Abstract
Periostin is an extracellular matrix protein. Five isoforms of human periostin cDNA have been reported, but the expression of periostin isoforms in the human thyroid tissue is by far unknown. A group of primer sets were designed to amplify the full length of cDNA sequence of periostin. Using human thyroid carcinoma and their paired non-neoplastic tissues together with anaplastic thyroid carcinoma cell lines, we examined the presence of periostin cDNA isoforms by RT-PCR and direct DNA sequence analysis. We identified eight coexisting cDNA isoforms in all the tissue samples and cell lines. Three of them were unique to this study. Especially two of them haven’t been previously reported in any species. The eight periostin isoforms differ in the C-terminus from exon XII to exon XXI where alternative splicing usually happens. This is the first report that demonstrates all the eight isoforms of periostin cDNA expressed in the human thyroid gland and identifies three novel isoforms.
Keywords: thyroid, carcinoma, periostin, isoform
Background
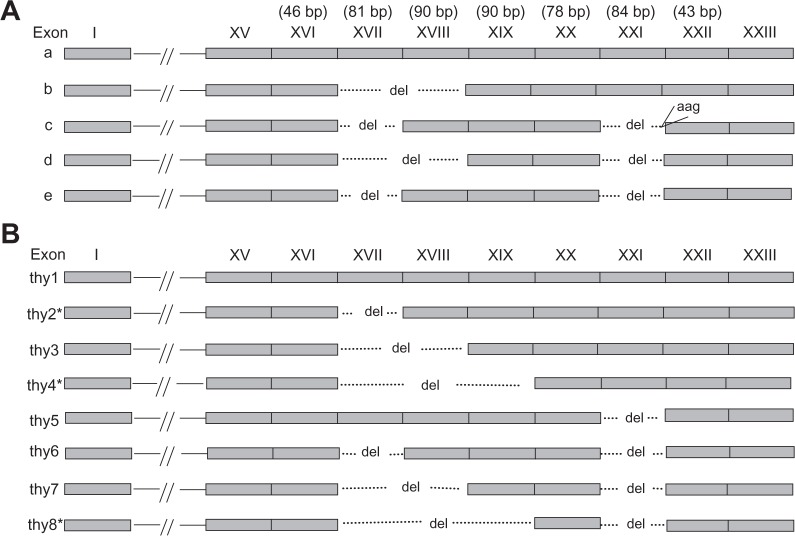
Periostin, also called osteoblast specific factor 2 (OSF-2) is an extracellular matrix protein, whose gene was first cloned from the mouse osteoblastic cell line MC3T3-E1, that shares homology with the insect protein fasciclin 1.1 Periostin is highly conserved between mouse and human, and has been suggested to act as a homophilic adhesion molecule in bone formation.1 Periostin contains a typical signal sequence, followed by a cysteine-rich domain, a 4-fold repeat structure of about 150 amino acids and a C-terminal domain.1 Five isoforms of human periostin (h-periostin) with available sequence have been reported from the literature.1–3 These isoforms differ at C-terminus, corresponding to cDNA exon XV∼XXIII. The five h-periostin isoforms (Fig. 1A) were identified from different tissues: human osteosarcoma for a, human placenta for b, epithelial ovarian carcinoma for c, and human bladder normal tissue or cancer tissue for b, d and e.1–3 The isolation of different h-periostin isoforms from different tissues suggested that their expression was tissue specific.
Figure 1.
Previous reported and newly identified h-periostin cDNA isoforms. A) Isoforms of h-periostin reviewed from the literature. Their accession numbers are D13666, D13665, AY140646, AY918092, and BC106709 respectively from isoforms a∼f. B) Eight isoforms (thy1–8) of h-periostin in thyroid tissue. Among them, three (marked with*) are novel isoforms specific in this study.
Abbreviation: del, deletion of cDNA sequence.
