Abstract
Background: Congenital chylous ascites is a rare condition that constitutes a challenge for the physician. It is defined as the accumulation of chyle into the peritoneal cavity in infants younger than 3 months. This condition is often refractory to therapy and it is responsible for serious malnutrition and immunological deficiency because of the loss of proteins and lymphocytes.
Material and methods: Four cases of congenital neonatal chylous ascites, were treated by our staff during the last two years. One case was treated conservatively and three with laparotomy. Two of them had intraabdominal cysts that were excised and one was treated with ligation of the left lumbar lymphatic trunk and cisterna chyli and the use of fibrin glue.
Results: All four cases were treated successfully. On follow up tests no one showed recurrence of the ascites. All children, except the one that treated conservatively and also had other problems due to prematurity, are growing up normally.
Conclusions: Congenital chylous ascites is a complex condition. Its diagnostic evaluation is difficult and its therapy of long duration. Conservative treatment is in most cases the initial choice, but when it fails, exploratory laparotomy could provide a successful alternative.
Keywords: chylous ascites, chyloperitoneum, cysterna chyli, mesenteric cyst, neonate, intestinal duplication
The first report of chylous ascites was by Morton, in 1694, in a two year old boy with tuberculosis however the theory that lymph is produced by diffusion from blood vessels was not accepted until 18491.
Chylous ascites is a rare condition (1:20464 admissions2). Congenital chylous ascites is even rarer and constitutes a challenge for the physician. It is defined as the accumulation of chyle into the peritoneal cavity in infants younger than three months3-5 and is believed that is caused by malformation of the lymphatics and lacteals6. This condition is often refractory to therapy and is responsible for serious malnutrition and immunological deficiency because of the loss of proteins and lymphocytes3. This paper presents our experience regarding the diagnostic evaluation and therapeutic approach in four cases of congenital neonatal chylous ascites that were treated successfully in our department in a two year period.
Case 1
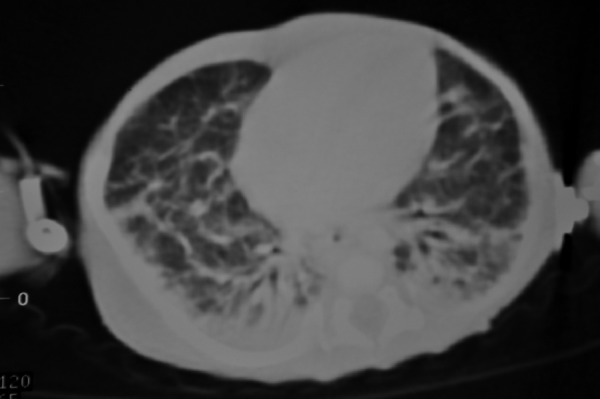
An extremely premature baby girl, born at 26 weeks gestation, developed abdominal distention and respiratory distress, while in NICU, on day 44. Thoracic and abdominal X-rays showed intrapleural collection of fluid on the right side and ascites. Abdominal US identified no underlying pathology. The fluid that was obtained by thoracentesis and abdominal paracentesis was chyle (abdominal: 4760cells/μL, 95% lymphocytes, triglycerides 1500mgr/dl pleural: 1400cells/μL, 70% lymphocytes, triglycerides 900mgr/dl ). Upper gastrointestinal study and abdominal CT were normal. Thoracic CT showed thickening of the interstitial space, a sign of possible pulmonary lymphangiectasia (Figure 1). Lymphoscintigraphy failed to reveal the site of leakage. Scintigraphy with IV administration of Tc99m-albumin confirmed the intraabdominal leakage of lymph and its passage to the right thoracic cavity through right hemidiaphragm. The patient was treated conservatively (fasting, total parenteral nutrition (TPN) for 125 days, multiple pleural and abdominal paracentesis, abdominal drainage tube, octreotide for 30 days at a dose of 3γ/kg/h). Ascites responded to this treatment, so the patient was started on MCT (medium chain triglycerides) milk diet. She was discharged from the hospital when she was 5.5 months old. Follow up US (1st, 5th and 8th month after discharge) showed a small amount of ascitic fluid but it was without clinical significance. The patient is below the 10th percentile for weight.
