Abstract
Background:The major cause of hereditary renal failure is autosomal dominant polycystic kidney disease (ADPKD). Many factors affect renal progression in these patients. Among these, hypertension and an increase in renal volume are interrelated in terms of their effects on renal progression. We aimed to investigate the effects of losartan and ramipril on renal volume and progression in patients with ADPKD.
Materials and Methods:Data from 18 hypertensive patients with ADPKD were evaluated. Eleven of the 18 hypertensive patients were on losartan and 7 on ramipril treatment. Demographic parameters, use of antihypertensives and other medications, the course of blood pressure (BP), biochemical parameters, creatinine clearance (CrCL), findings at computed tomography and renal volume were recorded at baseline and at 1 and 5 years.
Results: Target BP values were maintained over 5 years. The annual decrease in CrCL was 1.33 mL/min in the losartan group compared with 6.59 mL/min in the ramipril group. There was no significant difference between the groups in terms of annual decrease in CrCL. Annual increase in renal volume was 252.04 cm³ in the losartan group and 167.36 cm³ in the ramipril group. There was no significant difference between the groups in terms of the increase in renal volumes at 1 and 5 years.
Conclusion: Our study demonstrated that losartan and ramipril provided effective BP control. In addition, the results of our study demonstrated that despite the increase in renal volume, losartan and ramipril may have regressed renal progression via other factors.
Keywords: autosomal dominant polycystic kidney disease, losartan, renal volume, ramipril, renal progression
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary renal disease causing end-stage renal failure. ADPKD has an incidence of approximately 1/500-1000 in the USA1. The disease is characterized by continuous expansion of multiple cysts originating from renal tubules. Renal damage often cannot be identified until the third or fourth decade2,3. Hypertension and an increase in renal volume are interrelated with respect to their effects on pathogenesis and renal progression. Although there is no consensus regarding the pathogenesis of hypertension in ADPKD patients, it has been suggested that the renin-angiotensin system (RAS) is activated due to the pressure induced by expanded renal cysts 4-6. This study aimed to evaluate the effects of RAS blocking agents (losartan and ramipril) on blood pressure (BP) control, and also their clinical effects on renal volume and renal progression. We also compared the effects of losartan and ramipril on these parameters.
Materials and methods
We obtained data for patients aged 18-70 with creatinine clearance (CrCL) levels 30 ml/min or higher from the Karadeniz Technical University Clinic of Nephrology between 2003 and 2009. The hypertension limit before patients began treatment was ≥ 140/90 mmHg. Patients were started on antihypertensive treatment in our clinic, initial treatment consisting of losartan or ramipril, with dose titration performed until target blood pressure was achieved. Subjects given amlodipine 5-10 mg and doxazosin 4-8 mg treatment, and regularly monitored at 3-month intervals were included in the study. Patients with diabetes mellitus, congestive heart failure, liver disease, other renal disorders or psychiatric diseases, pregnant patients, those using cigarettes, caffeine, nonsteroidal anti-inflammatory drugs, or who had had a contrast test performed or had undergone surgery over the preceding 5-year period were excluded. Biochemical parameters, including levels of blood urea nitrogen (BUN), creatinine, sodium (Na), potassium (K), glucose, albumin, uric acid, calcium, phosphorous, low-density lipoprotein cholesterol (LDL-C), 24-hour urinary creatinine, protein, and hematocrit levels for patients meeting the inclusion criteria were recorded at baseline, and at 1 and 5 years. CrCL calculated using the 24-h urinary creatinine and simultaneous blood creatinine level was adjusted for a body surface area (BSA) of 1.73 m².
CrCL was calculated using the formula UV / P x 1.73 / BSA
U: Concentration of creatinine in urine in mg/dL
V: Volume of urine in ml divided by time of collection in minutes
P: Body surface area
BSA was calculated using the Dubois formula: BSA=[(weight in kg)0.425 x (height in cm)0.725] x 0.007184.