Recently, expression of h-periostin has been linked to tumor invasiveness and metastasis in a variety of types of carcinomas. Overexpression of h-periostin in thyroid papillary carcinoma, non-small cell lung carcinoma, epithelial ovarian carcinoma, breast carcinoma, pancreas cancer, colon cancer, head and neck and oral carcinoma, has been associated with greater invasiveness, angiogenesis or metastasis.2,4–10 Nevertheless, the expression of h-periostin is reduced in a variety of human cancer cell lines, human lung cancer tissues and bladder carcinoma tissues, and these cell lines had reduced anchorage-independent growth when infected with a recombinant retrovirus containing the periostin gene.11,12 In the above articles, the quantitative or semiquantitative expression of h-periostin in the tumor development was described, but the expression pattern of each periostin isoform in the tumor carcinogenesis or development have not been well established. By now, there is only one report describing the difference in the expression pattern of h-periostin between the tumor tissue and the non-neoplastic tissue, which focused on bladder.3
Thyroid carcinoma particularly that of follicular cell origin, is one of the most common malignancies of endocrine organs. Thus it would be of more potential therapeutic value to identify the molecular events underlying thyroid tumor invasiveness and metastasis. Expression of h-periostin mRNA has been reported in normal thyroid tissue and papillary thyroid carcinoma, and up-regulation of total h-periostin in the tumor tissue is significantly linked to extrathyroid invasion and lymph node metastasis.4,13,14 However, the expression of each h-periostin isoform is totally unknown either in the normal thyroid tissue or in the tumor tissue. This information led us to examine the expression pattern of all h-periostin isoforms in the normal thyroid gland and thyroid carcinoma tissue.
Methods
Patients and specimens
Adjacent neoplastic and non-neoplastic thyroid tissues were obtained from ten patients with primary thyroid carcinoma of follicular cell origin. All the specimens were obtained at surgery, snap-frozen immediately and stored at −80 °C until RNA extraction. The carcinoma tissue and paired non-neoplastic tissue were sampled more than 2 mm away from their interface to avoid contamination. Paired non-neoplastic tissues include seven normal thyroid tissues, one nodular hyperplasia and two Hashimoto’s disease. The histopathological diagnoses and clinical data are shown in Table 1. This study was approved by the ethics committee of Wakayama Medical University.
Table 1.
Clinical data of 10 cases of human thyroid carcinoma.
| Case no. | Gender | Age | Ex | LN | Diagnosis | Background |
|---|---|---|---|---|---|---|
| 1 | Male | 65 | + | + | PTC | Normal |
| 2 | Female | 54 | − | + | PTC | Normal |
| 3 | Female | 59 | + | + | PTC | Hashimoto |
| 4 | Female | 84 | + | − | FTC | Normal |
| 5 | Female | 55 | + | + | PTC | Normal |
| 6 | Female | 61 | − | + | PTC | Normal |
| 7 | Female | 34 | + | + | PTC | Hashimoto |
| 8 | Female | 32 | − | + | PTC | Normal |
| 9 | Female | 71 | + | + | PTC | Normal |
| 10 | Female | 61 | + | + | PTC | Ad |
Abbreviations: Ex, extrathyroid invasion; LN, lymph node metastasis; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma;
positive;
negative; Normal, Normal thyroid gland; Hashimoto, Hashimoto’s disease; Ad, adenomatous goiter.
Cell lines and culture conditions
Five human anaplastic thyroid carcinoma cell lines, 8305C, 8505C, KHM-5M, HTC-C3 and TCO-1, were obtained from the JCRB (Japanese Collection of Research Bioresources). All cell lines were maintained in the culture media recommended by the suppliers: Eagle’s minimal essential medium for 8305C and 8505C, RPMI-1640 for KHM-5M, and Dulbecco’s modified Eagle’s medium for HTC/C3 and TCO-1. The media were supplemented with 10% fetal calf serum and antibiotics in a humidified atmosphere with 5% CO2 and 95% air.
