Abstract
Aim:
Fish-oil omega-3 polyunsaturated fatty acids (n-3 PUFAs) are mostly esterified to the sn-2 position of triglycerides, while in seal-oil triglycerides, these are mostly esterified to the sn-1 and -3 positions. We investigated whether fish-oil and seal-oil feeding has a different effect on the regulation of lipid metabolism and oxidative stress in BioF1B hamsters.
Methods:
BioF1B hamsters were fed high fat diets rich in fish-oil or seal-oil for 4 weeks, and fasted for 14 hours prior to blood and tissue collection.
Results:
Plasma and hepatic lipids and lipid peroxidation levels were significantly lower in seal-oil-fed hamsters as compared to those fed fish-oil. There was a selective hindrance of clearance of lipids in fish-oil-fed hamsters as reflected by higher levels of plasma apoB48.
Conclusion:
Differences in the fatty acid composition and positional distribution of n-3 PUFAs in triglycerides of fish-oil and seal-oil are suggested to trigger metabolic differences.
Keywords: BioF1B hamsters, marine oils, fatty acids, lipids, cholesterol, oxidative stress
Introduction
Fish-oil is a rich source of long chain omega-3 (n-3) polyunsaturated fatty acids (PUFAs), especially eicosapentaenoic acid (EPA; 20:5) and docosahexaenoic acid (DHA; 22:6), which have the potential to be anti-atherogenic agents due to their hypolipidemic, anti-thrombotic and anti-atheromatous effects.1–3 Although the hypotriglyceridemic effects of fish oil are well known,4,5 the effects on plasma total cholesterol and low density lipoprotein (LDL)-cholesterol levels are not well established, because some studies have shown elevated total and LDL-cholesterol levels after the feeding of a diet rich in fish-oil.6–8
Another marine source that is rich in long chain n-3 PUFAs, with a comparable profile to fish-oil in terms of its n-3 PUFAs, is seal-oil. The Greenland Eskimo population not only consumed high amounts of fish, but seal and whale were also major components of their diet.9 Seal-oil has a different structural arrangement in its triglyceride molecule (TG) as compared to fish-oil in terms of the pattern of its intramolecular distribution of n-3 PUFAs.10–12 A major proportion of long chain n-3 PUFAs, such as EPA and DHA, in seal-oil TG are distributed mainly in the sn-1 and -3 positions, as opposed to the sn-2 position in fish oil TG.10–12 Differences in intramolecular distribution of n-3 PUFAs in these two marine oils have been suggested to cause different effects on the regulation of lipid metabolism.10,11 While n-3 PUFA from fish oil are primarily absorbed as sn-2 monoacylglycerols, these are absorbed as free fatty acids (FA) upon seal oil consumption. Differences in intramolecular distribution, however, were suggested to have no influence on the rate of absorption of n-3 PUFAs from these two marine oils.13
BioF1B hamster, a hybrid strain, is genetically susceptible to diet-induced hyperlipidemia and atherosclerosis. In our previous studies,14,15 we have shown that the feeding of a high fat diet supplemented with fish-oil resulted in milky plasma, with elevated plasma TG, very low-density lipoprotein (VLDL)- and (LDL)-cholesterol levels even after 14 hours of fasting, as compared to other mixed fat sources in BioF1B hamsters. The incidence of fish-oil-induced hyperlipidemia was more specific to the BioF1B hamsters compared to Golden Syrian hamsters.16 The BioF1B hamster was also found to have significantly lower post-heparin lipoprotein lipase (LPL) activity and reduced mRNA expression of adipocyte LPL, compared to the GS hamsters.16 The activity of LPL has been reported to have relative preference for the sn-1 and -3 positions as compared to the sn-2 position.17 Since the BioF1B hamster has significantly lower LPL activity as compared to the GS hamster, the BioF1B hamster provides an ideal experimental condition for the evaluation of metabolic differences, which could be triggered by differences in intramolecular distribution of long chain n-3 PUFAs in fish-oil and seal-oil.
We hypothesized that the regulation of lipid and lipoprotein metabolism in BioF1B hamsters fed with seal oil would be different from those fed with fish oil. It was further hypothesized that the level of oxidative stress would be lower in seal-oil-fed BioF1B hamsters, compared to fish-oil-fed hamsters. We found that the BioF1B hamsters fed seal oil did not have milky plasma as opposed to fish oil feeding. We further observed that the plasma and hepatic lipid levels were significantly lower in seal -oil-fed BioF1B hamsters, compared to fish-oil-fed hamsters, along with reduced levels of oxidative stress. Polymorphisms exist in the LPL gene in the human population, which are known to affect LPL activity.18 The BioF1B hamster may be an ideal animal model for human LPL deficiency, thus findings from this study will establish whether dietary treatments using n-3 PUFAs should also consider the intramolecular distribution of fatty acids in the TG molecule, especially under LPL deficiency conditions.