Biochemical parameters were measured with a Roche modular autoanalyzer using specific solutions. Patients’ ages, gender, duration of hypertension, use of medications and BP course were also recorded (Table 1). Non-contrast computed abdominal tomography (Siemens Volume 700 m device) performed for various indications was re-evaluated by two independent blinded radiologists, and renal volume was computed using the method described by Breiman et al7. Patients were divided into losartan or ramipril groups according to their use of the antihypertensive drugs.
Table 1. Baseline patient characteristics.
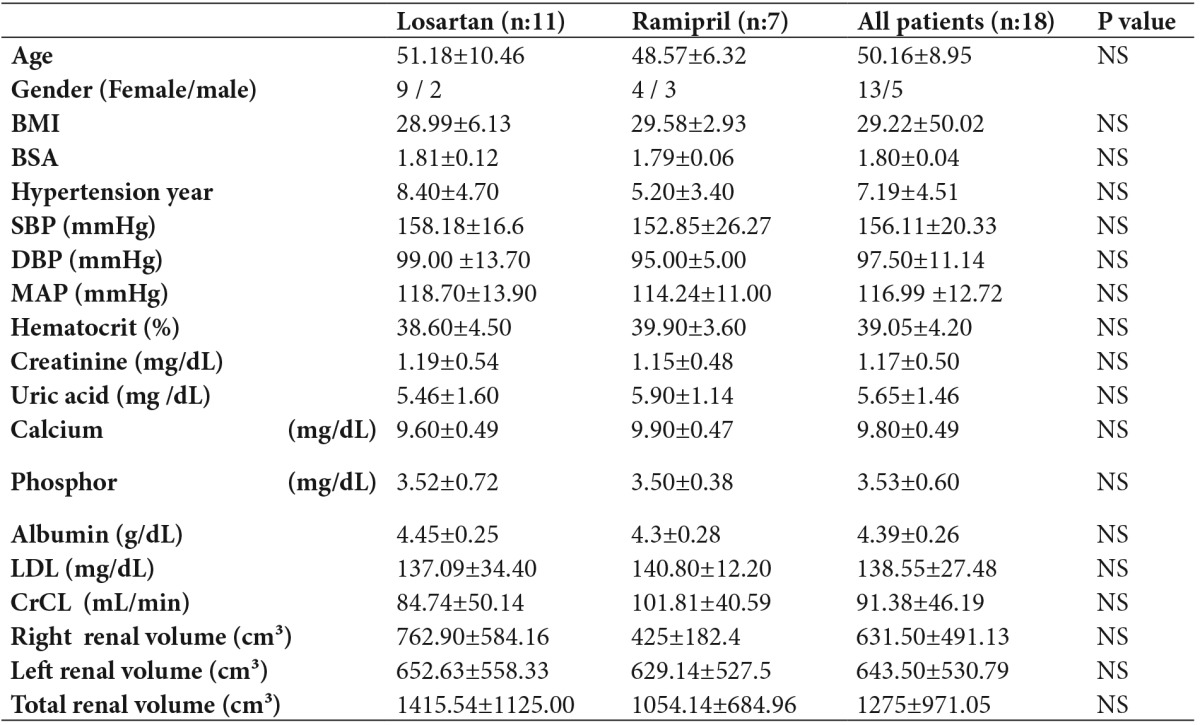
Statistical significance was p<0.05
Data is given as mean ± SD
BMI: Body mass index, BSA: Body surface area, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, MAP: Mean arterial pressure, LDL: Low density lipoprotein
P value: Losartan versus Ramipril
Eighteen patients (13 females and 5 males) with a mean age of 50.16 ± 8.95 years, and 11 receiving losartan and 7 ramipril treatment, were included in the study. In the losartan group, 7 patients were receiving losartan (100 mg), 3 patients were receiving losartan (100 mg) + amlodipine (5 mg), and 1 patient was receiving losartan (100 mg) + amlodipine (10 mg) + doxazosin (4 mg bid). In the ramipril group, 1 patient was receiving ramipril (2.5 mg), 1 patient was receiving ramipril (5 mg), 3 patients were receiving ramipril (10 mg), and 2 patients were receiving ramipril (10 mg) + amlodipine (5 mg)
Statistical analysis
Statistical analysis was performed using SPSS 12.0. Student’s t-test was used to compare mean BP, CrCL, creatinine level, and renal volumes of the losartan and ramipril groups. Comparison of the changes in these parameters from baseline to 1 and 5 years was performed using the Wilcoxon signed-ranks test. Results are expressed as mean ± standard deviation (SD). Significance was set at p<0.05.
