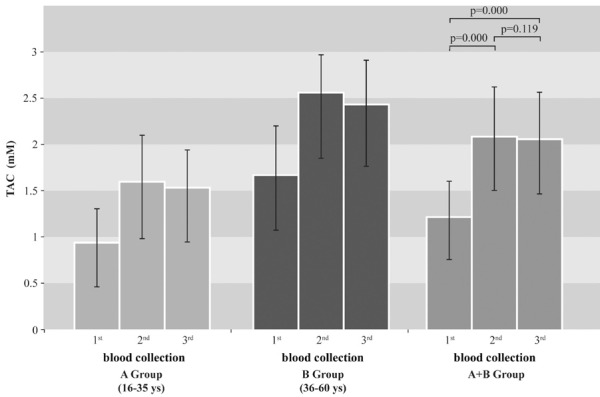
The correlation of blood antioxidant concentration with nutrition was examined (Table 2, Figure 3). Increased serum antioxidants levels were observed after the 30-days diet rich in antioxidants in 70% of the volunteers (p=0.000). The increase reaches in some subjects 270%. Thirty per cent of the subjects who did not present any increase, did not follow the diet as gathered by the dietary records they kept. After the third blood collection, there was a low not significant decrease in serum antioxidants (p=0.119).
Table 2. Serum total antioxidant Capacity and % change after 30day diet full in antioxidants (2nd blood collection) and after 15 day free diet (3d blood collection).
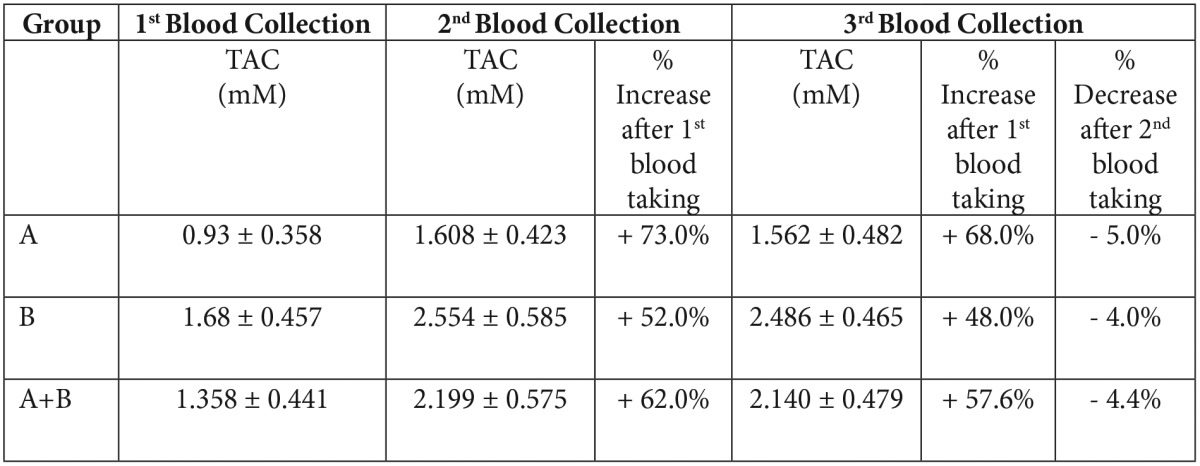
Data are means ± standard deviation (SD). The Student’s t test has been used for all samples.
Figure 3. Total antioxidant capacity (TAC) in blood serum before (1st blood collection) and after (2nd and 3d blood collection) diet. Significance, according to Student’s t-test. Data are representative of means ± SD TAC in serum after diet rich in antioxidants was significantly higher (2nd blood collection): p=0,000. TAC values remained high one week after the diet (3d blood taking): p=0.119.
Discussion
Oxidative stress and age
The present study analyzed the antioxidant capacity of three different age groups. As a primary result, the middle-aged and elderly subjects showed higher antioxidant capacity than the younger subjects. Previous studies have acknowledged age-related increase of oxidative stress25,27.