Reverse transcription-polymerase chain reaction (RT-PCR)
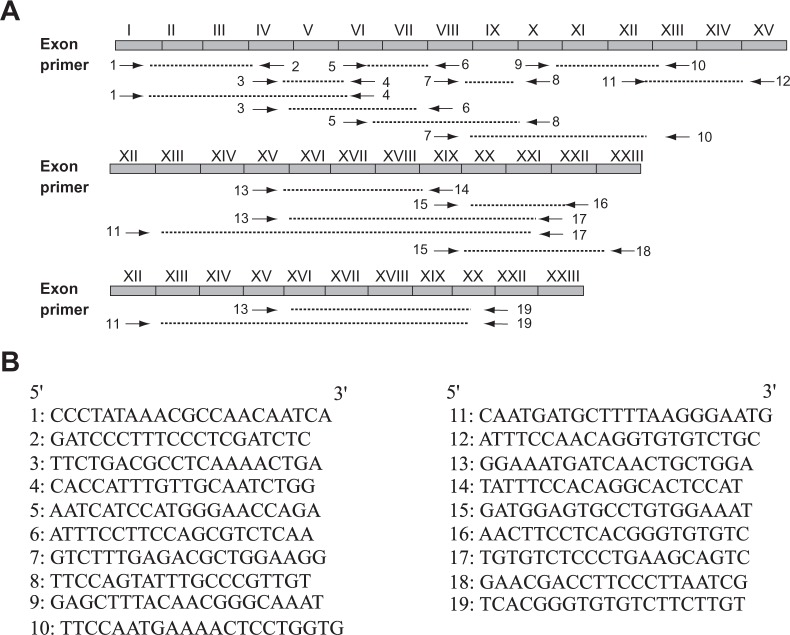
Total RNA was extracted from the frozen tissues and cell lines using the ULTRASPECTM RNA isolation system (Biotecx, TX) according to the manufacturer’s instruction. Their concentration was determined by standard spectrophotometric methods. First strand cDNA was synthesized using 3 ug total RNA as template and oligo (dT)12–18 as primer in a 20 ul reaction unit according to the introduction of SuperScript TM First-Strand Synthesis System for RT-PCR (Invitrogen, CA). An identical quantity of total RNA from tumor and paired non-neoplastic tissue samples was used to synthesize cDNA. 2 ul of the cDNA was subjected to a 25 ul PCR reaction mixture. PCR was performed using a thermal cycler (GeneAmp PCR system 9700, Tokyo, Japan) for 35 cycles. The PCR reaction primers used for the isoform analysis were designed using the software Primer3 (http://frodo.wi.mit.edu/), and their location and oligonucleotide sequences are shown in Figure 2. Reaction conditions of PCR were as follows: denaturation for 30 seconds at 95 °C for all the primers; annealing for 30 seconds at a temperature of 61 °C for primer 13/14, 60 °C for primer 15/16 and 57 °C for all the other primers; extension at 72 °C for 45 seconds for all the primers. RT-PCR analysis using β-actin primers (forward 5′-AAGAGAGGCATCCTCACCCT-3′ and reverse 5′-TACATGGCTGGGGTGTTGAA-3′) was used as an internal RNA control. The annealing temperature for the β-actin primers was 57 °C. The other PCR conditions for β-actin were the same as that used for analysis of all the other primers.
Figure 2.
Location and sequence of periostin primers within the periostin cDNA. A) There are total 23 exons in the wild type h-periostin (exon I∼XXIII). The position and direction of primers are shown by arrows. B) DNA sequences of oligonucleotide primers for h-periostin (from primer 1 to primer 19).
The PCR products were run on 2% agarose gel at 100 mV, and visualized by ethidium bromide staining.
Sequencing of PCR products
After electrophoresis, the bands containing objective PCR products were cut from the agarose gel carefully under the ultraviolet observation and collected into a 2 ml sterilized micro-tube. Strict care was taken to avoid contamination by the other bands during cut. Then PCR product was extracted from the gel and purified using QIAquick Gel Extraction Kit (QIAGEN, Tokyo, Japan), and sequenced on a 310 genetic analyzer (PerkinElmer) with utilization of the BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Warrington, UK).