Results
The effect of treatment on blood pressure
The mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) of the study population were 156.11±20.33 and 97.50±11.14 mmHg, respectively, at baseline. Eleven patients were on losartan and 7 on ramipril treatment. SBP and DBP were 158.18±16.62 and 99.09±13.75 mmHg, respectively, in the losartan group and 152.85±26.27 and 95.00±5.00, respectively, in the ramipril group. There was no significant difference between the groups in terms of BP measurements at baseline (Table 1). Target BP values (≤130/80 mmHg) were achieved at the end of 1 year, and this effect was maintained at the end of 5 years in both groups. There was no significant difference between the groups in terms of BP measurements at 1 and 5 years.
The effect of treatment on renal progression
Mean CrCL in our 18 patients was 91.38±46.19 mL/min at baseline, 82.98±40.11 mL/min at the end of 1 year, and 74.47±46.30 mL/min at the end of 5 years. While there was no significant decrease in CrCL at the end of 1 year (p=0.166), a significant decrease was noted at the end of 5 years compared to the baseline (p=0.030) (Figure 1), (Table 2). The mean decrease in CrCL was 8.39 mL/min at the end of 1 year, with an annual decrease in CrCL of 2.12 mL/min in the following 4 years. An annual decrease in CrCL of 3.38 mL/min was observed in the 18 patients over a 5-year period.
Figure 1. Five year follow-up of CrCL in the ramipril and losartan groups and all patients.
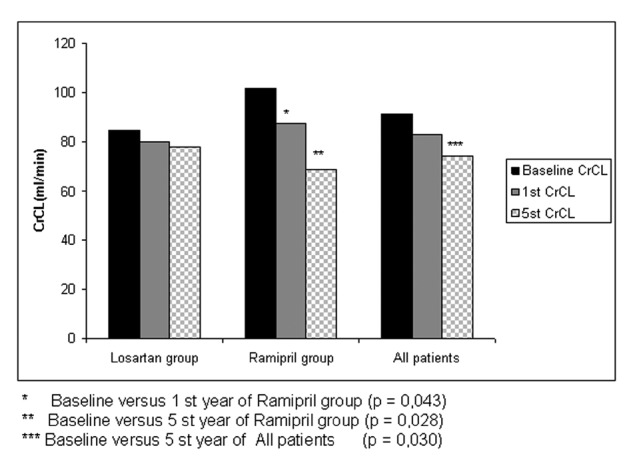
Table 2. Five-year follow-up of All patients.