Materials and Methods
Animals and diets
Bio F1B hamsters (male, 7 weeks old) were obtained from Bio Breeders Inc. (Watertown, MA). During the initial equilibration period, hamsters were kept on a regular chow diet for 1 week. Hamsters were then divided into 2 groups (n = 7) and kept on specified diets for 4 weeks. The specified diets consisted of custom-made fat-free semi-purified diet (ICN Biomedical Inc., OH, USA) that was either supplemented with 20% (w/w) fish-oil (Menhaden oil, Sigma-Aldrich, Ontario, Canada) or 20% (w/w) seal-oil (Ocean Choice Ltd., NL, Canada). The diet composition was modified from the previously published diet16 and is given in Table 1. Diets were stored at −20 °C; the FA composition of different diets was analyzed by Gas Liquid chromatography (GLC) using previously performed methods14–16 and is given in Table 2. The animals were housed in individual cages in a single room. The room conditions were maintained at 12–12 h light-dark cycles (room was lit from 07:00 to 19:00 h), temperature kept at 23 ± 1 °C and humidity at 35% ± 5%. Fresh diets were fed to the hamsters daily and experimental diets and water were given ad libitum for 4 weeks. Food intake was measured daily and body weight was measured once a week. No significant differences were observed in food intake (5.41 ± 0.21 vs. 6.05 ± 0.56 g/day), body weight gain (19.18 ± 3.58 vs. 17.07 ± 3.68 g) or liver weight (5.02 ± 0.9 vs. 4.32 ± 0.6 g) in BioF1B hamsters fed fish-oil or seal-oil respectively during the 4 weeks of feeding period. Institutional Animal Care Committee at Memorial University approved all experiments, complying with the guidelines of the Canadian Council for Animal Care.
Table 1.
Nutrient composition (g/kg) of fish-oil and seal-oil diets.1
| Components (g/kg) | Fish-oil | Seal-oil |
|---|---|---|
| Casein | 200 | 200 |
| DL-Methionine | 3 | 3 |
| Sucrose | 305 | 305 |
| Corn starch | 190 | 190 |
| Vitamin mix2 | 11 | 11 |
| AIN mineral mix3 | 40 | 40 |
| Fibre | 50 | 50 |
| Fat | 200 | 200 |
Notes:
Semi-purified diets were designed to obtain 200 g/kg fat;
Formulated to meet the national requirements (National Research Council, 1995). Vitamin A, 25,000 IU; ergocalciferol, 2500 IU; dl-tocopheryl acetate, 0.25 g; menadione, 6.25 mg; choline dihydrogen citrate, 6 g; P-aminobenzoic acid, 0.125 g; inositol, 0.125 g; niacin, 0.05 g; calcium pantothenate, 0.05 g; riboflavin, 0.01 g; thiamine, 0.00625 g; pyridoxin, 0.00625 g; folic acid, 0.0025 g; biotin 0.0005 g; cyanocobalamin, 0.0375 mg; anhydrous dextrose, 6 g;
Supplied as Alphacel non-nutritive bulk (ICN Biomedicals Inc., OH, USA). Teklad 40055 (per kg of reconstituted diet): Teklad 170750 (per kg of reconstituted diet): CaCO3, 1.34 g; MgO, 1.6 g; KH2PO4, 5.15 g; K2SO4, 4.32 g; NaCl, 1.95 g; NaH2PO4, 1.36 g; CuC6H5O7, 30 mg; FeC6H5O7, 0.35 g; MnC6H5O7, 0.53 g; KI, 0.46 mg; ZnC6H5O7, 85 mg; C6H8O7, 0.14 g.
Table 2.