Figure 1. Thoracic CT, showing thickening of the interstitial space, a sign of possible pulmonary lymphangiectasia.
Case 2
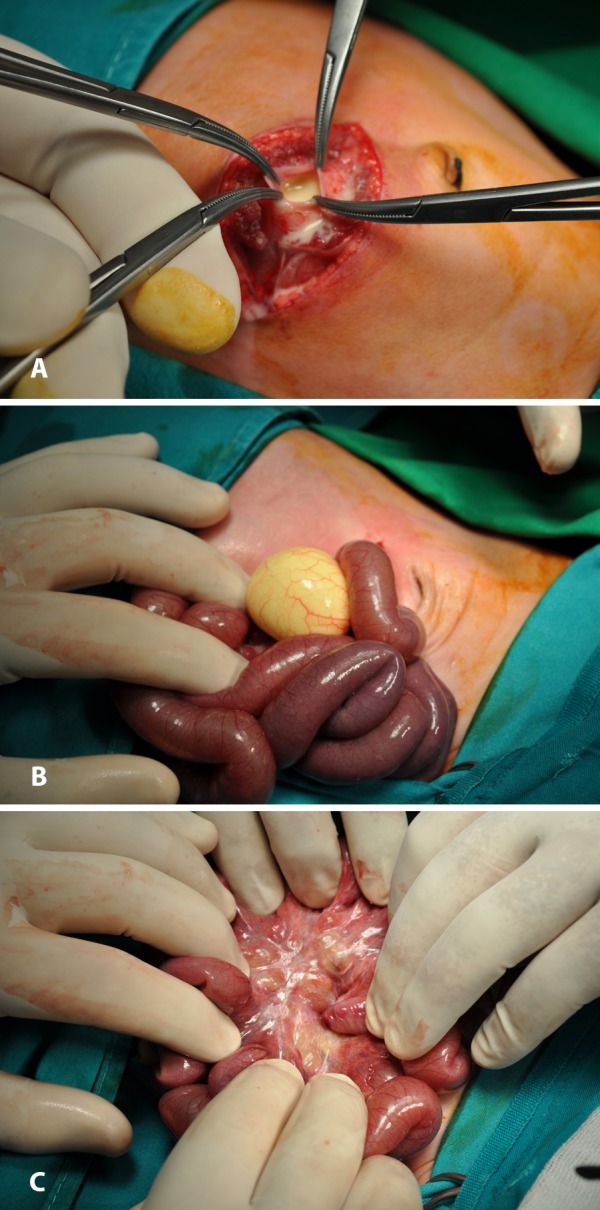
A 21-day-old, full-term boy was brought to the emergency department due to abdominal pain, vomiting and bloody stools. Abdominal US revealed a cystic formation (29×31mm) near the splenic flexure and MRI additionally showed edema of the bowel wall. The analysis of the ascitic fluid, obtained by paracentesis, revealed its chylous nature (cells>3300/μL, 75% lymphocytes, triglycerides:4480mgr/dL). The neonate underwent urgent exploratory laparotomy. The peritoneal cavity was filled with milky fluid (Figure 2A). A chylous mesenteric cyst was found 180 cm from the ileocolic valve at the jejunum (Figure 2B). The mesentery was thickened and its lymphatics were dilated (Figure 2C). The cyst was excised and what was left from its wall was cauterized. The pathologic examination showed thick, vascularized, fibrous connective tissue, consistent with cyst wall. On the 10th postoperative (PO) day, the patient was started on MCT milk diet. On the 17th PO day, he was dismissed from the hospital. Follow up US (3rd, 6th and 12th month PO) found no ascitic fluid. From the 7th month he has been fed with normal milk, he is growing up normally and he has normal feces.
Figure 2. A: Milky fluid filling the peritoneal cavity. B: Mesenteric cyst with chyle. C: Thickened mesentery with dilated lymphatics.
