Abstract
Background: Although left ventricular hypertrophy (LVH) is an independent predictor of mortality in patients with end stage renal disease, few have examined its prevalence before the initiation of dialysis. The aim of this study was to investigate the relationship between LVH, estimated glomerular filtration rate (GFR), and inflammatory markers in patients with chronic kidney disease (CKD).
Methods: Forty-one CKD patients (18 women, 23 men, mean age 53±17 years) with an estimated GFR between 15 and 59 mL/min (mean 34.2 mL/min) were enrolled and the following tests performed: routine serum biochemical analyses, high sensitivity C-reactive protein (hs-CRP), fibrinogen, ferritin, and homocysteine, and left ventricular mass index (LVMI), left ventricular ejection fraction (LVEF), and left ventricular fractional shortening (LVFS).
Results: LVH was diagnosed in 32/41 patients (78%). CKD patients with LVH (n=32) had significantly higher hs-CRP (p=0.012), fibrinogen (p=0.031), and lower serum albumin (p=0.028) levels than those without LVH (n=9). In all patients, LVMI correlated positively with hs-CRP (r=0.483, p=0.002) and serum fibrinogen (r=0.426, p=0.015). Estimated GFR correlated positively with LVEF (r=0.414, p=0.007) and LVFS (r=0.376, p=0.018).
Conclusions: Important positive associations exist between markers of inflammation and LVMI in patients with CKD. In addition to hs-CRP, elevated fibrinogen may portend the development of LVH in patients with CKD who are not yet on dialysis.
Keywords: chronic kidney disease, echocardiography, fibrinogen, hs-CRP, left ventricular hypertrophy
Patients with end stage renal disease (ESRD) are at high risk for vascular atherosclerosis and left ventricular hypertrophy (LVH)1,2. LVH is an independent predictor of cardiovascular mortality in patients with ESRD and appears to progress during dialysis therapy3-5. The high prevalence of LVH among ESRD patients at the start of dialysis therapy6 suggests that it might already be present in a significant proportion of chronic kidney disease (CKD) patients who are not yet on dialysis7.
Hypertension, hypervolemia, and anemia have been identified as major determinants of LVH in ESRD patients4,6. Other factors such as inappropriate activation of the renin-angiotensin-aldosterone system, oxidative stress, and inflammation may also play a role in left ventricular growth in ESRD8. Persistent activation of the inflammatory response has been recognized as an important independent risk factor for the development of cardiovascular complications in hemodialysis patients9. Levels of C-reactive protein (CRP), a marker of the reactant plasma protein component, correlate positively with LVH in patients receiving hemodialysis10,11. However, the number of studies examining the association between inflammatory markers and LVH in patients with CKD is limited.
In patients with CKD not yet on dialysis, the decline in creatinine clearance was associated with an increase in the left ventricular mass index12. Studies of LVH in CKD patients found that its prevalence increases with declining renal function7,12,13. Regression of LVH was noted in dialysis patients after successful kidney transplantion14. Both of these observations suggest that renal dysfunction is an important factor in the development of LVH.
The aim of this study was to investigate the relationship between estimated glomerular filtration rate (GFR), inflammatory markers, and left ventricular hypertrophy (LVH) in patients with CKD.
Subjects and methods
Patients
The study protocol was approved by our Faculty of Medicine Ethics Committee. Between June 2008 and December 2009, patients over 18 years old with CKD managed without dialysis presenting to our university hospital outpatient nephrology clinic were approached for participation in the study. Participation was allowed if creatinine clearance was between 15 and 59 mL/min and if they had carried a diagnosis of CKD for at least 3 months. Exclusion criteria included cancer, autoimmune disease, or any other known condition that would alter inflammatory status or use of medications such as cimetidine, trimethoprim or amiloride, which can affect estimated GFR by altering plasma creatinine concentration15. Patients who had atrial fibrillation or severe ischemic heart disease with abnormal left ventricular wall motion or severe valvular heart disease by echocardiography were also excluded. All patients gave written informed consent before entering the study.