Fatty acid (FA) composition of fish-oil (FO) and seal-oil (SO) diets.1
| Fatty acids | FO | SO |
|---|---|---|
| Total SFA | 32.1 | 18 |
| Total MUFA | 22.2 | 50.6 |
| Total PUFA | 34.6 | 28.8 |
| Total n-3PUFA | 31.9 | 26.5 |
| Total n-6PUFA | 2.7 | 2.3 |
| n-3/n-6 ratio | 11.8 | 11.5 |
| 14:0 | 11 | 6.5 |
| 16:0 | 18 | 10.1 |
| 16:1 n-7 | 12.2 | 16.8 |
| 18:0 | 3.1 | 1.4 |
| 18:1n-9 | 8.9 | 22.9 |
| 18:2n-6 | 1.9 | 1.8 |
| 18:3n-3 | 1.5 | 0.6 |
| 18:4n-3 | 3.5 | ND |
| 20:1n-9 | 1.1 | 10.9 |
| 20:4n-6 | 0.8 | 0.5 |
| 20:5n-3 | 12.6 | 9 |
| 22:5n-3 | 2.2 | 5.2 |
| 22:6n-3 | 12.1 | 11.7 |
Notes:
Lipids were extracted from fish-oil and seal-oil diets as explained under the methods. FA composition was determined by gas liquid chromatography (GLC). SFA, MUFA, PUFA indicates saturated, monounsaturated and polyunsaturated fatty acids, respectively. FAs are shown as % of the total fatty acids.
Abbreviation: ND, not detected.
Plasma lipid and lipoprotein profile
At the end of the 4 week feeding period, animals from each diet group were fasted for 14 h and sacrificed after euthanizing with halothane vapours in a closed chamber. Blood samples were obtained by cardiac puncture in tubes containing EDTA and centrifuged immediately at 3000 g for 15 min to separate plasma. Chylomicron fraction was separated by centrifugation at 15,500 g for 20 min at 12 °C. Density gradient ultracentrifugation was then performed on the remaining plasma to isolate various lipoprotein fractions, ie, VLDL, LDL and high-density lipoprotein (HDL).14 Plasma, chylomicrons and the individual lipoprotein fractions were assayed for TG and total cholesterol (Kit No. 225-S7 and 236-60, respectively, Diagnostic Chemicals Ltd, PE, Canada). Remaining plasma samples were kept frozen at −80 °C until further use.
Hepatic lipid analysis
Liver samples from BioF1B hamsters fed different diets were immediately freeze clamped in liquid nitrogen and stored at −80 °C until further use. Hepatic lipids were extracted19 and analyzed for TG and total-cholesterol (Kit No. 225-S7 and 236-60, respectively, Diagnostic Chemicals Ltd, PE, Canada), and free cholesterol (Kit No. 274-47109, Wako Chemicals, VA, USA). Cholesterol ester concentrations were calculated by subtracting free cholesterol concentrations from total cholesterol concentrations.
Fatty acid analysis of hepatic and chylomicron lipids
Lipids were extracted from the chylomicron fraction and liver samples using the Bligh and Dyer method.19 The FA composition of the samples was determined using GLC according to the previously published methods.14–16 Menhaden oil FA standards from Supelco (PUFA No.3, Sigma-Aldrich, Ontario, Canada) were used for identification and quantification of individual fatty acids.
Western blot analysis of apolipoprotein B (apoB48 and apoB100)
The western blot analysis was conducted by the modification of previously published methods.16 Plasma samples containing 30 μg protein were used for the immunoblotting of apoB. Proteins were separated using SDS-PAGE (6%) and transferred to a PVDF transfer membrane (PerkinElmer, Boston, USA). Antibodies against apoB were obtained from Calbiochem (California, USA) and for β-actin from Santa-Cruz (Santa-Cruz Biotechnology Inc., CA). Apoproteins (apoB48 and −100) and β-actin were visualized using a secondary bovine anti-goat immunoglobulin (IgG) conjugated to horseradish peroxidase at a dilution of 1:5000 (Santa Cruz, California, USA). A biotinylated protein ladder/marker (Cell Signaling, Danvers, USA) was run with each gel. ApoB48, −100 and β-actin were detected using Western Lightning Chemiluminescence Reagent Plus detection system (PerkinElmer, Boston, USA). The densitometric quantification of immunoreactivity was performed using Chemi-imager and data was expressed as apoB48 or −100/β-actin ratio.