Case 3
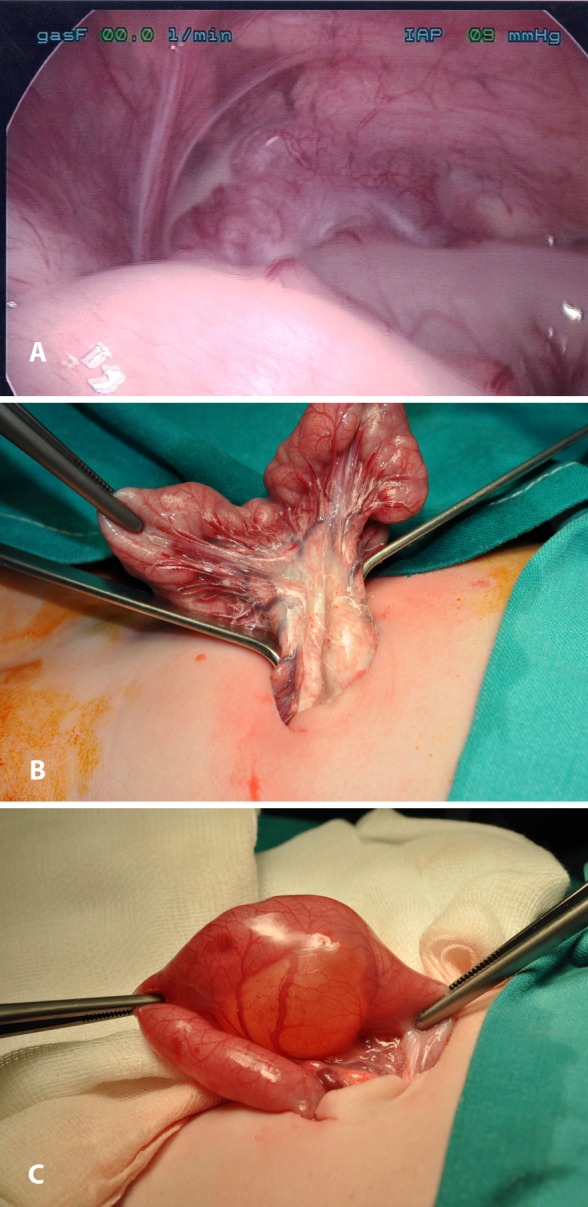
A new-born, full-term baby girl was transmitted to the NICU on the 2nd day of life due to bilious vomiting. Abdominal US revealed a cystic formation at the right lower quadrant of the abdomen. The same was found during MRI (dimensions: 36×32×27.5mm). Differential diagnosis included mesenteric cyst and cystic duplication of the intestine. During exploratory laparoscopy, a moderate amount of milky ascitic fluid (Figure 3A) and a cyst at the mesentery of the jejunum were discovered. The operation was converted to laparotomy through the umbilical incision. The lymphatics at the root of the mesentery and at the intestinal wall were dilated and the regional lymph nodes were enlarged (Figure 3B). The cyst was excised, along with the adjacent part of the intestine (Figure 3C). The biochemical analysis of the ascitic fluid showed concentration of triglycerides: 661mgr/dL. The pathologic examination of the cystic wall revealed that it was made from muscular fibers and intestinal epithelium, findings consistent with enteric duplication. Postoperative days were uneventful. She was discharged on MCT milk diet. At her first and second follow up examination (1st and 3rd month postoperatively) she was in excellent condition.
Figure 3. A: Laparoscopic image of milky fluid in the peritoneal cavity. B: Dilated lymphatics and enlarged lymph nodes of the mesentery. C: A cyst bonded to the enteric wall.