Demographic data were obtained, including age, gender, and tobacco usage. Antihypertensive medications prescribed previously were recorded; multiple agents of a single class (eg. angiotensin-converting enzyme inhibitors, beta-adrenergic antagonists) were coded as one agent unless they have recognized clinical utility in combination (eg. calcium channel antagonists).
Laboratory parameters
After fasting overnight, between 08.00 and 10.00 in the morning, venous blood samples were drawn from the antecubital vein of all patients before echocardiography was performed. For fibrinogen measurement, a sample was separately taken into an EDTA tube. Blood urea nitrogen (BUN), creatinine, hemoglobin concentration, serum albumin, cholesterol, triglycerides, low-density lipoprotein-cholesterol, and high-density lipoprotein-cholesterol levels were analyzed using standard laboratory methods. High sensitivity C-reactive protein (hs-CRP), fibrinogen, ferritin, and homocysteine levels were also measured. Hs-CRP was measured by immunonephelometry on an Immage 800 Immunochemistry System (Beckman Coulter Inc, Fullerton, USA). Plasma fibrinogen was measured by clotting with a commercially available kit (Albio, Diagnostica Stago, Seine, France). Serum ferritin was measured by electrochemilluminescence immunoassay with a Roche E-170® automatic analyzer (Hitachi Corporation, Osaka, Japan). Homocysteine levels were measured by a high-pressure liquid chromatography (HPLC) using a commercial kit (Recipe, Chemicals & Instruments, GmbH, Munich, Germany). Intact parathyroid hormone levels and quantitative total protein measurements in 24-h urine samples were obtained from patient files.
Renal function was determined by estimating GFR with the Cockroft and Gault formula16 using the serum creatinine concentration (Crs, mg/dL) as follows: GFR in males (mL/min) = [(140 – age) x body weight] / (Crs x 72) GFR in females (mL/min) = value for males x 0.85.
Echocardiography
Transthoracic echocardiography was performed on patients in the left decubitus position with a Vivid 7 Dimension ultrasound machine with a 3.5 MHz probe (GE Healthcare, Milwaukee, USA). A single experienced cardiologist (G.K.), blinded to the clinical details of the patients, made the M-mode echocardiographic measurements. Left ventricular mass (LVM, calculated by the Devereux formula17) was corrected by body surface area and expressed as LVM index (LVMI). Left ventricular hypertrophy was defined as LVMI >131 g/m2 for men and >100 g/m2 for women18. Left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) were calculated by using the Teichholz method. Blood pressures of the patients were measured from the right arm by a manual sphygmomanometer at the time of echocardiography, after 10 minutes of rest
Statistical analysis
Data were evaluated using SPSS version 13.0 for Windows® (SPSS Inc., Chicago, USA). Comparisons between groups were performed using Student-t test for normally distributed variables, whereas the Mann-Whitney U test was used for parametric variables with non-normal distributions. Chi-square testing was used to analyze categorical data. Correlations between estimated GFR and clinical, biochemical and echocardiographic parameters were investigated by Spearman’s correlation test. A p value of <0.05 was considered statistically significant.
Results
Forty-one patients (18 women, 23 men, mean age 53±17 years) participated in the study. Demographic and clinical characteristics of the patients are given in Table 1. The mean estimated GFR of the study cohort was 34.2 mL/min. Table 2 shows the clinical characteristics of patients stratified according to the level of renal function. By kidney disease outcome quality initiative (K/DOQI) guidelines, 63% (26 patients) had stage 3 and 37% (15 patients) had stage 4 CKD19. Patients with stage 4 CKD had a lower LVEF (66±7%) than patients with less severe CKD (71±12%, p=0.013). Stage 3 and stage 4 CKD patients did not differ significantly with regard to LVMI (p=0.904) and LVFS (P=0.160).
Table 1. Demographic, clinical and laboratory characteristics of 41 patients with chronic kidney disease. Values are expressed as mean±SD unless otherwise noted.