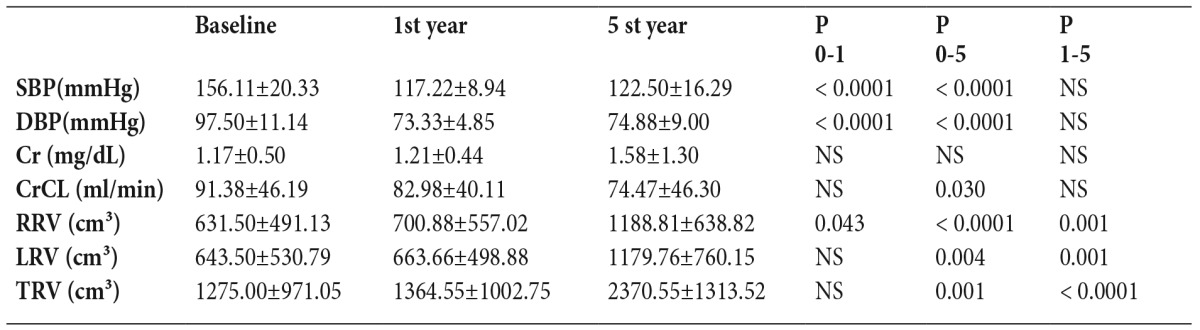
Statistical significance was p<0.05
Data is given as mean ± SD
SBP: Systolic blood pressure, DBP: Diastolic blood pressure, CrCL: Creatinine clarence, RRV: Right renal volume, LRV: Left renal volume, TRV: Total renal volume, Cr: Creatinine
P 0-1: Baseline versus 1st year
P 0-5: Baseline versus 5th year
P 1-5: 1st year versus 5th year
Mean CrCL at baseline was 84.74±50.14 mL/min in the losartan group and 101.81±40.59 mL/min in the ramipril group. There was no significant difference between the groups in terms of CrCL measurements at baseline (Table 1). The decrease in CrCL in the losartan group was 4.78 mL/min at the end of 1 year and 0.47 mL/min/year in the following 4 years, with an annual decrease in CrCL of 1.33 mL/min over a 5-year period (Table 3). The decrease in CrCL in the ramipril group was 14.09 mL/min at the end of 1 year and 4.72 mL/min/year in the following 4 years, with an annual CrCL decrease of 6.59 mL/min/1.73 m² over a 5-year period (Figure 1)(Table 4). There was no significant difference between the groups in terms of the mean change in CrCL from baseline to 1 year (p=0.53) or from baseline to 5 years (p=0.06).
Table 3. 5-year follow-up of Losartan group.
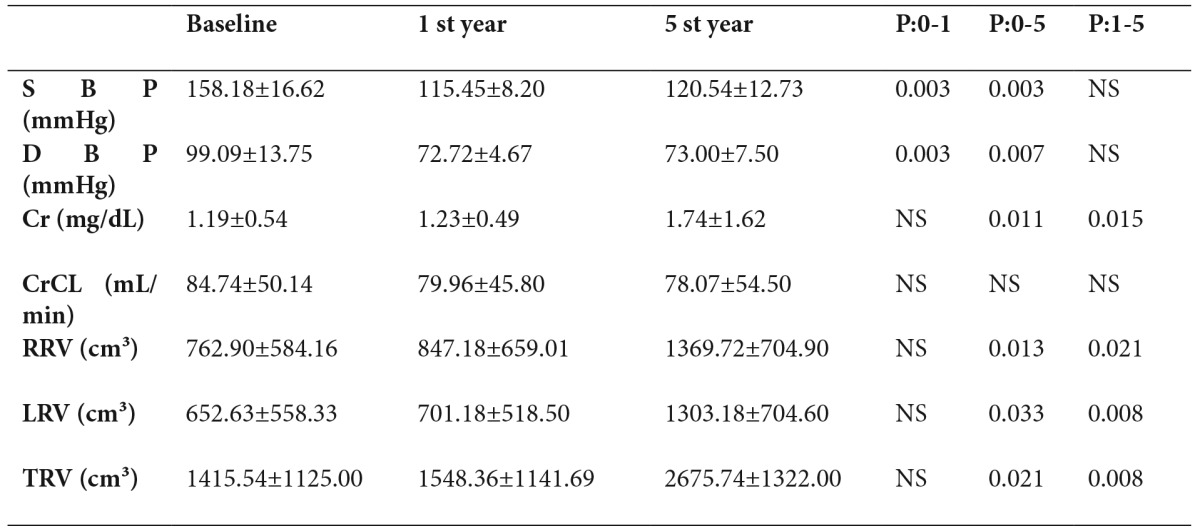
P 0-1: Baseline vs 1st year
P 0-5: Baseline vs 5th year
P 1-5: 1st year vs 5th year
Table 4. Five-year follow-up of Ramipril group.
