Abstract
Two modes of incretin-based therapy, incretin mimetics (ie, glucagon-like peptide-1 (GLP-1) agonists) and incretin enhancers (ie, inhibitors of dipeptidyl peptidase IV (DPP-IV)), have recently been introduced into the clinical use. From the viewpoint of evolutionary endocrinology of GLP-1 and their receptors, the incretin action of GLP-1 seems to be relatively recent. Exendin-3 and exendin-4 are paralogs of GLP-1 from the lizards, and the synthetic exendin-4, exenatide, is a paralog of GLP-1. It has recently been indicated that GLP-1 and its receptor are expressed in the taste buds of the tongue, suggesting their possible function in the taste sensing signal pathway. In order to elucidate unknown functions of GLP-1 and its agonists and enhancers, ie, other than incretin actions in humans, it is possibly useful to consider GLP-1 from the viewpoint of integrated systems biology and evolutionary endocrinology.
Keywords: incretin, evolution, taste, systems biology
Introduction
Over 100 years have passed since the discovery of the incretin concept, two gut hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), have been shown to act as incretins.1 Two novel classes of incretin-based antidiabetic medications have been introduced into the clinical use: the first representatives of these classes were incretin mimetics (GLP-1 agonists), such as exenatide and liraglutide. The second one is incretin enhancers (inhibitors of dipeptidyl peptidase IV(DPP-IV), such as sitagliptin and vildagliptin.2 In this minireview, we consider the evolution of GLP-1 and their receptors as well as GLP-1 agonists (exendin-4) and its receptors from the viewpoint of integrated systems biology and evolutionary endocrinology.3
Evolution of GLP-1 and its receptors
Evolution of ligands
GLP-1 is one of the peptide hormones encoded by the proglucagon gene, the others being glucagon and GLP-2 (Fig. 1). The proglucagon and GIP genes are both members of secretin-like hormones family.4,5 Separate genes for proglucagon and GIP were likely produced during a whole genome duplication that occurred very early in vertebrate evolution5–7 (Fig. 2).
Figure 1.
Processing of proglucagon in the pancreas (left panel) and the intestine (right panel).
Figure 2.
Evolution of incretin (GLP-1 and GIP) and its receptors.
Abbreviations: GLP-1, glucagon-like peptide-1; GIP, glucose-dependent insulinotropic polypeptide.
The sequence of GLP-1 is highly variable. The three peptides, glucagon, GLP-1 and GLP-2 originated before the earliest divergence of vertebrate species, and glucagon diverged first, nearly 1 billion years ago whole GLP-1 and GLP-2 diverged from each other approximately 600 million years ago, just before the divergence of earliest vertebrate lineages.5–7
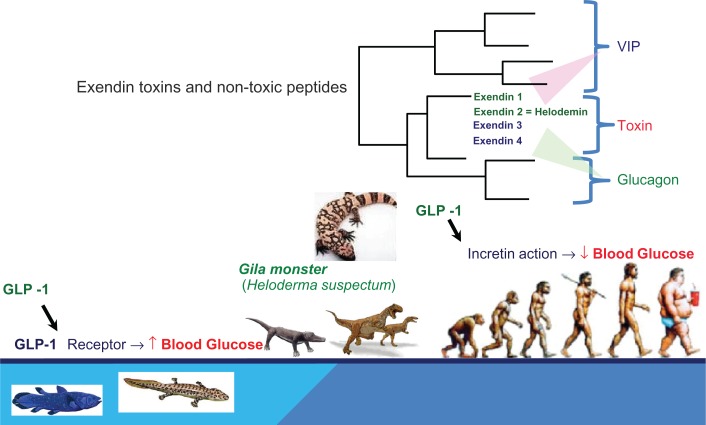
In contrast to mammals, there are multiple GLP-1 paralogs (ie, products of similar genes arising from a gene duplication),8 in the frog and venomous lizard Gila monster (Heloderma suspectum) (Fig. 3).9,10 Exendin-3 and exendin-4 are paralogs of GLP-1 from the lizards; one is highly similar to other vertebrate GLP-1 s, and a second peptide known as exendin-4 has weaker similarity and is found to be expressed only in the saliva glands.11,12 In contrast, exendin-1 and exendin-2 (ie, helodermin) have similarit to VIP (vasoactive intestinal polypeptide) and PACAP (Pituitary Adenylate Cyclase-Activating Polypeptide).13,14
Figure 3.
Evolution of exendin toxins and related peptides.