Case 4
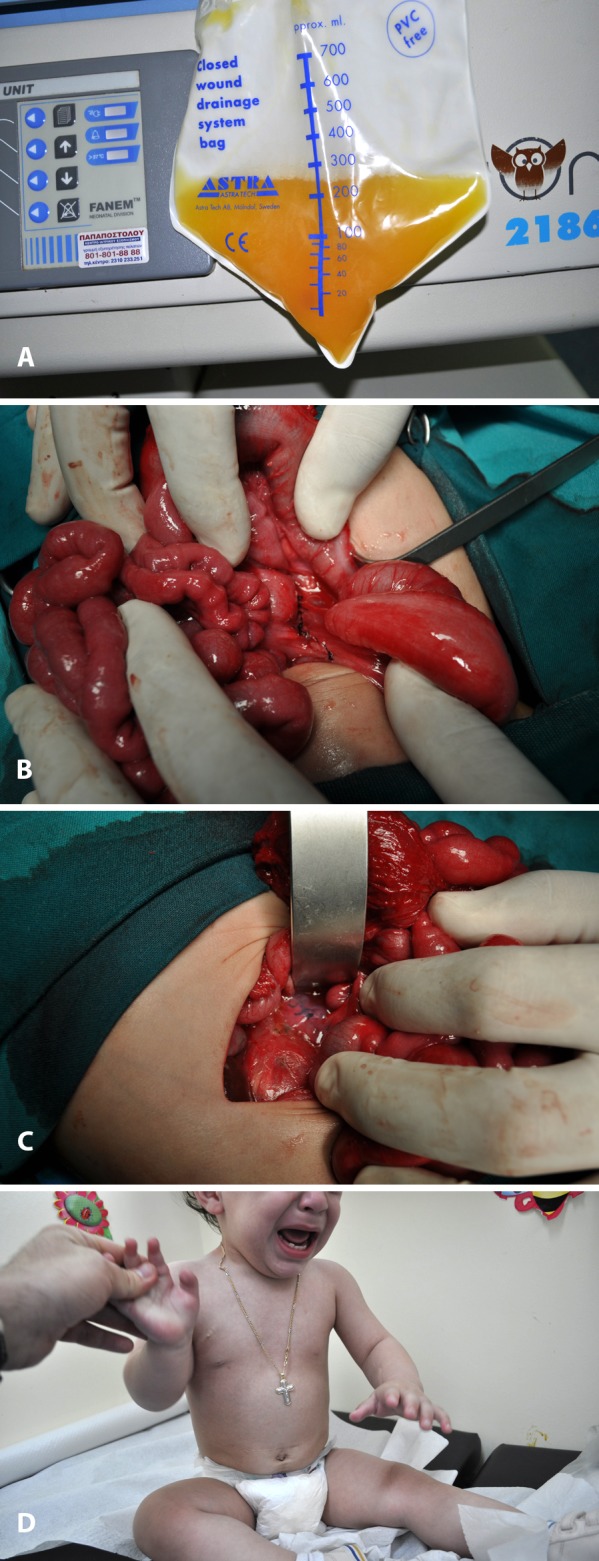
A boy was born prematurely by caesarian section at 32 weeks’ gestation because of fetal ascites and hydrops. The ascitic fluid obtained by paracentesis was straw-coloured, with 8120 cells/μL (93% lymphocytes). Diagnostic evaluation including abdominal CT, thoracic-retroperitoneal MRI and upper gastrointestinal study, was not suggestive of any abnormality. Initial treatment was conservative, with TPN, octreotide and multiple abdominal paracentesis. Finally, an abdominal drainage tube was inserted. The amount of ascetic fluid produced was 700ml/24h (Figure 4A). Since no decrease of chyle was accomplished, surgical intervention was decided. Lymphoscintigraphy failed to reveal the site of leakage. On day 50, surgery was performed. Six hours preoperatively 60 ml of fat rich formula were administered to the patient with 1.5gr of Sudan Black. Although the dye failed to reveal the site of leakage, the intra-abdominal lymphatics were better visualized because of the fat-rich meal. During exploratory laparotomy, the left lumbar lymphatic trunk was found dilated and ligated (Figure 4B). Interrupted sutures and fibrin glue were placed at the dilated cysterna chyli, after a Kocher maneuver was performed (Figure 4C). A drainage tube was placed in the abdominal cavity. The amount of ascitic fluid dramatically decreased postoperatively. The child was started on MCT milk diet on the day 10 post-operatively and discharged a month after surgery. Follow up abdominal US 1st, 3rd, 6th, 15th month after discharge revealed no accumulation of ascitic fluid. The child has normal growth and has been fed normally since 12th month of life (Figure 4D).
Figure 4. A: Straw-coloured fluid from the peritoneal cavity through the drainage tube. B: Continuous suturing of the dilated left lymphatic lumbar trunk. C: Sutures on the cysterna chyli, scattered with fibrin glue. D: The patient 12 months after the operation.