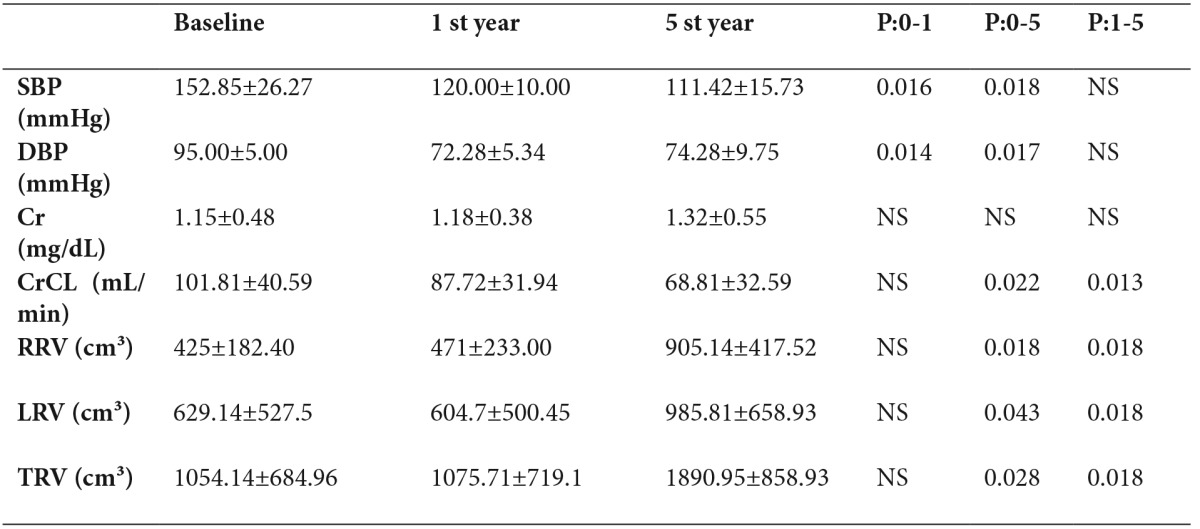
P 0-1: Baseline vs 1st year
P 0-5: Baseline vs 5th year
P 1-5: 1st year vs 5thyear
The effects of treatment on renal volume
The mean total renal volume in the 18 patients was 1275±971.05 cm³ at baseline (Table 1). While there was no significant increase at the end of 1 year (p=0.325), a significant increase was observed at the end of 5 years (p=0.001) (Figure 2), (Table 2).
Figure 2. Five year follow-up of renal volume in the ramipril and losartan groups and all patients.
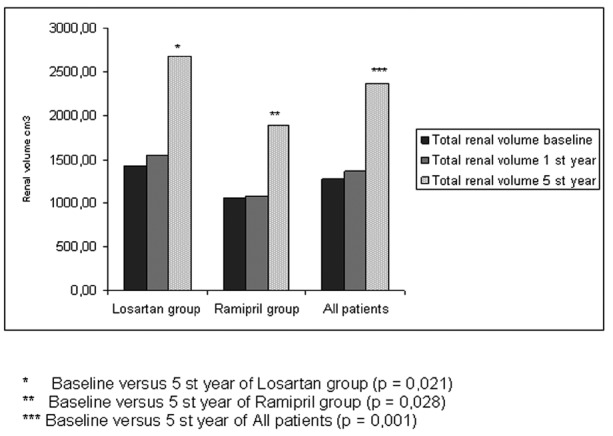
At baseline, the total renal volumes of the losartan and ramipril groups were 1415.54±1125.00 and 1054.14±684.96 cm³, respectively (Table 1). There was no significant difference between the groups in terms of baseline renal volumes at baseline (Table 1). While there was no significant increase in renal volume at the end of 1 year in either group, significant increases were noted at the end of 5 years. The annual total increase in renal volume was 219.11 ± 228.98 cm³ in the entire study population, 252.04 ± 271.10 cm³ in the losartan group and 167.36 ± 144.74 cm³ in the ramipril group (Figure 2), (Tables 2,3,4). There was no significant difference between the two groups in terms of the increase in renal volumes at 1 and 5 years.
No patients had proteinuria in 24-h urine collection; this parameter was therefore not included in the statistical evaluation.
Discussion
Although chronic renal failure has a diverse etiological distribution, the major cause of hereditary renal failure is ADPKD, which constitutes 7%-10% of dialysis patients in the USA 8,9. Hypertension, left ventricular hypertrophy and increased renal volume are interrelated in terms of their effects on pathogenesis and renal progression4-6.