Gene expression analysis
Liver total RNA was extracted using TRIZOL reagent (Invitrogen Life Technologies Inc., Gaithersburg, MD, USA) and mRNA expression levels for the following transcription factors were measured by reverse transcription and in vitro DNA amplification: Peroxisome proliferator activated receptor-α (PPAR-α) (Sense: 5′-GAGAAAGCAAAACTGAAAGCAGAGA-3′, antisense: 5′-GAAGGGCGGGTTATTGCTG-3′, liver X-receptor-α (LXR-α) (Sense: 5′-GCAACTCAATGA TGCCGAGTT-3′, antisense: 5′-CGTGGGAACATC AGTCGGTC-3′), sterol regulatory-element-binding protein (SREBP)-1c (Sense: 5′-GCGGACGCAGTC TGGG-3′, antisense: 5′-ATGAGCTGGAGCATGTC TTCAAA-3′) and SREBP-2 (Sense: 5′-AGCTGG CAAATCAGAAAAACAAG-3, antisense: 5′-GATT AAAGTCTTCAATCTTCAAGTCCAC-3′). β-actin was used as an internal control (Sense: 5′-GCTACAGCTTCACCACCACA-3′, antisense: 5′-CATCGTACTCCTGCTTGCTG-3′). The cDNA was synthesized from total liver RNA (2 μg) using Superscript reverse transcriptase (Life Technologies, Burlington, ON, Canada) and used as templates for in vitro DNA amplification reactions. Transcription factors and β-actin mRNA sequences were simultaneously amplified using specific primers. No amplification products were detectable in the absence of reverse transcriptase. The total number of cycles for each PCR reaction was chosen to remain within the exponential phase of the reaction. All PCR reactions were performed in triplicate, and the products were resolved on 1.2% agarose gel. The representative bands were quantitated using gel documentation system. The amount of various transcription factors mRNA was normalized to β-actin mRNA content and expressed as relative units.
Plasma and liver TBARS assay
Plasma TBARS were assayed using the method of Ohkawa et al.20 Liver TBARS were assayed similar to plasma TBARS with minor modifications.21 The standards were prepared with 0.1 mmol/L 1,1, 3,3-tetramethoxypropane (dissolved in absolute ethanol) and 29 mmol/L thiobarbituric acid (TBA) (dissolved in 8.75 mol/L acetic acid). Liver tissues (approximately 100 mg) were homogenized in 1 ml of phosphate buffered saline. The following were then added to 300 μl of the liver homogenate, 75 μl of plasma samples or the 1,1,3,3-tetramethoxypropane standards: 100 μl of 0.9% NaCl, 100 μl of 15% trichloroacetic acid (TCA), 100 μl of 2.5 mM BHT, 150 μl of TBA and 60 μl of 8.1% sodium dodecyl sulfate. Tubes were vortexed and kept in a water bath at 95 °C for 60 min. The samples were cooled on ice for 10 min, followed by addition of 750 μl n-butanol and centrifugation at 4400 rpm (4 °C) for 20 min. The absorbance of the supernatant was read at 532 nm, where liver supernatant was first filtered through 0.45 μm syringe filters. TBARS concentration was expressed as malondialdehyde (MDA) mmol/ml plasma or nmol MDA/mg tissue.
Statistical analysis
The results were analyzed using unpaired t-test (GraphPad Software Inc., CA). Results were expressed as group means (n = 7) and ± SEM (standard error of mean). Differences were considered statistically significant at P ≤ 0.05.
Results
Plasma and chylomicron lipid and lipoprotein profiles
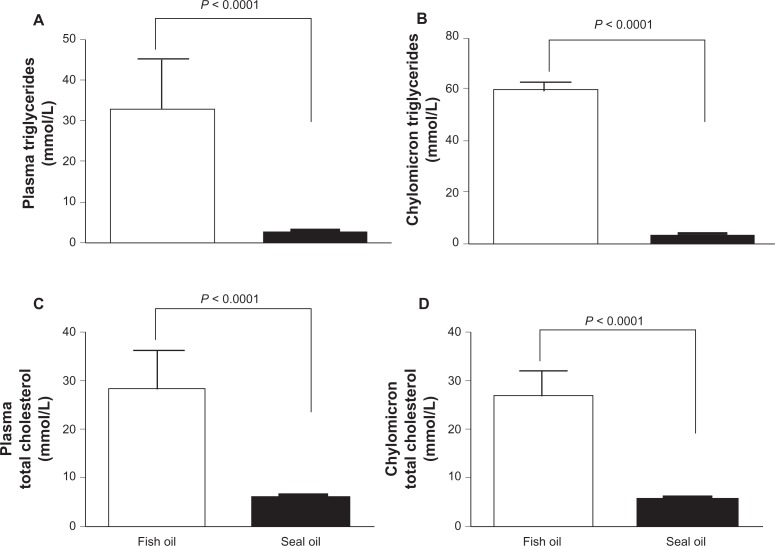
Plasma samples from BioF1B hamsters fed fish-oil showed a milky appearance, which is similar to our previously reported observations.16 However, it was interesting to notice that milky plasma was not evident in high fat seal-oil-fed BioF1B hamsters. Plasma and chylomicron triglyceride concentrations were significantly higher in fish-oil- fed hamsters as compared to the seal-oil-fed hamsters (P < 0.0001) (Fig. 1A and B respectively), the fold increase was dramatically greater in the chylomicron fraction as compared to the plasma fraction (∼20 fold increase as opposed to 11 fold increase). Plasma and chylomicron total cholesterol concentrations were also significantly higher in the fish-oil-fed hamsters as compared to the seal-oil-fed hamsters; the fold increase was however similar for plasma and chylomicron fraction (∼5 fold increase; P < 0.0001) (Fig. 1C and D respectively).