Exendin-4 is one of them isolated from H. suspectum, which has weak similarity to other vertebral GLP-1 and has unique feature of being highly stable to degradation by DPP-IV.1,15 The synthetic exendin-4, named as exenatide, was the first approved GLP-1 “analog” for clinical use in humans in 2005 in the United States and 2010 in Japan.1,2 Exactly, exenatide is not an analog of GLP-1, but a paraolog.8
The sequence of glucagon is relatively conserved among vertebrates.5–7 Furthermore, the function of glucagon is similar, like that of insulin, among vertebrates, ie, as the counter-regulatory hormone to insulin, stimulating glycogenolysis and gluconeogenesis in the liver.9,10
GLP-1 seems to have the opposite physiological roles in fish and mammals. In contrast to mammals, where GLP-1 acts through the pancreatic islets to increase insulin release, causing a decrease in blood glucose, in fish, GLP-1, which is released from both intestinal and pancreatic cells, acts directly on the liver causing the release of glucose into the blood like glucagon.4,9,10
Therefore, it is considered that GLP-1 acquired the incretin role after the divergence of fish and mammal. The function of GLP-2 is known in mammals, where it acts as an intestinal growth factor. In fish, the biological function of GLP-2 is unknown, but it dose not act like glucagon or GLP-1.4,9,10
Although the GIP and GLP-1 are equally redundant, the rate of evolution of GLP-1 is slower than that of GIP, possibly through it is under stronger constraints than GIP, suggesting a possibility of other functions than incretin actions of GLP-1.4,5,9,10
Evolution of receptors
The receptors of glucagon, GLP-1, and GLP-2 are all members of the secretin subclass (class B) of the G-protein coupled seven-transmembrane domain receptors, which includes receptors for GIP, VIP, and secretin. The genes of those receptors share a common evolutionary origin by gene duplications (Fig. 2).7,10
The phylogeny of receptors suggests that GLP-2 receptor may have diverged first. GLP-1 receptor has evolved more slowly than the glucagon and GIP receptors, while the GLP-2 receptor may have evolved faster. GLP-1 receptor shows greater identity to the VIP receptors, while GIP receptor is more closely related to glucagon receptor than either the GLP-1 or GLP-2 receptors.7,10
Furthermore, the GLP-1 type receptors diverged from the glucagon receptor gene before the divergence of fish and mammals, and the fish has lost a gene for a receptor orthologous to the mammalian GLP-1 receptor.7,10
These findings suggest that the GLP-1 receptors in mammals are under greater selective constraint than the glucagon, GLP-2 or GIP receptors. The greater selective constraint may be a consequence of the complex biological activities of GLP-1; GLP-1 stimulates insulin secretion in a glucose-dependent manner, inhibits gastric emptying, decreases food intake, inhibits glucagon secretion, stimulates β-cell proliferation, regulate bone resorption and turnover through a calcitonin-dependent pathway, exerts myocardial metabolic effects, and improve endothelial dysfunction.1,2
Significance of evolution of incretin from functional perspective
Thus, GLP-1 acquired the incretin functions and specific receptors after the divergence of fish and mammals. Sometimes, the actions of hormones are different among the species; ie, hormone may have species-specific actions. For example, ghrelin inhibits feeding in birds, while it stimulates feeding in mammals.16
Generally, the evolution of ligand and its receptor is considered to be co-evolution. In most cases, it appears that the ligands which we regard as hormones arose long before the receptors that they control. Consequently, the responses to a given hormone can vary widely across different species and even within species. The fate of peptide genes after duplication is classified into the three classes; non-functionalization, subfunctionalization (ie, specialization) and neofunctionalization.17
The difference in the phylogeny for the glucagon-like hormones and their receptors may indicate that the specificity of the receptor-ligand pair evolved much later than the peptides and/or receptors. In that sense, the evolution of steroid receptor family gives us some perspective. First, estrogen receptor (ER) appears first, which was evolved into the androgen receptor (AR) and mineralocorticoid receptor (MR), and the ancestor corticoid receptor (AcCR).18 Similarly, GLP-1 has a stimulating effect on blood glucose in fishes, while it gains the incretin action after the divergence between fish and mammals.