The results suggested that oxidative stress in young people is independent from their current stress (most of them were students and participated in the survey some days before their semester exams) and may be explained with their diet and daily routine (Figure 2). On the other hand, especially in Greece, elderly people have a better diet than the younger ones. The oxidative stress might increase with malnutrition and stress45 and is not so relevant with age. Previous studies have reported blood GSSG/GSH increase with age, suggesting that glutathione redox status inclines to a direction of the oxidation with age50,51. Another study, however, displayed constant balance in blood GSSG/GSH despite increase of plasma oxidative stress with age52. Another major serum component, which could be responsible for the increased TAC in elderly people, is urate. For example, TAC is increased in hemodialysis patients, despite the increased oxidative stress that characterizes them and this is due to increased urate levels.53 Our study presumed these findings. Although the cause of this hypothesis is not clear, it is assumed that good health and eating habits of these middle-aged and elderly subjects may be factors in well-balanced antioxidant capacity.
Figure 2. Total antioxidant capacity and cortisol in blood serum of the first age group according to random population.
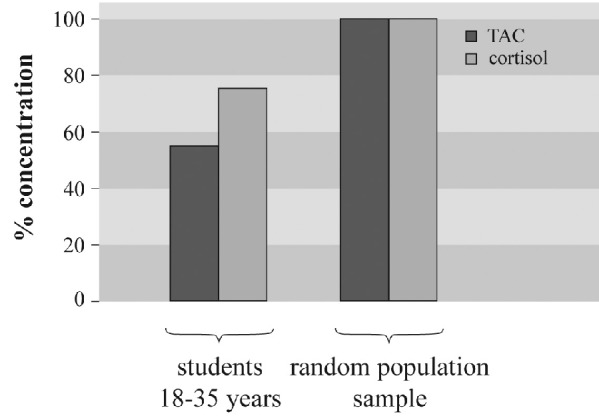
Young people and especially students are under biological and emotional stress. This may lead to high cortisol levels, which can be an additional cause for antioxidants reduction. From our results, we assume that stress is not the reason of lower antioxidant levels in young people.
Oxidative stress and nutrition
Our findings highlight the possibility that antioxidant activity in the organism could be increased by high dietary intakes of fruit, vegetables and other rich in antioxidants constituents23,24,36. A positive, statistically significant correlation with antioxidant rich diet was observed. The volunteers, who did not follow the diet, did not present any increase in serum TAC. Differences in each individual metabolism, daily activities36,38 and needs, as well as their preference in food combinations may explain the differences observed in diet response. The bioability and metabolism of food antioxidants are different and influence antioxidant response54,55. Vitamin E, for example, can act under special conditions as a prooxidant and not as an antioxidant. Such a condition takes place in vitro in the presence of sufficient amounts of vitamin E with concomitant depletion of other antioxidants that are necessary for vitamin E reduction, like ascorbate. This results in accumulation of the oxidized form of vitamin E that is able to oxidize lipids56,57. Besides certain food combinations may counteract the absorption and protective effects of flavonoids. Moreover, the diet may also contain prooxidants such as iron, copper and lipid peroxides58,59.
In conclusion, the significance of a balanced rich in antioxidants diet instead of single antioxidants administration is the major finding of the present study. There is also a need to take into account and more precisely study dietary combinations and habits to confirm these results.
References
- 1.Auten RL, Davis JM. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res. 2009;66:121–127. doi: 10.1203/PDR.0b013e3181a9eafb. [DOI] [PubMed] [Google Scholar]
- 2.Ochi H, Sakai K. Oxidative stress profile: OSP. Rinsho Byori. 2003;51:115–125. [PubMed] [Google Scholar]