Figure 1.
The plasma and chylomicron triglyceride and cholesterol concentrations were lower in seal-oil-fed hamsters. BioF1B hamsters were fed high fat diets enriched in fish-oil (open) or seal-oil (slid) for 4 weeks. Fasting plasma and chylomicron samples were collected to assay for triglycerides and total cholesterol. Triglyceride concentrations are shown in Panel A (plasma) and B (chylomicrons), and cholesterol concentrations are shown in panel C (plasma) and D (chylomicron).Values given are means ± SEM (n = 7) analyzed by unpaired student t-test.
The concentrations of TGs and total cholesterol in various lipoprotein fractions from BioF1B hamsters fed fish-oil or seal-oil are given in Figure 2 (A and B). The plasma lipid parameters showed significantly lower concentrations for seal-oil-fed BioF1B hamsters as compared to fish-oil-fed BioF1B hamsters. Seal-oil feeding as compared to fish-oil feeding resulted in significantly lower TGs in VLDL (1.50 ± 0.20 vs. 15.84 ± 2.85 mmol/L; P < 0.0001), LDL (0.85 ± 0.09 vs. 8.90 ± 1.54 mmol/L; P < 0.01) and HDL (0.32 ± 0.04 vs. 5.10 ± 1.7 mmol/L; P < 0.01) (Fig. 2A). Analysis of different lipoprotein fractions for total cholesterol concentration showed that seal-oil fed BioF1B hamsters had significantly lower concentrations as compared to fish-oil feeding for VLDL (1.56 ± 0.20 vs. 7.95 ± 1.06 mmol/L; P < 0.0001), LDL (2.17 ± 0.32 vs. 6.92 ± 1.09 mmol/L; P < 0.0001) and HDL (1.26 ± 0.09 vs. 4.25 ± 0.69 mmol/L; P < 0.005) (Fig. 2B).
Figure 2.
Individual lipoprotein fractions showed lower concentrations of triglyceride and cholesterol in seal-oil-fed hamsters. BioF1B hamsters were fed high fat diets enriched in fish-oil (open) or seal-oil (hatched) for 4 weeks. Fasting plasma samples were collected and lipoprotein fractions were separated by density gradient centrifugation as explained under the methods section. Plasma lipoprotein fractions were assayed for triglycerides (A) and total cholesterol (B). Values given are means ± SEM (n = 7) analyzed by unpaired student t-test.