Results
Expression of h-periostin mRNA in the thyroid tissue
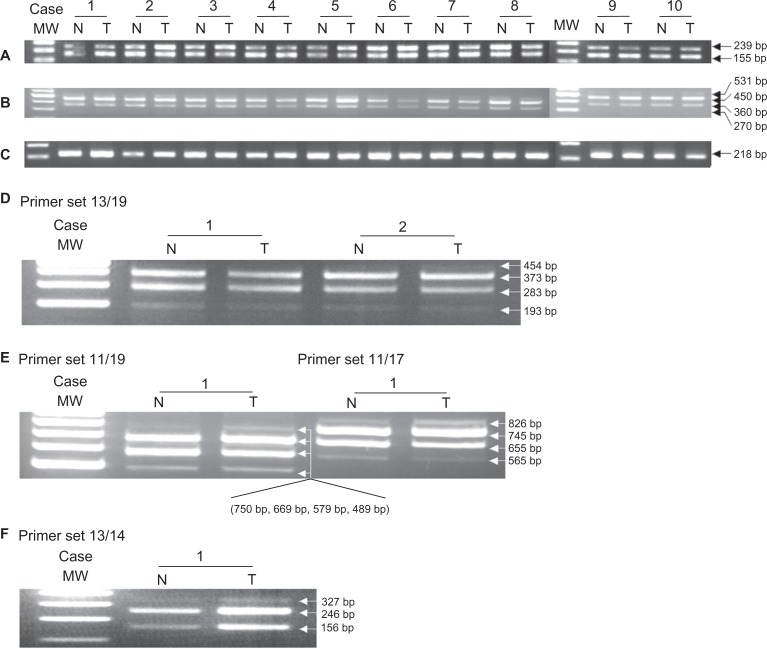
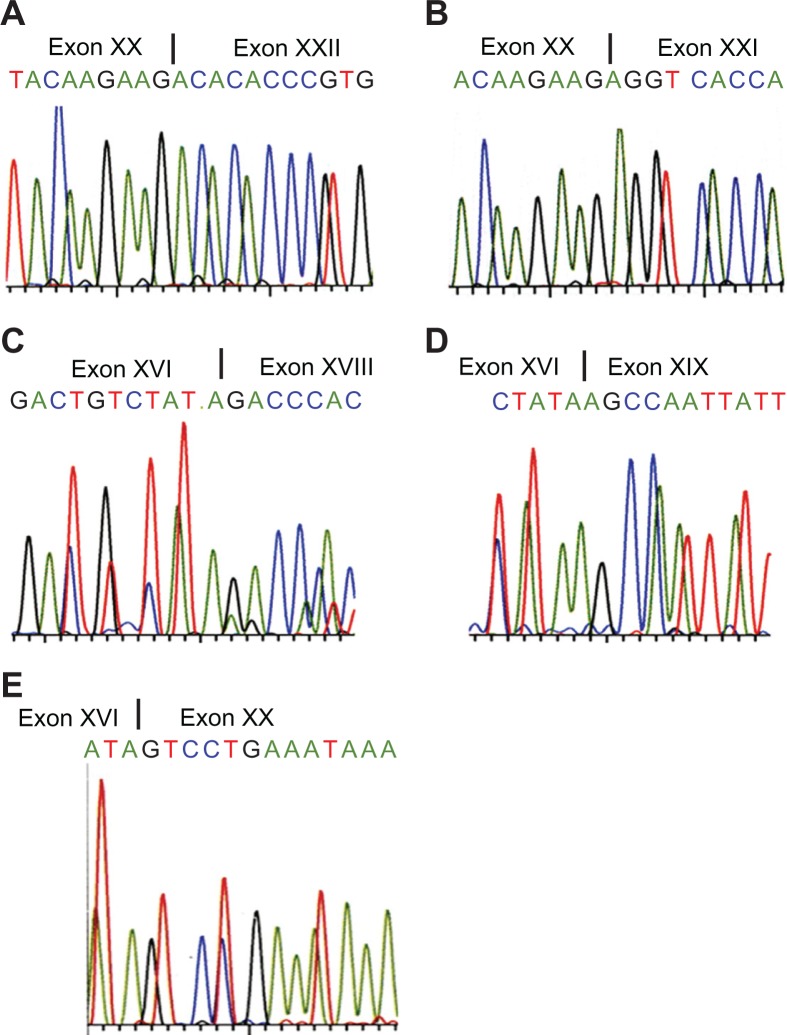
β-actin mRNA was strongly expressed in all ten cases of thyroid carcinoma and paired non-neoplastic tissue (Fig. 3C). RT-PCR analysis yielded products of 239 and 155 bps in all samples using the 15/16 periostin primer set (Fig. 3A). Direct DNA sequence was performed on cases 1 and 2. This analysis confirmed that the sequence of the 239 bp product corresponded to that of the reported sequence of wild type h-periostin (Isoform a, Fig. 1A), whereas the 155 bp fragment contained a deletion of 84 bp relative to the wild type sequence. The deleted 84 bp nucleotides are in accordance with the sequence of exon XXI (Fig. 4A and 4B). This result illuminates there is deletion of exon XXI in the 155 bp fragment.