- 3.Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease: randomised placebo-controlled trial. Lancet. 2000;356:1213–1218. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 5.Abudu N, Miller JJ, Attaelmannan M, Levinson SS. Vitamins in human arteriosclerosis with emphasis on vitamin C and vitamin E. Clin Chim Acta. 2004;339:11–25. doi: 10.1016/j.cccn.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Zureik M, Galan P, Bertrais S, Mennen L, Czernichow S, Blacher J, et al. Effects of long-term daily low-dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arterioscler Thromb Vasc Biol. 2004;24:1485–1491. doi: 10.1161/01.ATV.0000136648.62973.c8. [DOI] [PubMed] [Google Scholar]
- 7.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 8.Parslow RA, Sachdev P, Salonikas C, Lux O, Jorm AF, Naidoo D. Associations between plasma antioxidants and hypertension in a community-based sample of 415 Australians aged 60-64. J Hum Hypertens. 2005;19:219–226. doi: 10.1038/sj.jhh.1001809. [DOI] [PubMed] [Google Scholar]
- 9.Moriel P, Plavnik FL, Zanella MT, Bertolami MC, Abdalla DS. Lipid peroxidation and antioxidants in hyperlipidemia and hypertension. Biol Res. 2000;33:105–112. doi: 10.4067/s0716-97602000000200010. [DOI] [PubMed] [Google Scholar]
- 10.Dierckx N, Horvath G, van Gils C, Vertommen J, van de Vliet J, De Leeuw I, et al. Oxidative stress status in patients with diabetes mellitus: relationship to diet. Eur J Clin Nutr. 2003;57:999–1008. doi: 10.1038/sj.ejcn.1601635. [DOI] [PubMed] [Google Scholar]
- 11.Junqueira VB, Barros SB, Chan SS, Rodrigues L, Giavarotti L, Abud RL, et al. Aging and oxidative stress. Mol Aspects Med. 2004;25:5–16. doi: 10.1016/j.mam.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Tanabe K, Masuda K, Ajisaka R, Matsuda M, Hirayama A, Nagase S, et al. Relationships between Age, Daily Physical Activity, Antioxidant Capacity and Oxidative Stress among Middle-aged and Elderly People. Int J Sport Health Sci. 2006;4:515–527. [Google Scholar]
- 13.Kasapoglu M, Ozben T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp Gerontol. 2001;36:209–220. doi: 10.1016/s0531-5565(00)00198-4. [DOI] [PubMed] [Google Scholar]
- 14.Mecocci P, Polidori MC, Troiano L, Cherubini A, Cecchetti R, Pini G, et al. Plasma antioxidants and longevity: a study on healthy centenarians. Free Radic Biol Med. 2000;28:1243–1248. doi: 10.1016/s0891-5849(00)00246-x. [DOI] [PubMed] [Google Scholar]
- 15.Erden-Inal M, Sunal E, Kanbak G. Age-related changes in the glutathione redox system. Cell Biochem Funct. 2002;20:61–66. doi: 10.1002/cbf.937. [DOI] [PubMed] [Google Scholar]
- 16.Stanner SA, Hughes J, Kelly CN, Buttriss J. A review of the epidemiological evidence for the ‘antioxidant hypothesis’. Public Health Nutr. 2004;7:407–422. doi: 10.1079/phn2003543. [DOI] [PubMed] [Google Scholar]
- 17.Marusin AV, Salyukov VB, Yu Bragina E. Antioxidant Activity of Blood Plasma in Individuals with Neoplasms. Bull Exp Biol Med. 2002;133:481–483. doi: 10.1023/a:1019869922937. [DOI] [PubMed] [Google Scholar]
- 18.Behrens WA, Madere R. Alpha- and gamma tocopherol concentrations in human serum. J Am Coll Nutr. 1986;5:91–96. doi: 10.1080/07315724.1986.10720116. [DOI] [PubMed] [Google Scholar]
- 19.Andersen HR, Nielsen JB, Nielsen F, Gransdjean P. Antioxidative enzyme activities in human erythrocytes. Clin Chem. 1997;43:562–568. [PubMed] [Google Scholar]
- 20.Guemouri L, Artur Y, Herbeth B, Jeandel C, Cuny G, Siest G. Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clin Chem. 1991;37:1932–1937. [PubMed] [Google Scholar]
- 21.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 22.Cao G, Sofic E, Prior RL. Antioxidant capacity of tea and common vegetables. J Agric Food Chem. 1996;44:3426–3431. [Google Scholar]
- 23.Winklhofer-Roob BM, Rock E, Ribalta J, Shmerling DH, Roob JM. Effects of vitamin E and carotenoid status on oxidative stress in health and disease. Evidence obtained from human intervention studies. Mol Aspects Med. 2003;24:391–402. doi: 10.1016/s0098-2997(03)00035-9. [DOI] [PubMed] [Google Scholar]