Taste sensation and incretin
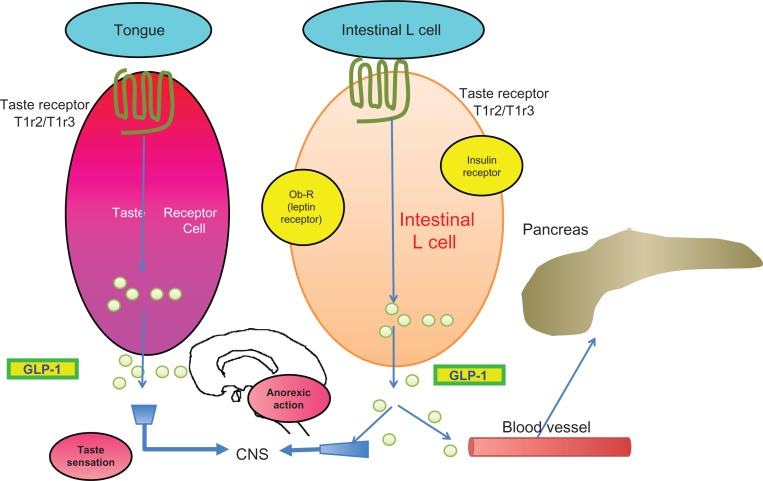
Recently the intestinal L cells have been indicated to express the sweet taste receptor (T1R2/T1R3) as well as taste G protein (gustducin), both of which regulate the release of GLP-1 from the L cells (Fig. 4).19
Figure 4.
Taste sensation and GLP-1. (From reference 19 with modifications).
GLP-1 and its receptor are expressed in the taste buds of the tongue. However, DPP-IV is absent in the taste bud cells, suggesting a longer duration of GLP-1 action in the taste buds than in the intestinal L cells.20
Functionally, GLP-1 may regulate the taste sensation through its novel paracrine action, since GLP-1 knock out mouse showed dramatically reduced taste response to sweetners.21,22 On the other hand, VIP knockout mouse exhibited an increased preference to sweet taste, hyperglycemia and an increased expression of GLP-1 receptor in the taste buds.23 GLP-1 was responsible for sweet taste preference in humans. These studies suggest that the tongue can play a direct role in modulating energy intake to correct peripheral glycemic imbalances. In this way, we could view the tongue as a sensory mechanism that is bidirectionally regulated and thus forms a bridge between available food stuffs and the intricate hormonal balance.24
In humans, ingestion of sugar or artificial noncaloric sweetner causes a release of GLP-1.25 Recently it has been reported that ingestion of diet soda exaggerated GLP-1 secretion after oral glucose tolerance test. This result suggests that sweet taste may synergize with glucose to enhance GLP-1 release in humans.26
Both intestinal L cells and taste buds of tongue are known to express leptin receptor and insulin receptor, both of which are known to activate GLP-1 secretion.
GLP-1 may be also involved in anticipation of meals, suggesting a cephalic increase of GLP-1.27 Furthermore, GLP-1R knockout mice exhibit a dramatic reduction in sweet taste sensitivity as well as an enhanced sensitivity to umami-tasting stimuli.28 These findings suggest that GLP-1 and its receptor system have an important role in the taste sense signal, and that there is a strong link between gustation and peripheral glycemic control. Further studies are required to elucidate unknown physiological functions of GLP-1 in non-classical target organs, such as a mediator of the taste sensation signal to satiety or insulin secretion.
Recent findings of exendin-4
One of the authors (Y.K.) has recently demonstrated that exendin-4 can protect pancreatic beta cells from the cytotoxic effect of rapamycin, and that its effect can be attributable to the inhibition of JNK and p38 phosphorylation by exendin-4.29 Since rapamycin has been used as an immunosuppressant in the pancreatic islet transplantation this protective effect of exendin-4 may be of clinical significance in humans. This is one example of hitherto unknown effects of exendin-4 in humans. Considering that the incretin action of GLP-1 is considered to be recently developed in the evolution, both GLP-1 and exendin-4 may have other unknown effects in humans.