Figure 3.
PCR products amplified from exon XVII to XXII in the C-terminal of h-periostin cDNA. RT-PCR analysis using primer sets 15/16 A) and 13/17 B) in 10 cases of thyroid carcinoma tissue (abbr. T) and their paired non-neoplastic tissue (abbr. N), using primer sets 13/19 D), 11/19 and 11/17 E) for case 1 and case 2, and primer set 13/14 for case 1 F). Level of β-actin was used as an internal control C).
Abbreviation: MW, molecular weight marker.
Figure 4.
Deletion of h-periostin cDNA sequence confirmed from direct DNA sequence analysis. Direct DNA sequence analysis performed on case 1 and case 2 showing deletion of exon XXI A), wide type of exon XXI B), deletion of exon XVII C), deletion of exons XVII and XVIII D) and deletion of exon XVII∼XIX E).
In order to check whether there were other deletions or insertions at the C-terminus of h-periostin, primer sets 13, 17 and 19 were designed as shown in Figure 2. Primer 17 encompassed the junction of exon XXI and XXII, and primer 19 encompassed the junction of exon XX and XXII. RT-PCR analysis using primer set 13/17 on ten cases of thyroid tissue revealed four products, although the longest and the shortest products were only faintly detected (Fig. 3B). RT-PCR analysis performed on cases 1 and 2 using primer set 13/19 produced four products, the longest of which was only faintly detected (Fig. 3D). The longest PCR product obtained using primer set 13/17 was consistent with the sequence of wild type h-periostin. It differed from the longest PCR product produced with primer set 13/19 by the presence of sequences corresponding to exon XXI. We isolated the two mid-sized PCR products shown in Figure 3B and the three smaller products shown in Figure 3D, which were then subjected to direct DNA sequence analysis. The results showed there was deletion of exon XVII DNA sequence (81 bp) (Fig. 4C) in the second longest PCR product produced using primer sets 13/17 and 13/19, and deletion of sequences corresponding to exons XVII + XVIII (171 bp) (Fig. 4D) in the third longest PCR product produced from both primer sets. Sequence analysis of the shortest PCR product produced using primer set 13/19 revealed deletion of exons XVII + XVIII + XIX (261 bp) (Fig. 4E) was informed from sequencing the in the fourth band from primer 13/19. We were unable to sequence the shortest PCR product produced using primer set 13/17, because it was expressed in an extreme low level. To clarify which sequence was deleted in the shortest PCR product produced using primer set 13/17 and to confirm the deleted sequences in the PCR products using primer sets 13/17 and 13/19, we thus designed and used primer sets 11/19, 11/17 and 13/14 for RT-PCR analysis. We observed four products using primer sets 11/19 and 11/17 (Fig. 3E), and three products using primer set 13/14 (Fig. 3F). With all the three primer sets, the yielded longest products corresponded to wild type h-periostin except for additional deleted sequence of exon XXI in the longest product using primer set 11/19. Since we did not detect deletion of exon XVI or XX in the periostin mRNA isolated from thyroid tissue following RT-PCR using primer sets 13/14 and 15/16 respectively, we conclude that the shortest PCR product obtained using primer set 13/17 should be an isoform lacking exons XVII, XVIII and XIX.
No deletion was detected in the sequence of exon I∼ exon XV and exon XXII by RT-PCR analysis (data not shown).
Expression of h-periostin cDNA isoforms in the thyroid tissue
We identified four isoforms of h-periostin in the thyroid tissue by RT-PCR analysis using primer sets 13/17 and 11/17. These isoforms corresponded to wild type h-periostin, an isoform with a deletion of exon XVII, an isoform with a deletion of exon XVII + XVIII, and an isoform with a deletion of exon XVII + XVIII + XIX. We additionally detected four isoforms using primer sets 13/19 and 11/17 were detected. DNA sequence analysis revealed that these isoforms corresponded to fragments with a deletion of exon XXI, deletions of exon XVII + XXI, deletions of exons XVII + XVIII + XXI, and deletions of exons XVII + XVIII + XIX + XXI. We thus identified a total of eight isoforms of h-periostin in thyroid tissue, which we denote as thy1∼thy8 (Fig. 1B). The four products generated using primer sets 13/17 and 11/17 corresponded to isoforms thy1∼thy4, and the four products generated from primer sets 13/19 and 11/19 corresponded to isoforms thy5∼thy8.