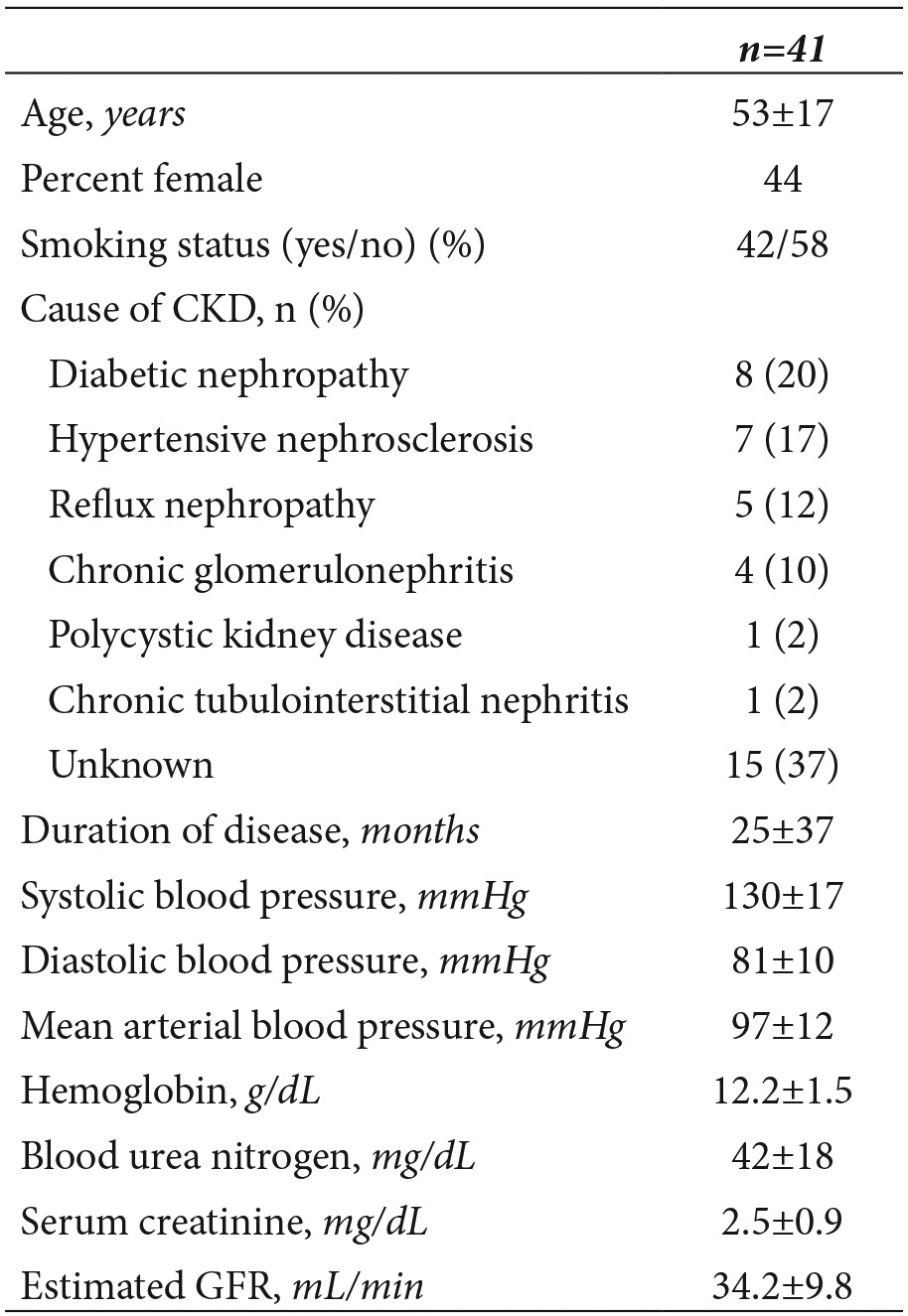
Table 2. Characteristics and echocardiographic measurements of the 41 study subjects, stratified according to level of kidney function: stage 3 if the estimated GFR was 30-59 mL/min, and stage 4 if the estimated GFR was 15-29 mL/min. Values are expressed as mean±SD unless otherwise noted. Statistically significant values are italicized.