- 24.Huang HY, Appel LJ. Supplementation of diets with alpha-tocopherol reduces serum concentrations of gamma- and delta-tocopherol in humans. J Nutr. 2003;133:3137–3140. doi: 10.1093/jn/133.10.3137. [DOI] [PubMed] [Google Scholar]
- 25.Mutlu-Turkoglu U, Ilhan E, Oztezcan S, Kuru A, Aykac-Toker G, Uysal M. Age-related increases in plasma malondialdehyde and protein carbonyl levels and lymphocyte DNA damage in elderly subjects. Clin Biochem. 2003;36:397–400. doi: 10.1016/s0009-9120(03)00035-3. [DOI] [PubMed] [Google Scholar]
- 26.Tanabe K, Masuda K, Sugawara J, Ajisaka R, Matsuda M, Kono I, et al. Effects of daily physical activity on oxidative stress in middle-aged and elderly people. Jpn J Phys Fitness Sports Med. 2002;51:325–336. [Google Scholar]
- 27.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 28.Gupta R, Sharma M, Lakshmy R, Prabhakaran D, Reddy SK. Improved method of total antioxidant assay. Indian J Biochem Biophys. 2009;46:126–129. [PubMed] [Google Scholar]
- 29.Οstka T, Drai J, Berthouze SE, Lacour JR, Bonnefoy M. Physical activity, fitness and intergrated antioxidant system in healthy active elderly women. Int J Sports Med. 1998;19:462–467. doi: 10.1055/s-2007-971945. [DOI] [PubMed] [Google Scholar]
- 30.Sen CK, Packer L. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr. 2000;72:653–669. doi: 10.1093/ajcn/72.2.653S. [DOI] [PubMed] [Google Scholar]
- 30.Tanabe K, Masuda K, Sugawara J, Ajisaka R, Matsuda M, Kono I, et al. Effects of different type of training on blood antioxidant capacity and redox balance in middle-aged and elderly women. J Exerc Physiol. 2003;10:65–76. [Google Scholar]
- 32.Marangon K, Herbeth B, Lecomte E, Paul-Dauphin A, Grolier P, Chancerelle Y, et al. Diet, antioxidant status, and smoking habits in French men. Am J Clin Nutr. 1998;67:231–239. doi: 10.1093/ajcn/67.2.231. [DOI] [PubMed] [Google Scholar]
- 33.Millen AE, Dodd KW, Subar AF. Use of vitamin, mineral, nonvitamin, and nonmineral supplements in the United States: The 1987, 1992, and 2000 National Health Interview Survey results. J Am Diet Assoc. 2004;104:942–950. doi: 10.1016/j.jada.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Balluz LS, Kieszak SM, Philen RM, Mulinare J. Vitamin and mineral supplement use in the United States. Results from the third National Health and Nutrition Examination Survey. Arch Fam Med. 2000;9:258–262. doi: 10.1001/archfami.9.3.258. [DOI] [PubMed] [Google Scholar]
- 35.Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 36.Block G, Patterson BH, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg ER. Vitamin E Supplements: Good in Theory, but Is the Theory Good? Ann Intern Med. 2005;142:75–76. doi: 10.7326/0003-4819-142-1-200501040-00112. [DOI] [PubMed] [Google Scholar]
- 38.Omaye ST, Burri BJ, Swendseid ME, Henning SM, Briggs LA, Bowen HT. Blood antioxidant changes in young women following beta-carotene depletion and repletion. J Am Coll Nutr. 1996;15:469–477. doi: 10.1080/07315724.1996.10718626. [DOI] [PubMed] [Google Scholar]
- 39.Prior RL. Fruits and vegetables in the prevention of cellular oxidative damage. Am J Clin Nutr. 2003;78:570–578. doi: 10.1093/ajcn/78.3.570S. [DOI] [PubMed] [Google Scholar]
- 40.Takamatsu S, Takamatsu M, Satoh K, Imaizumi T, Yoshida H, Hiramoto M, et al. Effects on health of dietary supplementation with 100 mg D-alpha-tocopheryl acetate, daily for 6 years. J Int Med Res. 1995;23:342–357. doi: 10.1177/030006059502300504. [DOI] [PubMed] [Google Scholar]
- 41.Van den Berg R, van Vliet T, Broekmans W M, Cnubben NH, Vaes WH, Roza L, et al. A vegetable/fruit concentrate with high antioxidant capacity has no effect on biomarkers of antioxidant status in male smokers. J Nutr. 2001;131:1714–1722. doi: 10.1093/jn/131.6.1714. [DOI] [PubMed] [Google Scholar]
- 42.John J, Ziebland S, Yudkin P, Roe L, Neil H. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: a randomised controlled trial. Lancet. 2002;359:1969–1974. doi: 10.1016/s0140-6736(02)98858-6. [DOI] [PubMed] [Google Scholar]