Among the eight h-periostin isoforms identified in thyroid tissue, the sequences of thy1, thy3, thy6 and thy7 were identical to those of isoforms a, b, e, and d respectively (Fig. 1). Thy5 was most probably identical to one of the five isoforms identified by Takeshita et al, since the five isoforms differ at two sites in the C-terminal domain of periostin, exons XVII + XVIII and exon XXI. The expression of isoforms corresponding to isoforms thy2, thy4, and thy8 in human was unique in this study, which haven’t been reported in any other human tissue (Genbank accession number: EU262883, EU262884, and EU262886 respectively). Thy2 has the similar cDNA sequence with one of the mouse periostin isoforms identified by Horiuchi et al,15 while thy4 and thy8 haven’t been previously reported in any species.
Correlation between h-periostin cDNA isoforms and thyroid carcinoma
A total of eight isoforms of h-periostin cDNA were identified universally in the ten cases of thyroid carcinoma and the paired non-neoplastic thyroid tissue. We did not detect differences in the expression pattern of the eight isoforms between the thyroid carcinoma and the paired non-neoplastic tissues. The ten cases of thyroid carcinoma included nine cases of thyroid papillary carcinoma and one case of follicular carcinoma. Among the ten cases, seven cases were identified with extrathyroid invasion and nine cases were positive with lymph node metastasis, but no distant metastasis was detected at surgery. The results suggest the expression pattern of these isoforms is not linked to thyroid carcinoma carcinogenesis, invasion or lymph node metastasis.
Expression of h-periostin cDNA isoforms in the anaplastic thyroid carcinoma cell lines
The eight h-periostin cDNA isoforms were all identified in the five anaplastic thyroid carcinoma cell lines (Fig. 5), which indicates that the thyroid neoplastic follicular epithelial cells could produce periostin. The expression pattern of h-periostin isoforms did not change during the dedifferentiation of thyroid follicular-originated carcinoma, from well differentiated carcinoma to anaplastic carcinoma, since the same expression pattern was identified not only in the non-neoplastic thyroid tissue, well differentiated thyroid carcinoma, but also in the anaplastic thyroid carcinoma cell lines in the current study.
Figure 5.
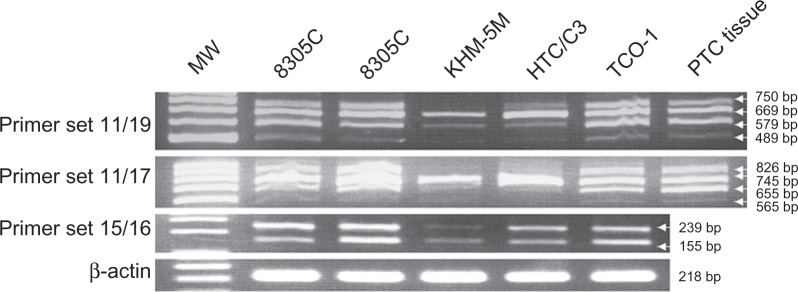
Expression of h-periostin isoforms in five anaplastic thyroid carcinoma cell lines. Eight h-periostin cDNA isoforms were identified in the above cell lines, same as those identified in thyroid tissue. cDNA from papillary thyroid carcinoma tissue was used as positive control.
Abbreviation: MW, molecular weight marker.
Discussion
In this study, we report novel identification and expression of h-periostin cDNA isoforms in the thyroid gland. The deletion of certain cDNA sequence in our novel isoforms does not cause shift of opening reading frame, since the number of deleted nucleotides is divisible by three in all the deleted fragments. To the best of our knowledge, this is the first report which demonstrated the expression of eight h-periostin isoforms in the thyroid gland. Previous studies have reported alternative splicing in periostin mRNA.1,3,15,16 Takeshita et al isolated five isoforms of h-periostin from human osteosarcoma and placenta cDNA libraries.1 Kim et al reported three h-periostin isoforms in either normal bladder mucosa or urothelial carcinoma tissue.3 Litvin et al identified an isoform of mouse periostin expressed in bone.16 Horiuchi et al described four isoforms of mouse periostin preferentially expressed in periosteum and periodontal ligament.15 Among our newly identified three h-periostin isoforms, thy2, thy4 and thy8, thy2 has the similar cDNA sequence with one of the mouse periostin isoforms identified by Horiuchi et al,15 while thy4 and thy8 haven’t been previously reported in any species.