GFR, glomerular filtration rate; Hs-CRP, high sensitivity C reactive protein; LVMI, Left ventricular mass index; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening
In patients with CKD, the mean LVMI was 154±52 g/m2 (range 60–307 g/m2) and LVH was diagnosed in 32 patients (78%). These patients with LVH had significantly higher hs-CRP (P=0.012), fibrinogen (p=0.031) and lower albumin (p=0.028) levels than those without LVH (n=9). However, estimated GFR (p=0.865), and daily protein excretion (p=0.106) were not significantly different between the two groups, as shown in Table 3. Patients with and without LVH were not significantly different with respect to serum ferritin (p=0.772) and homocysteine levels (p=0.134). In the entire cohort, LVMI correlated positively with hs-CRP (r=0.483, p=0.002) and serum fibrinogen (r=0.426, p=0.015).
Table 3. Characteristics and echocardiographic measurements of the 41 CKD patients, stratified according to the presence of left ventricular hypertrophy (defined as left ventricular mass index of >131 g/m2 for men and >100 g/m2 for women). Values are expressed as mean±SD unless otherwise noted. Statistically significant values are italicized.
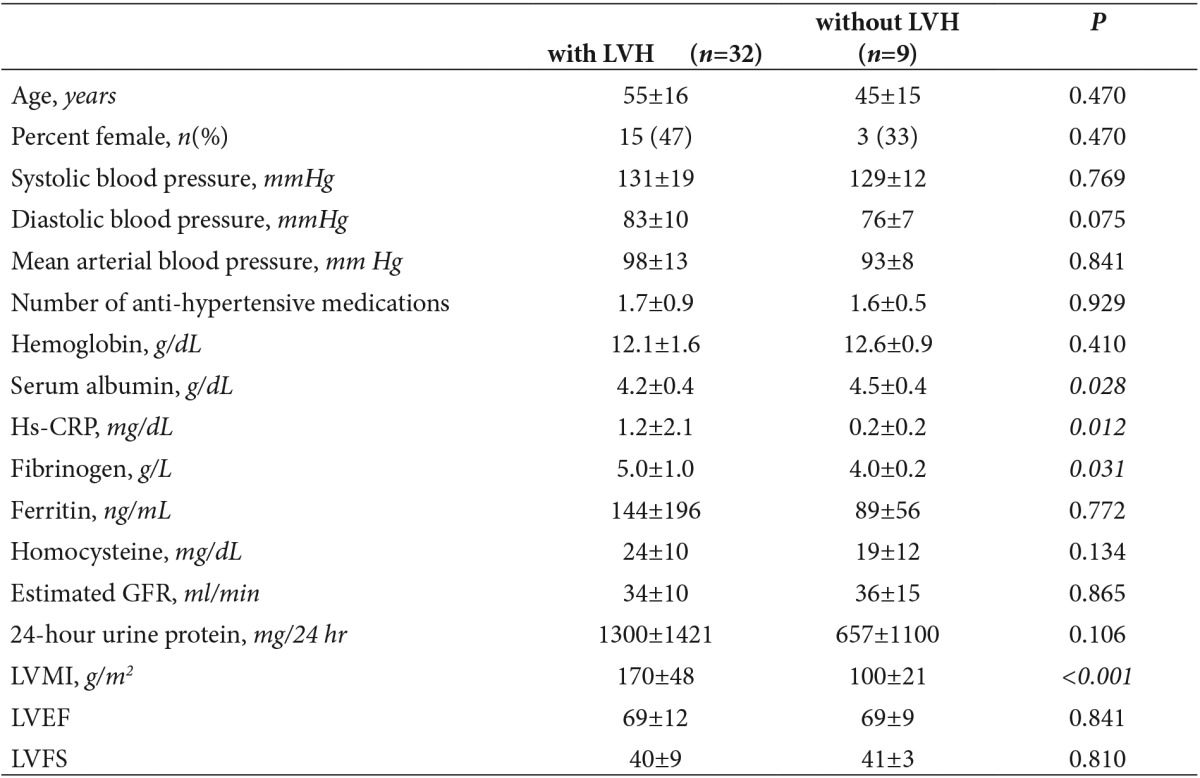
GFR, glomerular filtration rate; Hs-CRP, high sensitivity C reactive protein; LVMI, left ventricular mass index; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening
In CKD patients, estimated GFR correlated positively with hemoglobin (r=0.444, p=0.004), and serum albumin (r=0.441, p=0.004), as shown in Table 4. Estimated GFR was significantly and inversely related to mean arterial blood pressure (r=-0.384, p=0.013), serum ferritin (r =-0.334, p=0.038) and serum parathyroid hormone levels (r=-0.343, p=0.032). Importantly, estimated GFR correlated positively with LVEF (r=0.414, p=0.007) and LVFS (r=0.376, p=0.018) but not to LVMI (r=0.083, p=0.607). Among patients with LVH (n=32), estimated GFR also correlated with LVEF (r=0.388, p=0.028) and LVFS (r=0.383, p=0.033), but not to LVMI (r=0.157, p=0.392).