Incretin network
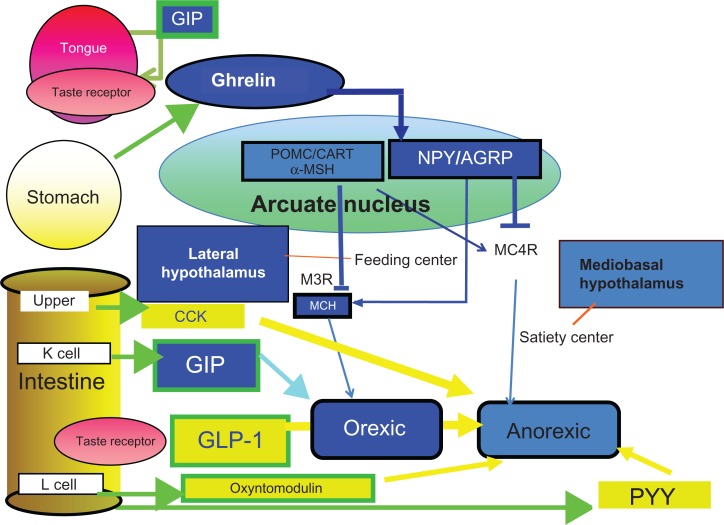
Thus, from the viewpoint of evolutionary endocrinology3,31,32,39 the incretin actions of GLP-1 and GIP seem to be relatively recent, suggesting hitherto unknown functions, such as a mediator of the taste sensation signal to satiety or insulin secretion. Furthermore, from the viewpoint of systems biology,3,31,32,39 GLP-1 and GIP, as well as their receptors, can be considered to form network system with other peptides in regulation of appetite32,33 (Fig. 5).
Figure 5.
Network system regulating appetite from gastrointestinal tract.
Conclusion
Incretin-based therapy, incretin mimetics (GLP-1 agonists) and incretin enhancers (inhibitors of DPP-IV), has recently been introduced into the clinical use. In order to elucidate unknown functions of GLP-1 agonists and enhancers, it is possibly useful to consider GLP-1 from the viewpoint of integrated systems biology and evolutionary endocrinology.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. All authors have received grant support from Merck. Y. H. and H. K. have received grant support and honoraria from Novartis Pharma. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: similarities and differences. J Diabetes Invest. 2010;1:8–23. doi: 10.1111/j.2040-1124.2010.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nauck MA, Vandarli I. Comparative evaluation of incretin-based antidiabetic medications and alternative therapies to be added to metformin in the case of monotherapy failure. J Diabetes Invest. 2010;1:24–36. doi: 10.1111/j.2040-1124.2010.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koshiyama H, Ogawa Y, Tanaka K, Tanaka I. Integrated Network Systems and Evolutionary Developmental Endocrinology. Medical Hypotheses. 2010;4:132–8. doi: 10.1016/j.mehy.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Skovgaard M, Kodra JT, Gram DX, Knudsen SM, Madsen D, Liberles DA. Using evolutionary information and ancestral sequences to understand the sequence-function relationship in GLP-1 agonists. J Mol Biol. 2006;363:977–88. doi: 10.1016/j.jmb.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 5.Irwin DM. Molecular evolution of mammalian incretin hormone genes. Regul Peptides. 2009;155:121–30. doi: 10.1016/j.regpep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Chow BKC, et al. Endocrinology. 2004;145:3273–88. doi: 10.1210/en.2003-0597. [DOI] [PubMed] [Google Scholar]
- 7.Sivarajah P, Wheeler MB, Irwin DM. Evolution of receptors for proglucagon receptors. Comp Biochem Physiol. 2001;128B:517–27. doi: 10.1016/s1096-4959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 8.Gomperts BD, Kramer MI, Tatham PER. Signal transduction. 2nd ed. Academic Press; 2009. [Google Scholar]
- 9.Mommsen TP, Conlon JM, Irwin DM. Amphibian glucagon family peptides: potent metabolic regulators in fish hepatocytes. Regul Peptides. 2001;99:111–8. doi: 10.1016/s0167-0115(01)00241-5. [DOI] [PubMed] [Google Scholar]
- 10.Irwin DM, Wong K. Evolution of new hormone function: loss and gain of a receptor. J Heredity. 2005;96:205–11. doi: 10.1093/jhered/esi024. [DOI] [PubMed] [Google Scholar]
- 11.Christel CM, deNardo DF, Secor SM. Metabolic and digestive response to food ingestion in a binge-feeding lizard, the Gila monster (Helodema suspectum) J Biol Chem. 2007;210:3430–9. doi: 10.1242/jeb.004820. [DOI] [PubMed] [Google Scholar]
- 12.Fry BG, Roelants K, Winter K, et al. Novel venom proteins produced by differential domain-expression strategies in beated lizards and Gila monsters (genus Heloderma) Mol Biol Evol. 2010;27:395–407. doi: 10.1093/molbev/msp251. [DOI] [PubMed] [Google Scholar]
- 13.Vandermeers A, Vandermeers-Piret M-C, Robberecht M, et al. Purification of a novle pancreatic secretory facgor (PSF) and a novel peptide with VIP- and secretin-like properties (helodermin) from Gila monster venom. FEBS Lett. 1984;166:273. doi: 10.1016/0014-5793(84)80094-0. [DOI] [PubMed] [Google Scholar]