Discussion
Congenital chylous ascites may be due to a variety of reasons. The most common (45-60%) is the malformation of the lymphatic vessels, as in atresia or stenosis of the major lacteals, mesenteric cysts and lymphangiomatosis4,7. But in 50% of neonates, none of the above is identified, and the chylous ascites is thought to be due to a condition called “leaky lymphatics”, probably because of a delayed maturation of the lacteals8-11. Another reason (20-25%) is external compression which causes obstruction of the lymphatics, as in malrotation, incarcerated hernia, intussusception, inflammatory enlargement of lymph nodes and malignancy. In 15-20% of cases, the chylous ascites is caused by trauma during surgery, accidents or child abuse4,7.
The diagnostic tool for the type of ascites is the analysis of the fluid obtained by abdominal paracentesis. If the patient is being orally fed, the fluid has a milky appearance, otherwise it is straw-colored. Nevertheless, sometimes the fluid doesn’t become milky, but cloudy, despite enteral feeding12. It is odorless, alkaline, with specific gravity>1012 and with bacteriostatic properties. Its fat globules get stained with Sudan Red. Its concentration of proteins is 2.5-4mgr/dL, of cells >500/μL (70-90% lymphocytes)1,4,13 and of triglycerides>110mgr/dL with the predominance of chylomicrons14,15. The true challenge is determining the underlying cause of the chylous ascites. The initial diagnostic investigation includes US and CT or MRI of the abdomen, MRI of the retroperitoneum, and upper and lower gastrointestinal studies to exclude conditions that demand immediate surgical intervention. If everything comes out negative, malformation of the lymphatics is the most probable cause7,10,14-16. Further diagnostic tests are important if surgery is decided, with the purpose of identifying the site of the leakage of chyle preoperatively.
The imaging study of choice is lympho-scintigraphy1,11,13,15. It involves the injection of 99mTc-dextran, 99mTc-labelled sulfur colloid or 99mTc-labelled human albumin into the interdigital web spaces of the feet. This study helps to evaluate the patency of the lymphatic system and possibly to identify the site of leakage1,11,15. Lymphangiography is avoided because of the technical difficulties in such small children and because of the many serious side effects1,7,11,13,14,17. It is indicated only in complicated, refractory to therapy cases 18. Also, the laparoscopic exploration of the abdomen after the administration of fat-rich formula with a lipophilic dye, such as Sudan Black or Sudan III could be of important use1,7,14,17. Laparoscopy allows the surgeon to explore a magnified image of the abdominal cavity, in its natural state17. It could also be used to place a drainage tube 14.
The treatment, when there is no identifiable surgical cause, is primarily conservative. Initially, it is necessary to relieve the patient from the symptoms by abdominal paracentesis or by placing a drainage tube and to restore fluid and protein losses1,11,13,14. Ascites should be drained by simultaneous IV administration of 500ml NaCl 0.9% with 10gr of human albumin for every liter of ascitic fluid that is being removed14. The purpose of therapeutic approach is to maintain nutrition and decrease the production and flow of chyle1,10. Chronic loss of chyle leads to anemia, hypoproteinemia, hypocalcemia, hypolipidemia, immunocompromise and malnourishment. So, initially we begin with a low fat, high protein diet, with MCT milk and parenteral nutrition if needed. Medium chain triglycerides (fatty acids with carbon chain length ≤12) are thought to be absorbed directly into the portal venous system, without requiring reesterification or packaging , so they do not induce the production of chyle8,13. Nevertheless, sometimes this isn’t enough and discontinuance of oral feeding with the support of total parenteral nutrition is necessary1,8,10,11. In refractory cases, the administration of somatostatin or its analogue octreotide is needed3,6,11,13,14,19. It is not completely understood how these drugs work in this case. It is speculated that they reduce the absorption of fatty acids from the intestine, decrease the gastric, pancreatic