Table 4. Correlation between estimated glomerular filtration rate (GFR, mL/min) and clinical, laboratory, and echocardiographic parameters in 41 CKD patients. Statistically significant values are italicized.
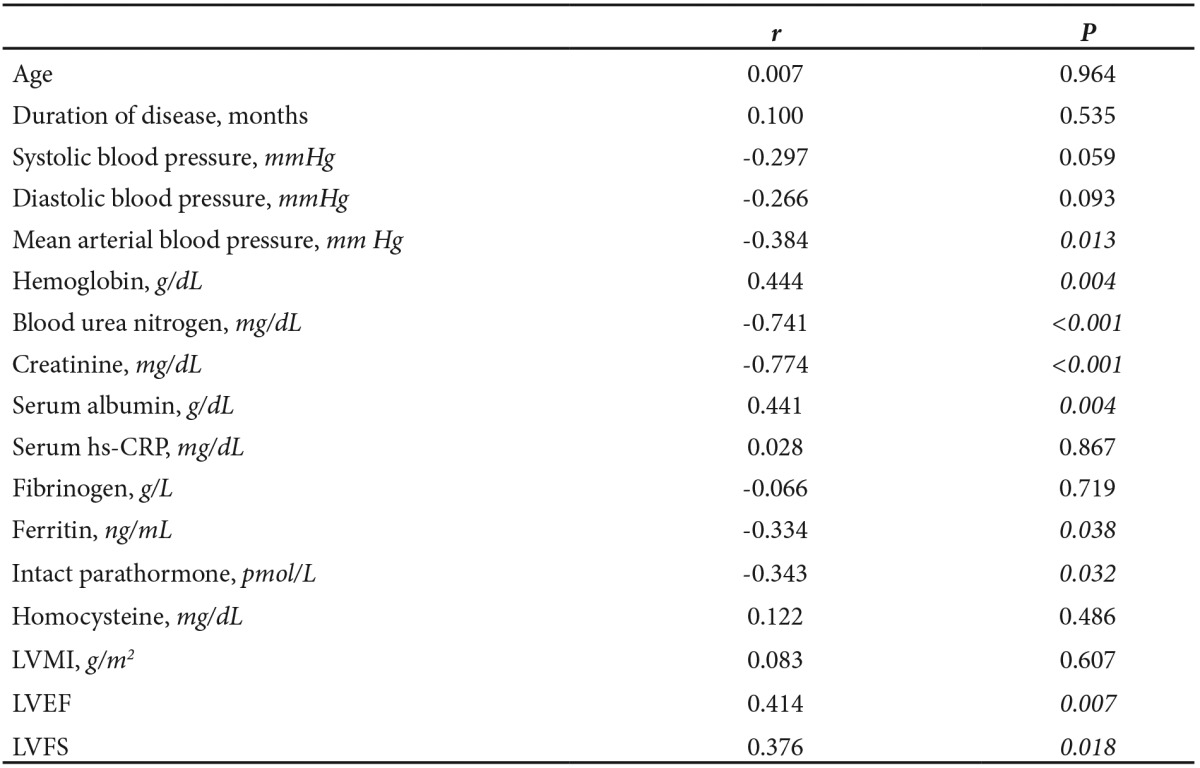
Hs-CRP, high sensitivity C reactive protein; LVMI, left ventricular mass index; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening
Discussion
In this cross-sectional study, patients with CKD who had more kidney dysfunction had a lower mean LVEF. In the entire cohort, estimated GFR was positively correlated with LVEF and LVFS. CKD patients with LVH had significantly lower levels of serum albumin and higher levels of hs-CRP and fibrinogen compared to their non-LVH counterparts. LVMI correlated positively with hs-CRP and serum fibrinogen. Since serum albumin is a negative acute-phase reactant, and hs-CRP and fibrinogen are biomarkers of inflammation, our results demonstrate that mean left ventricular mass in CKD patients increases in parallel with the degree of inflammation.