- 14.Koshiyama H, Kato Y, Inoue T, Christophe J, Yanaihara N, Imura H. Helodermin stimulates prolactin secretion in the rat. Eur J Pharmac. 1987;141:319–21. doi: 10.1016/0014-2999(87)90279-2. [DOI] [PubMed] [Google Scholar]
- 15.Pohl M, Wank SA. Molecular cloning of the helodermin and exendin-4 cDNAs in the lizard. J Biol Chem. 1998;273:9778–84. doi: 10.1074/jbc.273.16.9778. [DOI] [PubMed] [Google Scholar]
- 16.Kaiya H, Furuse M, Miyazato M, Kangawa K. Current knowledge of the roles of ghrelin in regulating food intake and energy balance in birds. Gen Comp Endocrinol. 2009 Sep 1;163(1–2):33–8. doi: 10.1016/j.ygcen.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Larhammer D, Ocampo Daza D, Larsson TA, Dreborg S, Sundström G. Chromosome duplication in neuropeptide evolution. The abstracts of The 7th International Congress of Neuroendocrinoology; 2010 Jul 11–15; Rouen, France. [Google Scholar]
- 18.Bridgham JT, Carroll SM, Thornton JW. Evolution of hormone-receptor complexity by molecular exploitation. Science. 2006;312:97–101. doi: 10.1126/science.1123348. [DOI] [PubMed] [Google Scholar]
- 19.Jang H-J, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;38:15069–74. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartel S, Gossrau R, Hanski C, Reutter W. Dipeptidyl peptidase (DPP) IV in rat organs. Histochemistry. 1988;89:151–61. doi: 10.1007/BF00489918. [DOI] [PubMed] [Google Scholar]
- 21.Shin Y-K, Martin B, Golden E, et al. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008;106:455–63. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin B, Dotson CD, Shin YK, et al. Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann NY Acad Sci. 2009;1170:98–101. doi: 10.1111/j.1749-6632.2009.03920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin B, Shin Y-K, White CM, et al. Vasoactive intestinal peptide null mice demonstrate enhanced sweet taste preference, dysreglycemia and reduced taste but leptin receptor expression. Diabetes. 2010;59:1143–52. doi: 10.2337/db09-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim GE, Brubaker PL. Glucagon-like peptide 1 secretion by the L-cell: the view from within. Diabetes. 2006;55S:70–7. [Google Scholar]
- 25.Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol. 2009;587:27–32. doi: 10.1113/jphysiol.2008.164012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown RJ, Walter K. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1. Diabetes Care. 2009;32:2184–6. doi: 10.2337/dc09-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vahl TP, Drazen DL, Seeley RJ, Alessio DA, Woods SC. Meal-anticipatory glucagon-like peptide-1 secretion in rats. Endocrinology. 2010;151:569–75. doi: 10.1210/en.2009-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massolt ET, van Haard PM, Rehfeld JF, Posthuma EF, van der Veer E, Schweitzer DH. Appetite suppression through smelling of dark chocolate correlates with changes in ghrelin in young women. Regul Peptides. 2010;161:81–6. doi: 10.1016/j.regpep.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki Y, Harashima S, Sasaki M, et al. Exendin-4 protects pancreatic beta cells from the cytotoxic effect of rapamycin by inhibiting JNK and p38 phosphorylation. Horm Metab Res. 2010;42:311–7. doi: 10.1055/s-0030-1249035. [DOI] [PubMed] [Google Scholar]
- 30.Szallasi Z, Stelling J, Periwal V, editors. System Modeling in Cellular Biology: from Concepts to Nuts and Bolts. The MIT Press; 2006. [Google Scholar]
- 31.Koshiyama H, Ogawa Y, Tanaka K, Tanaka I. Diabetes mellitus as dysfunction of interactions among all organs: “ominous orchestra of organs”. Clinical Medicine: Endocrinology and Diabetes. 2008;1:1–6. [Google Scholar]
- 32.Koshiyama H. Integrated Network Systems and Evolutionary Developmental Endocrinology (INS-EDEN) Mihara Igakusya Co; (in press) [DOI] [PubMed] [Google Scholar]
- 33.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]