- 43.Lovallo WR, Farag NH, Vincent AS, Thomas TL, Wilson MF. Cortisol responses to mental stress, exercise, and meals following caffeine intake in men and women. Pharmacol Biochem Behav. 2006;83:441–447. doi: 10.1016/j.pbb.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruzic B, Tomaskovic I, Trnski D, Kraus O, Bekavac-Beslin M, Vrkic N. Systemic stress responses in patients undergoing surgery for benign prostatic hyperplasia. BJU international. 2005;95:77–80. doi: 10.1111/j.1464-410X.2004.05276.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Muxin G, Nishida H, Shirakawa C, Sato S, Konishi T. Psychological Stress-Induced Oxidative Stress as a Model of Sub-Healthy Condition and the Effect of TCM. Evid Based Complement Alternat Med. 2007;4:195–202. doi: 10.1093/ecam/nel080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thakor AS, Giussani DA. Antioxidants enhance the adrenocortical response to stress in the fetus. Endocrine Abstracts. 2005;10:OC22. [Google Scholar]
- 47.Janaszewska A, Bartosz G. Assay of total antioxidant capacity: comparison of four methods as applied to human blood plasma. Scand J Clin Lab Invest. 2002;62:231–236. doi: 10.1080/003655102317475498. [DOI] [PubMed] [Google Scholar]
- 48.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Perry LA, Gossman AB. The role of the laboratory in the diagnosis of Cushing’s syndrome. Ann Clin Biochem. 1997;34:345–359. doi: 10.1177/000456329703400403. [DOI] [PubMed] [Google Scholar]
- 50.Wang QL, Wang SR, Ding Y, Peng KJ, Lin X, Qiao XR, et al. Age-related changes of the redox state of glutathione in plasma. Chin Med J (Engl) 2005;118:1560–1563. [PubMed] [Google Scholar]
- 51.Lang CA, Naryshkin S, Schneider DL, Mills BJ, Lindeman RD. Low blood glutathione levels in healthy aging adults. J Lab Clin Med. 1992;120:720–725. [PubMed] [Google Scholar]
- 52.Tanabe K, Masuda K, Ajisaka R, Matsuda M, Hirayama A, Nogase S, et al. Relationship between age, daily physical activity antioxidant activity and oxidative stress among middle-aged and elderly people. Int J Sport Health Sci. 2006;4:515–527. [Google Scholar]
- 53.Antoniadi G, Eleftheriadis T, Liakopoulos V, Kakasi E, Kartsios Ch, Passadakis P, et al. Effect of One-year Oral α-Tocopherol Administration on the Antioxidant Defense System in Hemodialysis Patient. Ther Apher Dial. 2008;12:237–242. doi: 10.1111/j.1744-9987.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 54.Morishita H, Ohnishi M. Absorption, metabolism and biological activities of chlorogenic acids and related compounds. Stud Nat Prod Chem. 2001;25:919–953. [Google Scholar]
- 55.Gorinstein S, Medina Vargas O, Jaramillo N, Salas I, Martinez Ayala AL, Arancibia-Avila P, et al. The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. Eur Food Res Technol. 2010;225:321–328. [Google Scholar]
- 56.Doba T, Burton GW, Ingold KU. Antioxidant and co-antioxidant activity of vitamin C. The effect of vitamin C, either alone or in the presence of vitamin E or a water-soluble vitamin E analogue, upon the peroxidation of aqueous multilamellar phospholipid liposomes. Biochim Biophys Acta. 1985;835:298–303. doi: 10.1016/0005-2760(85)90285-1. [DOI] [PubMed] [Google Scholar]
- 57.Kagan VE, Serbinova EA, Forte T, Scita G, Packer L. Recycling of vitamin E in human low density lipoproteins. J Lipid Res. 1992;33:385–397. [PubMed] [Google Scholar]
- 58.Swain J, Gutteridge JM. Prooxidant iron and copper, with ferroxidase and xanthine oxidase activities in human atherosclerotic material. FEBS Lett. 1995;368:513–515. doi: 10.1016/0014-5793(95)00726-p. [DOI] [PubMed] [Google Scholar]
- 59.Yoshida Y, Tsuchiya J, Niki E. Interaction of alpha-tocopherol with copper and its effect on lipid peroxidation. Biochim Biophys Acta. 1994;1200:85–92. doi: 10.1016/0304-4165(94)90121-x. [DOI] [PubMed] [Google Scholar]