Hypertension is quite common in this patient group and occurs in 60% of subjects before the development of renal dysfunction10. Although the pathogenesis of hypertension in patients with ADPKD has not been fully elucidated, the most favored mechanism is activation of the RAS resulting from ischemia due to pressure induced by expanded renal cysts11. Although there is no consensus as to which group of medications to select for the treatment of hypertension, several authors have advocated that hypertension observed in polycystic kidney disease is renin-dependent hypertension and that blocking this system will be effective in BP control10-14 . In an earlier study we showed that effective blood pressure was provided with RAS blocking in hypertensive ADPKD patients15. We also observed that target BP values (≤130/80 mmHg) were achieved at the end of 1 year, and that this effect was maintained at the end of 5 years via RAS blockade induced by two different drugs in the 18 hypertensive patients with ADPKD we retrospectively evaluated in this study.
One of the important factors affecting renal progression in polycystic kidney disease is the pressure exerted on surrounding parenchymal tissues induced by expanding cysts. Expansion of cysts and the resulting increase in renal volume has a negative influence on renal progression16,17. Recent studies have therefore focused on therapeutic alternatives which prevent or retard cyst expansion. These studies have evaluated the effects of sirolimus, everolimus, vasopressin and somatostatin on renal volume and renal progression in ADPKD 18. Serra et al, recently evaluated the effect of sirolimus on renal volume in ADPKD patients. In that study, during the 18-month study period the median total kidney volume increased by 99 cm3 in the sirolimus group and 97 cm3 in the control group. They found no evidence that sirolimus slowed polycystic kidney growth19. Additionally, Walz et al, recently evaluated the effect of everolimus on renal volume. Mean changes in total kidney volume were 101 ml and 239 ml for the everolimus group and 157 ml and 319 ml for the placebo group at years 1 and 2, respectively. Estimated GFR decreased by 8.9 ml per min in the everolimus group and 7.7 ml per min in the placebo group over the 2-year study period20. In our study of 18 patients in whom renal volume assessment was performed using non-contrast computed abdominal tomography, mean renal volume at baseline was 1275 cm³ in the entire study population, 1415.54 cm³ in the losartan group and 1054.14 cm³ in the ramipril group. Annual increase in renal volume was 219.11 cm³ in all subjects, 252.04 cm³ in the losartan group and 167.36 cm³ in the ramipril group. Despite effective BP control, the increase in renal volume was greater compared to that in these other studies19,20. However, there is currently no clear-cut information about the annual increase in renal volume in patients with ADPKD. Direct comparisons among current studies are rather difficult due to methodological differences in calculation of renal volume. Ethnic group differences are also present. Moreover, it is likely that greater renal volume increase would occur in patients without treatment; however, assessment of renal volume increase without administration of antihypertensive treatment would not be ethical. In addition, although this was not statistically significant, the greater increase in the losartan group may be ascribed to greater, albeit statitistically insignificant, basal TRVs.