Periostin is an extracellular matrix protein, whose gene was first cloned from the mouse osteoblastic cell line MC3T3-E1, showing its role in the recruitment and attachment of osteoblast precursors in the mouse periosteum.1,17 Recently, periostin was reported to contribute to the tumor development through interacting with the extracellular matrix molecules integrin αvβ3 and/or αvβ5 in a series of malignant tumors, including ovarian carcinoma, colon cancer, breast cancer and thyroid papillary carcinoma.2,4,6,8 Periostin transfected cells showed an increase in the expression of connective tissue molecules such as vimentin, epidermal growth factor receptor, and matrix metalloproteinase-9.18 We successfully detected the expression of periostin mRNA not only in the anaplastic thyroid carcinoma cell lines but also in the papillary and follicular thyroid carcinoma cell lines (data not shown), suggesting that periostin is produced by thyroid follicular cells. Although the biological role of periostin in normal thyroid glands is still unknown, we identified that up-regulation of periostin mRNA in papillary thyroid carcinomas than their matched non-neoplastic thyroid tissue was significantly correlated with extrathyroid invasion, pT and lymph node metastasis by amplifying the common sequence of all periostin isoforms.19 In this study, we showed expression of h-periostin isoforms was present in normal thyroid tissue, thyroid carcinoma and non-neoplastic thyroid diseases, including papillary carcinoma, follicular carcinoma, Hashimoto’s disease and adenomatous goiter. These results indicated that expression of periostin isoforms was universally observed in physiological condition and diseases including neoplasm. Our results failed to demonstrate a significant link between the expression pattern of periostin isoforms and carcinogenesis, tumor growth or invasiveness of thyroid carcinoma. It may suggest that all periostin isoforms are essential proteins for cell survival and maintenance and they may play unknown roles in the basic physiology of both normal and neoplastic thyroid tissues.
Our finding of eight periostin isoforms in human thyroid will show its importance in attribute to the understanding of role of each periostin isoforms in the future. Two isoforms, Variant I (lack exon XVII, XVIII and XXI) and Variant II (lack exon XVII and XXI), were reported to coexpress in the transitional cell carcinomas in the bladder.3 Variant I and II have the same cDNA sequence with isoform d and e in our nomination shown in Figure l. The suppressor function for in vitro cell invasiveness and in vivo metastasis of cancer cells was lost in Variant I, but maintained in Variant II.3 The eight periostin isoforms in the thyroid may also likely play different role in thyroid tumor invasiveness or metastasis even they coexist in the same tissue.
Periostin is regarded as a potential anti-cancer therapy target.20 The demonstration of all reported h-periostin isoforms and the identification of novel h-periostin isoforms will facilitate to analyze the expression pattern of periostin isoforms in other tissues and other malignancies, and further detect the role of every perostin isoform, which will possibly guide anti-cancer therapy.
Conclusions
This report is the first time that reviews all the reported h-periostin isoforms and describes the expression of h-periostin isoforms in the thyroid neoplastic tissue, no-neoplastic tissue and anaplastic thyroid carcinoma cell lines. We identified eight isoforms successfully, among which, three were first discovered in the human tissue, especially two have not been described in any species. Furthermore, we found the eight isoforms were coexpressed in both thyroid carcinoma and paired non-neoplastic tissues, suggesting that the expression pattern of periostin isoforms is not linked to thyroid carcinogenesis.
Acknowledgments
We are grateful to Mr. Yoshida and Ms. Maekawa, Department of Pathology, Kuma Hospital, Japan, for their technical assistance to prepare tissue samples for this study. YB received a scholarship from Rotary Yoneyama Memorial Foundation, Japan.
Abbreviations
- OSF-2
osteoblast specific factor 2
- h-periostin
human periostin
- JCRB
Japanese Collection of Research Bioresources
- PCR
polymerase chain reaction
- RT-PCR
reverse transcription-polymerase chain reaction
Footnotes
Author’s contributions
YB mainly attended the study design, carried out the study and drafted the manuscript. MN provided technological assistance and modified the manuscript. TO, IM, YL, and ZL served as assistants. KK and GZ were the principal investigators responsible for the whole study design and manuscript preparation.