In this study, LVH was present in 78% of CKD patients, a higher proportion than is usually reported in renal patients12,20,21. The high prevalence of LVH in our study cohort may be due to our inclusion of diabetic patients, who are known to have a higher prevalence of vascular disease and cardiac hypertrophy than the normal population22. However, similar to our findings, the recent study by Paoletti et al. also reported a high prevalence (78%) of LVH in their population of stage 3, 4, and 5 CKD patients7.
Inflammation, as demonstrated by low serum albumin and high hs-CRP and fibrinogen levels, appears to play an important role in the development of LVH in CKD patients. Elevated CRP levels and hypoalbuminemia is associated with progressive LVH in dialysis patients10,11,23,24. In 2007, Cottone et al. was the first to report the positive correlation (r=0.58, P<0.0001) between left ventricular mass index and hs-CRP in patients with moderate CKD25. They also showed that levels of fetuin-A, which is a negative acute phase reactant like albumin26, were inversely correlated with LVMI (r=-0.41, p=0.001). We did not measure fetuin-A levels, but measured other markers of cardiovascular risk such as fibrinogen and homocysteine.27 To date, only one study of dialysis patients has been published which reported the association between serum fibrinogen and LVH28. The current study is the first to describe the relationship between left ventricular mass index and serum fibrinogen in patients with CKD who are not yet on dialysis. Thus, we feel that malnutrition and inflammation should be added to the list of well-known risk factors (hypertension, extra-cellular fluid volume expansion and anemia) for the development of LVH in patients with CKD, as is the case of patients on dialysis25,29,30.
Chronic kidney disease patients with malnutrition are known to exhibit elevated plasma inflammatory cytokine levels, which may in turn cause poor nutritional status and trigger cardiovascular co-morbidities26,29. These patients should be followed with the counseling of a dietitian from the early stage of renal disease to prevent malnutrition. Several pathophysiological mechanisms by which inflammation might contribute to development of ventricular hypertrophy have been proposed31. Inflammation may promote the development of LVH via changes in morphology and function of vascular smooth muscle cells which increase arterial stiffness31,32. In addition, subclinical inflammation can lead to adverse left ventricular geometry by altering the equilibrium that regulates cell growth, apoptosis, phenotype, and matrix turnover of cardiac tissue31.
In this study, estimated GFR was positively correlated with LVEF and LVFS, suggesting a close relationship between the degree of renal function and left ventricular systolic function. However, we could not demonstrate a correlation between estimated GFR and LVMI either in the entire cohort or in the subgroup of patients with LVH. Conversely, two consecutive studies by Levin et al., with larger numbers of patients, clearly demonstrated an increase in the prevalence of LVH as renal function (defined by low creatinine clearance) decreased12,20. In our study, a larger patient population may have resulted in the detection of a clear relationship between low estimated GFR and high LVMI.
Timed (24-hour) urine collections have long been used clinically to measure creatinine clearance, and, hence GFR33. Difficulties in obtaining reliable 24-hour urine specimens account for the imprecision of this test. Errors resulting from under-collection or over-collection of urine introduce significant mistakes in calculated creatinine clearance.33 For these reasons, we used the Cockroft-Gault equation to estimate GFR, which is a more sensitive marker and accurate measure of renal function34.
Limitations of this study include its relatively small sample size (n=41) and its cross-sectional design. While causality cannot be proven in cross-sectional studies, associations can be demonstrated. Another limitation of this study is our inclusion of patients with CKD secondary to systemic hypertension, which itself is a well-known predisposing factor for LVH. We also failed to test for the presence of other pathology associated with LVH, such as myocardial ischemia.