Hepatic lipid profile
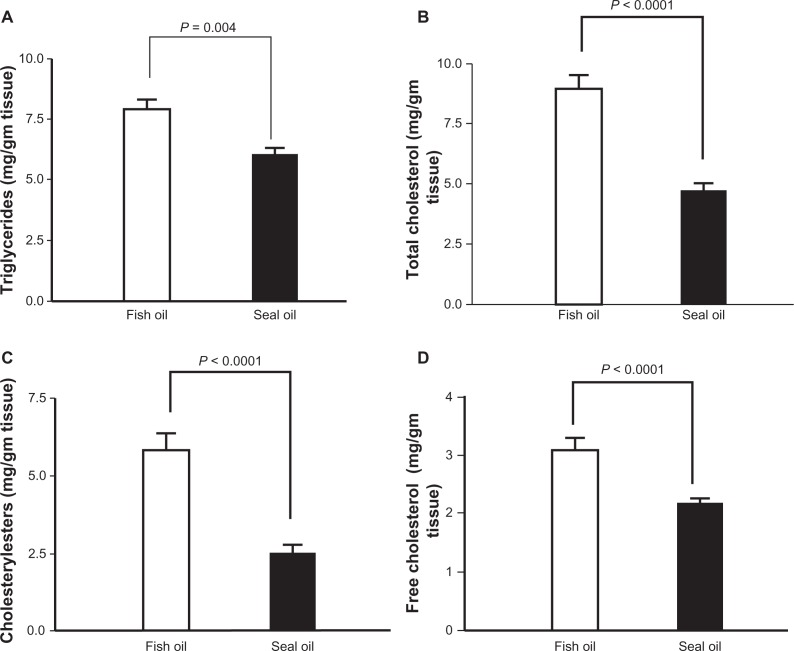
Hepatic TG concentrations were significantly lower in seal oil fed BioF1B hamsters as compared to the fish-oil-fed hamsters (6.0 ± 0.48 vs. 7.9 ± 0.75 mg/g tissue; P = 0.004) (Fig. 3A). Significant differences were also observed for hepatic total cholesterol, cholesteryl esters and free cholesterol concentrations in response to different marine oils (Fig. 3B–D), where seal-oil-fed BioF1B hamsters had significantly lower concentrations as compared to the fish-oil-fed hamsters for total cholesterol (4.74 ± 0.57 vs. 8.95 ± 1.00 mg/g tissue; P < 0.0001), cholesteryl esters (2.56 ± 0.45 vs. 5.83 ± 0.50 mg/g tissue; P < 0.0001) and free cholesterol (2.18 ± 0.12 vs. 3.13 ± 0.28 mg/g tissue; P < 0.0001). While hepatic total cholesterol and cholesteryl ester concentrations were higher by 89% and 128% respectively in fish-oil-fed hamsters as compared to seal-oil-fed hamsters, relatively smaller increases were observed for TG (32% increase) and free cholesterol (43% increase).
Figure 3.
Hepatic lipid concentrations were lower in BioF1B hamsters fed a diet enriched in seal-oil. BioF1B hamsters were fed high fat diets enriched in fish-oil (open) or seal-oil (solid) for a period of 4 weeks. Liver lipids were isolated and assayed for triglycerides (A), total cholesterol (B), cholesteryl esters (C) and free cholesterol (D) as mentioned in the methods section. Values given are means ± SEM (n = 7) analyzed by unpaired t-test.
Fatty acid composition of lipids in chylomicron fractions
Table 3 shows the FA composition of lipids of chylomicron fractions of the two experimental groups. Fish-oil-fed BioF1B hamsters had significantly higher proportion of long chain n-3 PUFAs such as C20:5 and C22:6 as compared to the seal-oil- fed hamsters. In contrast, those fed with seal oil had higher amounts of C16:0, 18:0, 18:1 n-9 and C18:2n-6. The sum total of n-3 PUFAs in fish-oil-fed hamsters was almost twice that of seal-oil-fed hamsters.
Table 3.
Fatty acid composition of chylomicron lipids in fish-oil (FO) and seal-oil (SO)1 fed groups.
| Fatty acids | FO | SO |
|---|---|---|
| 14:0 | 2.25 ± 0.17 | ND |
| 16:0 | 15.40 ± 1.22b | 22.64 ± 5.33a |
| 16:1 n-7 | 5.25 ± 0.10 | 5.78 ± 0.58 |
| 18:0 | 5.18 ± 0.43b | 7.86 ± 1.89a |
| 18:1n-9 | 9.67 ± 0.86b | 22.57 ± 2.73a |
| 18:2n-6 | 2.95 ± 0.58b | 8.39 ± 1.23a |
| 18:3n-3 | 0.88 ± 0.01 | ND |
| 20:1n-9 | ND | 1.91 ± 0.75 |
| 20:4n-6 | 1.91 ± 0.75 | 3.57 ± 0.09 |
| 20:5n-3 | 26.36 ± 1.26a | 12.49 ± 2.45b |
| 22:5n-3 | 1.86 ± 0.28 | 1.86 ± 0.28 |
| 22:6n-3 | 19.81 ± 0.99a | 15.28 ± 2.65b |
| Total SFA | 22.83 ± 1.32b | 30.49 ± 2.80a |
| Total MUFA | 16.57 ± 1.89b | 30.51 ± 2.34a |
| Total PUFA | 58.87 ± 3.23a | 36.47 ± 2.8b |
| Total n-3PUFA | 50.95 ± 3.54a | 28.39 ± 1.78b |
| Total n-6PUFA | 6.13 ± 0.45b | 10.77 ± 1.0a |
| n-3/n-6 ratio | 8.31 ± 0.67a | 2.63 ± 0.21b |
Notes:
Lipids were extracted from plasma of BioF1B hamsters fed a high fat diet enriched in fish-oil or seal-oil as explained under the methods. FA composition was determined by gas liquid chromatography (GLC). SFA, MUFA, PUFA indicates saturated, monounsaturated and polyunsaturated FA, respectively. The values are expressed as Mean ± SEM and values in the same row that do not represent the same superscript letters are significantly different at P < 0.05. FAs are shown as % of the total fatty acids.