Several studies have evaluated the effects of various antihypertensives on renal progression in patients with polycystic kidney disease. But these have not evaluated the effects of antihypertensives on renal volume. Ecder et al9 compared the effects of enalapril and amlodipine in hypertensive patients with polycystic kidney disease. After 5 years of follow-up, the mean annual decrease in CrCL was reported as 3.6 mL/min. Nutahara et al10 compared the effectiveness of amlodipine and candesartan in 49 hypertensive polycystic kidney patients and determined a mean annual decrease in CrCL of 5.5 mL/min in the amlodipine group and 1.6 mL/min in the candesartan group. In the polycystic patient subgroup of the Modification of Diet in Renal Disease (MDRD) study21, the annual GFR decrease was 5.9 mL/min in Group A (mean GFR, 37.8 ± 9 mL/min) and 4.4 mL/min in Group B (mean GFR, 17.4 ± 3.2 mL/min. There is also an ongoing HALT Progression of Polycystic Kidney Disease (PKD) trial evaluating the efficacy of dual vs. single RAS blockade, activated by cyst-induced surrounding tissue pressure, on cyst growth and renal progression22. In our study, mean CrCL in our 18 patients was 91.38 mL/min at baseline; the mean decrease in CrCL was 8.39 mL/min at the end of 1 year and 2.12 mL/min/year in the following 4 years, and an annual decrease in CrCL of 3.38 mL/min was observed over the 5-year period. The rapid decrease in GFR in the first year might be attributed to the sudden decrease in filtration due to RAS blockade. The mean decrease in CrCL was 2.12 mL/min in the following years. The decrease in CrCL in our study was considerably lower than that in those in previous studies. When the CrCL results were compared between the groups, CrCL was 84.74 mL/min in the losartan group and 101.81 ± 40 mL/min in the ramipril group at baseline in our study. Comparing the losartan and ramipril groups, the mean decrease in CrCL was 4.77 vs. 14.09 mL/min at the end of 1 year, 0.47 vs. 4.72 mL/min in the following 4 years, and 1.33 vs. 6.59 mL/min over the 5-year period. There was no statistically significant difference between the groups in terms of changes in CrCL. However, the annual decrease in CrCL in the losartan group was lower compared to that reported in previous studies9,10,21. Although no statistically significant difference was determined, the increase in renal volume in the losartan group was greater than that in the ramipril group. At this point, the question arises whether losartan has an independent renoprotective effect other than renal volume, since it has no beneficial effect on renal volume increase.
This evaluation of 5-year follow-up of 18 ADPKD patients has a number of limitations. One is the low patient number involved. This was due to the long follow-up period, groups being homogeneous and the exclusion of comorbid conditions that might affect renal progression. Another limitation is that the study is retrospective. However, the patients we enrolled had commenced treatment in our clinic and attended regularly for follow-up. Finally, we performed renal progression with 24-h urine collection and CrCL calculated on that basis. We selected this method since these patient data were available. GFR could also have been calculated with MDRD.
In conclusion, RAS blockade, either by losartan or ramipril, provides effective BP control. Although renal volume increase cannot be prevented with these treatments, it may be that both losartan and ramipril retard renal progression. It would be more appropriate to wait for the results of the HALT PKD study investigating the efficacy of these two medications in a larger patient population in order to draw more definitive conclusions.
Conflict of interest
We received no financical support for this study and have no conflict of interest to report.
References
- 1.Poster D, Kistler AD, Krauer F, Blumenfeld JD, Rennert H, Weishaupt D, et al. Kidney function and volume progression in unilateral autosomal dominant polycystic kidney disease with contralateral renal agenesis or hypoplasia: a case series. Am J Kidney Dis. 2009;54:450–458. doi: 10.1053/j.ajkd.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Bennett WM. Autosomal dominant polycystic kidney disease: 2009 update for internists. Korean J Intern Med. 2009;24:165–168. doi: 10.3904/kjim.2009.24.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae KT, Grantham JJ. Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2010;6:96–106. doi: 10.1038/nrneph.2009.214. [DOI] [PubMed] [Google Scholar]