and enteral secretions, the intestinal motility and the visceral blood flow and so they decrease the production of chyle. It is also believed that these drugs inhibit specific receptors found in the normal lymphatic vessels of the intestinal wall and they prevent the excretion of lymph3,13,19. Conservative treatment should be continued for 1-2 months before considered ineffective10,11,17,19. If the conservative treatment is not sufficient to resolve the ascites, surgery is the appropriate choice, since it is found that 58% of children with intractable chylous ascites have a lesion amenable to surgical intervention7. Important for the success of the operation is to recognize the leakage site17. As it has been mentioned previously, lymphoscintigraphy and laparoscopy are the tools for that. Laparoscopy could also be used as a final treatment, by laparoscopically ligating the leaking lymphatic vessel7. Otherwise, laparotomy is the next step. Six to three hours before the operation, the patient should be administered a fatty meal with lypophilic dye, because this could help the surgeon to identify the leak intraoperatively7,17. During exploratory laparotomy, all major lacteals should be examined for evidence of a leak requiring suturing. If a specific cause is found, the surgical cure rate may reach 85%20. If there is no obvious lymphatic leakage after exploring all the intraperitoneal surfaces, then the duodenum and the head of the pancreas should be mobilized (Kocher maneuver) to expose the root of the mesentery and identify the cysterna chyli. The intestinal, descending thoracic, hepatic and right and left lumbar trunks unite to form the cysterna chyli, which is a retroperitoneal structure tightly wedged between the aorta, inferior vena cava and the right crus of the diaphragm, and overlies the upper two lumbar vertebrae. This is a region where a leakage may often be identified 1,14. Sometimes, though, the surgeon cannot find a specific leak or he cannot control the excretion of lymph in spite of the suturing. In these cases, the use of fibrin glue seems to have excellent results 3,21. When there is a recurrence even after surgical intervention or in the case of general lymphangiomatosis, a peritoneovenous shunt could be performed. Unfortunately, this method has many complications, most important being the occlusion of the shunt4,15,18.
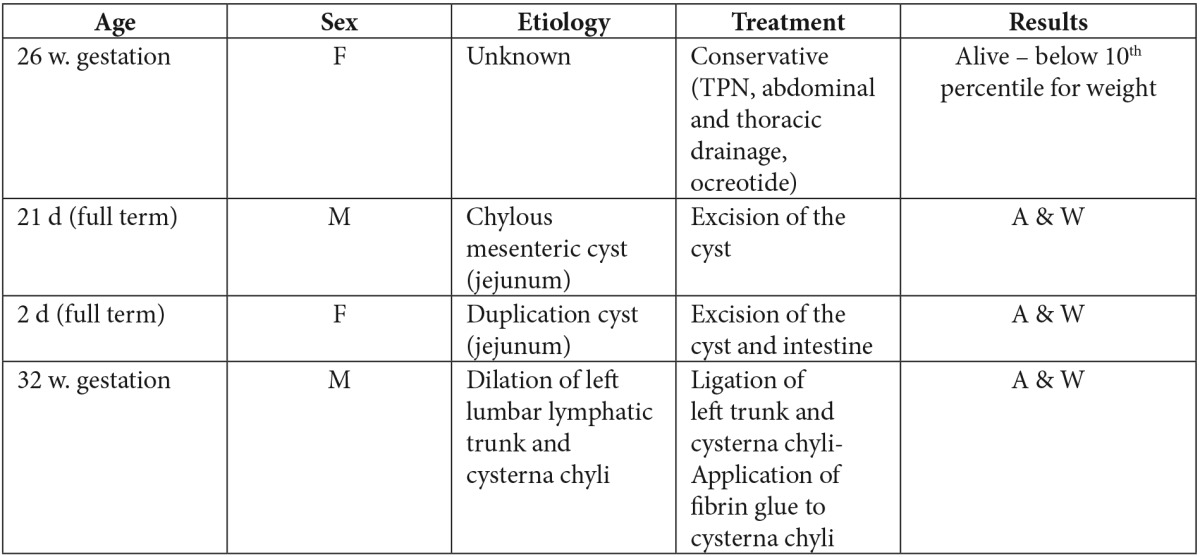
Our first case (Table 1) was treated conservatively with total parenteral nutrition, ocreotide, multiple thoracic and abdominal paracentesis and abdominal drainage tube. The rare combination of chylous ascites and chylothorax resulted from passage of chyle through transdiaphragmatic lymphatic channel in a manner similar to Meigs syndrome, which is also called porous diaphragmatic syndrome22,23. In the second case (Table 1) a blockage of the lymphatics somewhere in the mesentery in combination with weakness of the vessel wall may lead to the formation of a cyst. Extravasation of chyle may produce ascites24. That is probably the cause of thickened mesentery with dilated lymphatics and of the mesenteric cyst filled with milky fluid (Figure 2A, 2B, 2C). Cyst excision usually resolves the problem. Also we report (3rd case) (Table 1) a neonate with chylous ascites and intestinal duplication cyst (Figure 3A, 3B, 3C). To the best of authors’ knowledge, this is the first report in English-language literature of this combination. The pressure of the intestinal duplication cyst on the lymphatics may produce increased intralymphatic pressure and contribute to the leakage of chyle into the peritoneum. Another possible explanation is mesenteric lymphatic obstruction due to early embryonic adhesions between the gut and more dorsal structures (retroperitoneal sac). Both cystic and tubular duplications with independent muscular coats are dorsal enteric remnants caused by embryonic adhesions25. In the last of our neonates (4th case) (Table 1), the chylous ascites was caused by retroperitoneal megalymphatics and a possible lymphoperitoneal fistula. The surgical exploration was done according to a defined strategy that includes visualization of the retroperitoneal origin of the superior mesenteric artery.