In summary, we found important positive associations between LVMI and markers of inflammation such as hs-CRP and fibrinogen in patients with CKD. In addition to hs-CRP, elevated fibrinogen was associated with cardiac hypertrophy and dysfunction in patients with CKD not yet on dialysis. Clinical studies should be performed in CKD patients to evaluate the effects of anti-inflammatory treatments on the development of cardiac hypertrophy and dysfunction.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Chertow GM, Raggi P, Chasan-Taber S, Bommer J, Holzer H, Burke SK. Determinants of progressive vascular calcification in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1489–1496. doi: 10.1093/ndt/gfh125. [DOI] [PubMed] [Google Scholar]
- 2.Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS. Congestive heart failure in dialysis patients: prevalence, incidence, prognosis and risk factors. Kidney Int. 1995;47:884–890. doi: 10.1038/ki.1995.132. [DOI] [PubMed] [Google Scholar]
- 3.Parfrey PS, Griffiths SM, Harnett JD, Taylor R, King A, Hand J, et al. Outcome of congestive heart failure, dilated cardiomyopathy, hypertrophic hyperkinetic disease and ischemic heart disease in dialysis patients. Am J Nephrol. 1990;10:213–221. doi: 10.1159/000168084. [DOI] [PubMed] [Google Scholar]
- 4.Harnett JD, Kent GM, Barre PE, Taylor R, Parfrey PS. Risk factors for the development of left ventricular hypertrophy in a prospectively followed cohort of dialysis patients. J Am Soc Nephrol. 1994;4:1486–1490. doi: 10.1681/ASN.V471486. [DOI] [PubMed] [Google Scholar]
- 5.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant. 1996;11:1277–1285. [PubMed] [Google Scholar]
- 6.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 7.Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G. Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis. 2005;46:320–327. doi: 10.1053/j.ajkd.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Cerasola G, Nardi E, Palermo A, Mule G, Cottone S. Epidemiology and pathophysiology of left ventricular abnormalities in chronic kidney disease: a review. J Nephrol. 2011;24:1–10. doi: 10.5301/jn.2010.2030. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim BS, Jeon DS, Shin MJ, Kim YO, Song HC, Lee SH, et al. Persistent elevation of C-reactive protein may predict cardiac hypertrophy and dysfunction in patients maintained on hemodialysis. Am J Nephrol. 2005;25:189–195. doi: 10.1159/000085585. [DOI] [PubMed] [Google Scholar]
- 11.Shi B, Ni Z, Cai H, Zhang M, Mou S, Wang Q, et al. High-sensitivity C-reactive protein: an independent risk factor for left ventricular hypertrophy in patients with lupus nephritis. J Biomed Biotechnol. DOI: 10.1155/2010/373426 doi: 10.1155/2010/373426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125–134. doi: 10.1016/s0272-6386(99)70118-6. [DOI] [PubMed] [Google Scholar]
- 13.Nardi E, Palermo A, Mule G, Cusimano P, Cottone S, Cerasola G. Left ventricular hypertrophy and geometry in hypertensive patients with chronic kidney disease. J Hypertens. 2009;27:633–641. doi: 10.1097/HJH.0b013e3283220ecd. [DOI] [PubMed] [Google Scholar]
- 14.Rigatto C, Foley RN, Kent GM, Guttmann R, Parfrey PS. Long-term changes in left ventricular hypertrophy after renal transplantation. Transplantation. 2000;70:570–575. doi: 10.1097/00007890-200008270-00006. [DOI] [PubMed] [Google Scholar]
- 15.Stevens LA, Shastri S, Levey AS. Assessment of renal function. In: Floege J, Johnson RJ, Feehally J, editors. Comprehensive clinical nephrology. 4th ed. St. Louis. Elsevier Saunders. 2003;7 [Google Scholar]