- 4.van Dijk MA, Peters DJ, Breuning MH, Chang PC. The angiotensin-converting enzyme genotype and microalbuminuria in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1999;10:1916–1920. doi: 10.1681/ASN.V1091916. [DOI] [PubMed] [Google Scholar]
- 5.Schrier RW, McFann KK, Johnson AM. Epidemiological study of kidney survival in autosomal dominant polycystic kidney disease. Kidney Int. 2003;63:678–685. doi: 10.1046/j.1523-1755.2003.00776.x. [DOI] [PubMed] [Google Scholar]
- 6.Masoumi A, Reed-Gitomer B, Kelleher C, Bekheirnia MR, Schrier RW. Developments in the management of autosomal dominant polycystic kidney disease. Ther Clin Risk Manag. 2008;4:393–407. doi: 10.2147/tcrm.s1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sise C, Kusaka M, Wetzel LH, Winklhofer F, Cowley BD, Cook LT, et al. Volumetric determination of progression in autosomal dominant polycystic kidney disease by computed tomography. Kidney Int. 2000;58:2492–2501. doi: 10.1046/j.1523-1755.2000.00433.x. [DOI] [PubMed] [Google Scholar]
- 8.Ecder T, Edelstein CL, Chapman AB, Johnson AM, Tison L, Gill EA, et al. Reversal of left ventricular hypertrophy with angiotensin converting enzyme inhibition in hypertensive patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1999;14:1113–1116. doi: 10.1093/ndt/14.5.1113. [DOI] [PubMed] [Google Scholar]
- 9.Ecder T, Chapman AB, Brosnahan GM, Edelstein CL, Johnson AM, Schrier RW. Effect of antihypertensive therapy on renal function and urinary albumin excretion in hypertensive patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;35:427–432. doi: 10.1016/s0272-6386(00)70195-8. [DOI] [PubMed] [Google Scholar]
- 10.Nutahara K, Higashihara E, Horie S, Kamura K, Tsuchiya K, Mochizuki T, et al. Calcium channel blocker versus angiotensin II receptor blocker in autosomal dominant polycystic kidney disease. Nephron Clin Pract. 2005;99:18–23. doi: 10.1159/000081790. [DOI] [PubMed] [Google Scholar]
- 11.Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005;68:57–65. doi: 10.1111/j.1523-1755.2005.09911.x. [DOI] [PubMed] [Google Scholar]
- 12.Qian Q, Harris PC, Torres VE. Treatment prospects for autosomal-dominant polycystic kidney disease. Kidney Int. 2001;59:2005–2022. doi: 10.1046/j.1523-1755.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- 13.Hateboer N, Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet. 1999;353:103–107. doi: 10.1016/s0140-6736(98)03495-3. [DOI] [PubMed] [Google Scholar]
- 14.Woo D. Apoptosis and loss of renal tissue in polycystic kidney diseases. N Engl J Med. 1995;333:18–25. doi: 10.1056/NEJM199507063330104. [DOI] [PubMed] [Google Scholar]
- 15.Ulusoy S, Ozkan G, Orem C, Kaynar K, Kosucu P, Kiris A. A comparison of the effects of ramipril and losartan on blood pressure control and left ventricle hypertrophy in patients with autosomal dominant polycystic kidney disease. Ren Fail. 2010;32:913–917. doi: 10.3109/0886022X.2010.502277. [DOI] [PubMed] [Google Scholar]
- 16.Fick-Brosnahan GM, Belz MM, McFann KK, Johnson AM, Schrier RW. Relationship between renal volume growth and renal function in autosomal dominant polycystic kidney disease: a longitudinal study. Am J Kidney Dis. 2002;39:1127–1134. doi: 10.1053/ajkd.2002.33379. [DOI] [PubMed] [Google Scholar]
- 17.Seeman T, Dusek J, Vondrichova H, Kyncl M, John U, Misselwitz J, et al. Ambulatory blood pressure correlates with renal volume and number of renal cysts in children with autosomal dominant polycystic kidney disease. Blood Press Monit. 2003;8:107–110. doi: 10.1097/01.mbp.0000085762.28312.4a. [DOI] [PubMed] [Google Scholar]
- 18.Wuthrich RP, Serra AL. Mammalian target of rapamycin and autosomal dominant polycystic kidney disease. Transplant Proc. 2009;41:18–20. doi: 10.1016/j.transproceed.2009.06.097. [DOI] [PubMed] [Google Scholar]
- 19.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 20.Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 21.Klahr S, Breyer JA, Beck GJ, Dennis VW, Hartman JA, Roth D, et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group. J Am Soc Nephrol. 1995;5:2037–2047. doi: 10.1681/ASN.V5122037. [DOI] [PubMed] [Google Scholar]
- 22.Chapman AB. Approaches to testing new treatments in autosomal dominant polycystic kidney disease: insights from the CRISP and HALT-PKD studies. Clin J Am Soc Nephrol. 2008;3:1197–1204. doi: 10.2215/CJN.00060108. [DOI] [PubMed] [Google Scholar]