Abbreviation: ND, not detected.
Apolipoprotein-B expression
To determine the origin of various lipoproteins, western blot analysis was performed for plasma apoB48 and apoB100 of BioF1B hamsters fed with fish-oil and seal-oil rich diets (Fig. 4). As compared to fish-oil-fed BioF1B hamsters, the seal-oil- fed hamsters had significantly lower levels of apoB48, as well as apoB100 (P < 0.0001). The apoB48/apoB100 ratio for seal-oil and fish-oil hamsters was 0.27 and 0.66 respectively.
Figure 4.
ApolipoproteinB (apoB) in BioF1B hamsters fed fish-oil or seal-oil enriched diets. BioF1B hamsters were fed high fat diets enriched in fish-oil or seal-oil for a period of 4 weeks. Proteins were separated using SDS-PAGE (6%) and transferred to a nitrocellulose membrane. ApoB and β-actin proteins were detected by western blotting as described in the methods section. Two representative samples from each dietary treatment are shown (A). The expression of apoB100 (B) and apoB48 (C) was normalized to the expression of β-actin after densitometric scanning of western blots of plasma from fish-oil (open) and seal-oil (solid) fed hamsters. Values given are means ± SEM (n = 7) analyzed by unpaired t-test.
Gene expression analysis
The hepatic mRNA expression of various transcription factors involved in lipid and lipoprotein metabolism was measured to investigate whether fish-oil or seal-oil supplementation had a differential effect. Seal-oil feeding significantly reduced the levels of SREBP-1c mRNA expression in BioF1B hamsters as compared to fish-oil feeding (20% reduction in seal oil fed compared to fish oil fed hamsters) (Fig. 5), however no changes were observed for PPAR-α, LXR-α and SREBP-2 (data not shown).
Figure 5.
Seal oil feeding inhibited the gene expression of SREBP-1c. BioF1B hamsters were fed high fat diets enriched in fish-oil (open) or seal-oil (solid) for a period of 4 weeks. Hepatic total RNA was extracted and SREBP-1c mRNA levels were measured using specific primers as described under the methods section. The expression of SREBP-1c was normalized to β-actin and expressed as relative units. Values given are means ± SEM (n = 7) analyzed by unpaired t-test.
Plasma and liver TBARS
The extent of lipid peroxidation in fish-oil and seal-oil-fed hamsters was evaluated using TBARS assay (Fig. 6). Hamsters fed seal-oil showed significantly lower levels of lipid peroxides (P < 0.001) in plasma as compared to fish-oil-fed hamsters (68% reduction in seal-oil fed hamsters as compared to fish-oil fed hamsters) (Fig. 6A). Furthermore, seal-oil-fed hamsters had significantly reduced levels of liver TBARS (Fig. 6B) as compared to fish-oil-fed hamsters (27% reduction in seal-oil fed hamsters as compared to fish-oil fed hamsters) (P < 0.01).
Figure 6.
TBARS levels were lower in BioF1B hamsters fed a diet enriched in seal-oil. BioF1B hamsters were fed high fat diets enriched in fish-oil (open) or seal-oil (solid) for a period of 4 weeks. Plasma (A) and liver (B) samples were assayed for TBARS as mentioned in the methods section. Values given are means ± SEM (n = 7) analyzed by unpaired t-test.