- 16.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 17.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 18.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Study. J Am Coll Cardiol. 1995;25:879–884. doi: 10.1016/0735-1097(94)00473-4. [DOI] [PubMed] [Google Scholar]
- 19.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:1–266. [PubMed] [Google Scholar]
- 20.Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in predialysis population: identifying opportunities for intervention. Am J Kidney Dis. 1996;27:347–354. doi: 10.1016/s0272-6386(96)90357-1. [DOI] [PubMed] [Google Scholar]
- 21.McMahon LP, Roger SD, Levin A. Slimheart Investigators Group. Development, prevention, and potential reversal of left ventricular hypertrophy in chronic kidney disease. J Am Soc Nephrol. 2004;15:1640–1647. doi: 10.1097/01.asn.0000130566.69170.5e. [DOI] [PubMed] [Google Scholar]
- 22.Foley RN, Culleton BF, Parfrey PS, Harnett JD, Kent GM, Murray DC, et al. Cardiac disease in diabetic end-stage renal disease. Diabetologia. 1997;40:1307–1312. doi: 10.1007/s001250050825. [DOI] [PubMed] [Google Scholar]
- 23.Moon KH, Song IS, Yang WS, Shin YT, Kim SB, Song JK, et al. Hypoalbuminemia as a risk factor for progressive left-ventricular hypertrophy in hemodialysis patients. Am J Nephrol. 2000;20:396–401. doi: 10.1159/000013625. [DOI] [PubMed] [Google Scholar]
- 24.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J Am Soc Nephrol. 1996;7:728–736. doi: 10.1681/ASN.V75728. [DOI] [PubMed] [Google Scholar]
- 25.Cottone S, Nardi E, Mule G, Vadala A, Lorito MC, Riccobene R, et al. Association between biomarkers of inflammation and left ventricular hypertrophy in moderate chronic kidney disease. Clin Nephrol. 2007;67:209–216. doi: 10.5414/cnp67209. [DOI] [PubMed] [Google Scholar]
- 26.Dervisoglu E, Kir HM, Kalender B, Caglayan C, Eraldemir C. Serum fetuin--a concentrations are inversely related to cytokine concentrations in patients with chronic renal failure. Cytokine. 2008;44:323–327. doi: 10.1016/j.cyto.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Cases A. New cardiovascular risk factors and chronic kidney disease. Hypertrophy of the left ventricle. Atrial fibrillation. Smoking. Obesity. Emerging cardiovascular risk factors: homocysteine, Reactive C protein. Fibrinogen. Nefrologia. 2004;24:62–72. [PubMed] [Google Scholar]
- 28.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Cutrupi S, Parlongo S, et al. Fibrinogen, inflammation and concentric left ventricular hypertrophy in chronic renal failure. Eur J Clin Invest. 2003;33:561–566. doi: 10.1046/j.1365-2362.2003.01169.x. [DOI] [PubMed] [Google Scholar]
- 29.Drueke TB. Aspects of cardiovascular burden in pre-dialysis patients. Nephron. 2000;85:9–14. doi: 10.1159/000045704. [DOI] [PubMed] [Google Scholar]
- 30.Cottone S, Lorito MC, Riccobene R, Nardi E, Mule G, Buscemi S, et al. Oxidative stress, inflammation and cardiovascular disease in chronic renal failure. J Nephrol. 2008;2:175–179. [PubMed] [Google Scholar]
- 30.Ratto E, Leoncini G, Viazzi F, Falqui V, Parodi A, Conti N, et al. C-reactive protein and target organ damage in untreated patients with primary hypertension. J Am Soc Hypertens. 2007;1:407–413. doi: 10.1016/j.jash.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46:1118–1122. doi: 10.1161/01.HYP.0000185463.27209.b0. [DOI] [PubMed] [Google Scholar]
- 33.Hsu CY. Clinical evaluation of kidney function. In: Greenberg A, editor. Primer on kidney diseases. 5th ed. Philadelphia. Saunders Elsevier. 2009 [Google Scholar]
- 34.Coresh J, Toto RD, Kirk KA, Whelton PK, Massry S, Jones C. Creatinine clearance as a measure of GFR in screenees for the African-American Study of Kidney Disease and Hypertension pilot study. Am J Kidney Dis. 1998;32:32–42. doi: 10.1053/ajkd.1998.v32.pm9669421. [DOI] [PubMed] [Google Scholar]