Table 1. Profile of our patients with chylous ascites. w: weeks, d: days, F: Female, M: Male, TPN: Total Parenteral Nutrition, A&W: Alive and Well.
Conclusions
It is obvious that congenital chylous ascites is a complex condition. Its diagnostic evaluation is difficult and its therapy of long duration. Conservative treatment is in most cases the initial choice, but when it fails, exploratory laparotomy could provide a successful alternative. An adequate surgical exploration for chylous ascites in infants should always consist of a thorough search of all intraperitoneal surfaces for an obvious lymphatic leakage or other pathology. In all other situations, complete mobilization of the duodenum and head of the pancreas is necessary in order to explore the root of the mesentery and identify the cysterna chyli.
All participating authors have no conflict of interest to declare.
References
- 1.Aalami OO, Allen DB, Organ CH Jr. Chylous ascites: A collective review. Surgery. 2000;128:761–768. doi: 10.1067/msy.2000.109502. [DOI] [PubMed] [Google Scholar]
- 2.Press OW, Press NO, Kaufman SD. Evaluation and management of chylous ascites. Ann Intern Med. 1982;96:358–364. doi: 10.7326/0003-4819-96-3-358. [DOI] [PubMed] [Google Scholar]
- 3.Qi H, Bu-jun G, Li-ming L, Zhi-yuan T, Guo-fen Z, Yue-zu F. Successful management of chylous ascites with total parenteral nutrition, somatostatin and fibrin glue. Chin Med J. 2007;120:1847–1849. [PubMed] [Google Scholar]
- 4.Petropoulos AS, Sfougaris DK, Mouravas VK. Birth defects of the lymphatic system. In: Gaslem NA, editor. New developments in birth defects research. New York. Nova Science Publishers, Inc. 2007:1–67. [Google Scholar]
- 5.Jalili F. Medium-chain triglycerides and total parenteral nutrition in the management of infants with congenital chylothorax. South Med J. 1987;80:1290–1293. doi: 10.1097/00007611-198710000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Gaty MG, Hilfiker ML, Azizkhan RG, Glick PL. Successful treatment of congenital chylous ascites with a somatostatin analogue. Pediatr Surg Int. 1996;11:396–397. doi: 10.1007/BF00497824. [DOI] [PubMed] [Google Scholar]
- 7.Kuroiwa M, Toki F, Suzuki M, Suzuki N. Successful laparoscopic ligation of the lymphatic trunk for refractory chylous ascites. J Pediatr Surg. 2007;42:E15–E18. doi: 10.1016/j.jpedsurg.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 8.Alliet P, Young C, Lebenthal E. Chylous ascites: total parenteral nutrition as primary therapeutic modality. Eur J Pediatr. 1992;151:213–214. doi: 10.1007/BF01954387. [DOI] [PubMed] [Google Scholar]
- 9.Chye JK, Lim CT, Van der Heuvel M. Neonatal chylous ascites-report of three cases and review of the literature. Pediatr Surg Int. 1997;12:296–298. doi: 10.1007/BF01372154. [DOI] [PubMed] [Google Scholar]
- 10.Cochran WJ, Klish WJ, Brown MR, Lyons JM, Curtis T. Chylous ascites in infants and children: a case report and literature review. J Pediatr Gastroenterol Nutr. 1985;4:668–673. doi: 10.1097/00005176-198508000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Xu H. Treatment of congenital chylous ascites using total parenteral nutrition and somatostatin: a case report. Zhonghua Er Ke Za Zhi. 2005;43:152–153. [PubMed] [Google Scholar]