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294:271–8. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62:5358–64. [PubMed] [Google Scholar]
- 3.Kim CJ, Isono T, Tambe Y, Chano T, Okabe H, Okada Y, Inoue H. Role of alternative splicing of periostin in human bladder carcinogenesis. Int J Oncol. 2008;32:161–9. [PubMed] [Google Scholar]
- 4.Puppin C, Fabbro D, Dima M, et al. High periostin expression correlates with aggressiveness in papillary thyroid carcinomas. J Endocrinol. 2008;197:401–8. doi: 10.1677/JOE-07-0618. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki H, Dai M, Auclair D, et al. Serum level of the periostin, a homologue of an insect cell adhesion molecule, as a prognostic marker in nonsmall cell lung carcinomas. Cancer. 2001;92:843–8. doi: 10.1002/1097-0142(20010815)92:4<843::aid-cncr1391>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 6.Shao R, Bao S, Bai X, et al. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol. 2004;24:3992–4003. doi: 10.1128/MCB.24.9.3992-4003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erkan M, Kleeff J, Gorbachevski A, et al. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447–64. doi: 10.1053/j.gastro.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Bao S, Ouyang G, Bai X, et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329–39. doi: 10.1016/s1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 9.Kudo Y, Ogawa I, Kitajima S, et al. Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res. 2006;66:6928–35. doi: 10.1158/0008-5472.CAN-05-4540. [DOI] [PubMed] [Google Scholar]
- 10.Siriwardena BS, Kudo Y, Ogawa I, et al. Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer. Br J Cancer. 2006;95:1396–403. doi: 10.1038/sj.bjc.6603431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshioka N, Fuji S, Shimakage M, et al. Suppression of anchorage-independent growth of human cancer cell lines by the TRIF52/periostin/OSF-2 gene. Exp Cell Re. 2002;279:91–9. doi: 10.1006/excr.2002.5590. [DOI] [PubMed] [Google Scholar]
- 12.Kim CJ, Yoshioka N, Tambe Y, Kushima R, Okada Y, Inoue H. Periostin is down-regulated in high grade human bladder cancers and suppresses in vitro cell invasiveness and in vivo metastasis of cancer cells. Int J Cancer. 2005;17:51–8. doi: 10.1002/ijc.21120. [DOI] [PubMed] [Google Scholar]
- 13.Tai IT, Dai M, Chen LB. Periostin induction in tumor cell line explants and inhibition of in vitro cell growth by anti-periostin antibodies. Carcinogenesis. 2005;26:908–15. doi: 10.1093/carcin/bgi034. [DOI] [PubMed] [Google Scholar]
- 14.Fluge Ø, Bruland O, Akslen LA, Lillehaug JR, Varhaug JE. Gene expression in poorly differentiated papillary thyroid carcinomas. Thyroid. 2006;16:161–75. doi: 10.1089/thy.2006.16.161. [DOI] [PubMed] [Google Scholar]
- 15.Horiuchi K, Amizuka N, Takeshita S, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–49. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 16.Litvin J, Selim AH, Montgomery MO, et al. Expression and function of periostin-isoforms in bone. J Cell Biochem. 2004;92:1044–61. doi: 10.1002/jcb.20115. [DOI] [PubMed] [Google Scholar]
- 17.Horiuchi K, Amizuka N, Takeshita S, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–49. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 18.Yan W, Shao R. Transduction of a mesenchyme-specific gene periostin into 293T cells induces cell invasive activity through epithelial-mesenchymal transformation. J Biol Chem. 2006;281:19700–8. doi: 10.1074/jbc.M601856200. [DOI] [PubMed] [Google Scholar]
- 19.Bai Y, Kakudo K, Nakamura M, et al. Loss of cellular polarity/cohesiveness in the invasive front of papillary thyroid carcinonma and periostin expression. Cancer Lett. 2009;281:188–95. doi: 10.1016/j.canlet.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 20.Kudo Y, Siriwardena BS, Hatano H, Ogawa I, Takata T. Periostin: novel diagnostic and therapeutic target for cancer. Histol Histopathol. 2007;22:1167–74. doi: 10.14670/HH-22.1167. [DOI] [PubMed] [Google Scholar]