- 12.Amini E, Sohrabvand F, Molaian M, Shabani M. Chylous ascites and gut malrotation. Arch Iran Med. 2005;8:323–325. [Google Scholar]
- 13.Gardenas A, Chopra S. Chylous ascites. Clinical review. Am J Gastr. 2002;97:1896–1899. doi: 10.1111/j.1572-0241.2002.05911.x. [DOI] [PubMed] [Google Scholar]
- 14.Campisi C, Bellini C, Eretta C, Zilli A, da Rin E, Davini D, et al. Diagnosis and management of primary chylous ascites. J Vasc Surg. 2006;43:1244–1248. doi: 10.1016/j.jvs.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 15.Noel AA, Gloviczki P, Bender CE, Whitley D, Stanson AW, Deschamps C. Treatment of symptomatic primary chylous disorder. J Vasc Surg. 2001;34:785–791. doi: 10.1067/mva.2001.118800. [DOI] [PubMed] [Google Scholar]
- 16.McGahren ED. Ascites. In: Grosfeld JL, O’Neill JA Jr, Fonkalsrud EW, Coran AG, editors. Pediatric Surgery. 6th edition. Philadelphia: Mosby Elsevier. 2006:pp. 1407–1413. [Google Scholar]
- 17.Mitsunaga T, Yoshida H, Iwai J, Matsunaga T, Kouchi K, Ohtsuka Y, et al. Successful surgical treatment of two cases of congenital chylous ascites. J Pediatr Surg. 2001;36:1717–1719. doi: 10.1053/jpsu.2001.27973. [DOI] [PubMed] [Google Scholar]
- 18.Fishman SJ, Burrows PE, Upton J, Hendren WH. Life-threatening anomalies of the thoracic duct: anatomic delineation dictates management. J Pediatr Surg. 2001;36:1269–1272. doi: 10.1053/jpsu.2001.25792. [DOI] [PubMed] [Google Scholar]
- 19.Huang Q, Jiang Z, Jiang J, Li N, Li J. Chylous ascites: Treated with total parenteral nutrition and somatostatin. World J Gastroenterol. 2004;10:2588–2591. doi: 10.3748/wjg.v10.i17.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unger SW, Chandler JG. Chylous ascites in infants and children. Surgery. 1983;93:455–461. [PubMed] [Google Scholar]
- 21.Antalo B, Croaker D, Squire R. Successful management of congenital chyloperitoneum with fibrin glue. J Pediatr Surg. 2003;38:E7–E8. doi: 10.1016/j.jpedsurg.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Plummer JM, McFarlane ME, McDonald AH. Chylous ascites associated with chylothorax; a rare sequela of penetrating abdominal trauma: a case report. J Med Case Reports. 2007;1:149. doi: 10.1186/1752-1947-1-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takagi Y, Sato T, Morio Y, Kumasaka T, Mitani K, Miyamoto H, et al. A pleuro-peritoneal communication through the diaphragm affected with lymphangioleiomyomatosis. Intern Med. 2010;49:439–445. doi: 10.2169/internalmedicine.49.2714. [DOI] [PubMed] [Google Scholar]
- 24.Servelle M. Congenital malformation of the lymphatics of the small intestine. J Cardiovasc Surg. 1991;32:159–165. [PubMed] [Google Scholar]
- 25.Skandalakis JE, Gray SW, Rickets R, Richardson DD. The small intestine. In: Skandalakis JE, Gray SW, editors. Embryology for surgeons: The embryological basis for the treatment of congenital anomalies. Baltimore: Williams&Wilkins. 1994:pp. 184–241. [Google Scholar]