Discussion
Seal-oil, a rich source of n-3 fatty acids, differs from fish-oil both in the type of FA and the positional distribution of fatty acids in the TGs.10–13 The n-3 PUFAs in seal-oil are mainly located at the sn-1 and sn-3 positions, while those in fish-oil are mainly located at the sn-2 position.10–12 We investigated the metabolic actions of dietary seal-oil as compared to fish-oil under conditions of low LPL activity as found in the BioF1B hamster. BioF1B hamster, a hybrid strain, is genetically susceptible to diet-induced hyperlipidemia and atherosclerosis.22,23 We report, for the first time, that in contrast to the feeding of a high fat diet with fish-oil, feeding seal-oil did not cause milky plasma after an overnight fast. The concentrations of TGs and total cholesterol levels in plasma, chylomicron and individual lipoprotein fractions, as well as hepatic lipid levels were significantly lower in seal-oil-fed hamsters as compared to fish-oil-fed hamsters. Moreover, seal-oil-fed hamsters showed lower levels of oxidative stress as compared to the fish-oil-fed hamsters. Differences in the intramolecular distribution of n-3 PUFAs in two marine oils are likely responsible for the differential effects in in-vivo metabolism of lipids.10–13
Lack of milky plasma in seal-oil-fed hamsters as compared to fish-oil feeding suggests rapid clearance of chylomicron remnants and/or (VLDL) from seal-oil-fed hamsters. LPL has a relative preference for the sn-1 and sn-3 positions in TG molecules as compared to the sn-2 position.17 The FA profile of chylomicron lipids showed that EPA and DHA concentrations in fish-oil-fed BioF1B were significantly higher as compared to those fed seal-oil suggesting that the hydrolysis of these fatty acids from TG in chylomicron particles may not be efficient in fish-oil-fed BioF1B hamsters as compared to those fed with seal oil. Ackman24 has previously suggested that when EPA and DHA are present at the sn-2 position of TG of the chylomicrons, then these are transported to the liver, whereas, those at the sn-1 and sn-3 position are transported to peripheral tissues thus resulting in a different rate of clearance of chylomicrons. It is likely that n-3 PUFA at the sn-1 and -3 positions from seal-oil are distributed to various tissues differently as compared to fish-oil, which needs to be investigated in the future.
Seal oil feeding in BioF1B hamsters also showed markedly lower levels of plasma apoB48 and apoB48/100 ratio as compared to fish-oil feeding, indicating lower levels of intestinally derived lipoproteins. Previously, we reported that apoB48 and -100 protein levels were elevated in BioF1B hamsters fed fish-oil as compared to MUFA.16 Elevated plasma apoB48 expression in fish-oil-fed BioF1B hamsters even after a 14 h fast indicated the presence of lower-density lipoproteins, seen as milky plasma, which was absent in seal-oil-fed BioF1B hamsters. Reduced post-heparin LPL activity was previously shown to be associated with an increased accumulation of chylomicron-like particles in BioF1B hamsters.16,23 Overall, a combined effect of low LPL activity and differences in positional distribution of n-3 PUFAs may have resulted in milky plasma in hamsters fed with fish-oil.
Besides other differences in seal-oil and fish-oil, seal-oil also contains higher levels of MUFA as compared to fish-oil. As shown in Table 2, the MUFA content in seal-oil diet is 51% as compared to 22% in fish-oil diet. The increased MUFA content of seal-oil may have also contributed to reduction of hepatic and plasma lipids due to the known hypolipidemic effects of MUFA.26,27 We have previously shown that a diet rich in MUFA had significantly lower levels of plasma and hepatic lipids as compared to fish-oil feeding.16 Comparison of our previous data from BioF1B hamsters fed a MUFA rich diet to seal-oil-fed BioF1B hamsters revealed that the plasma cholesterol levels of various lipoprotein fractions were similar to the levels of MUFA fed hamsters (data not shown). However the plasma TG levels were dramatically lower in seal-oil-fed hamsters as compared to the MUFA fed hamsters (data not shown) suggesting a combined effect of MUFA and n-3 fatty acids. It will be important to delineate the effects of individual fatty acids along with the positional distribution of fatty acids on the regulation of lipid metabolism in the future studies.
In addition to the plasma parameters, the BioF1B hamsters fed with seal-oil also showed significantly lower hepatic lipid levels as compared to the fish-oil-fed hamsters. Seal-oil-fed BioF1B hamsters showed 50% reduction in total hepatic cholesterol, suggesting that seal-oil specifically alters the regulation of metabolic pathways involved in hepatic cholesterol metabolism. It has been shown that dietary seal-oil inhibits the action of fatty acid synthase (FAS), glucose-6-phosphate dehydrogenase (G6PDH) and hepatic triacylglycerol lipase (HTGL).11 Liver is the most important organ that is involved in the regulation of lipid metabolism. Recent studies indicate the involvement of several transcription factors in this regulatory mechanism such as: peroxisome proliferator activated receptors (PPAR), liver-x receptor (LXR-α), SREBP-1c and SREBP-2.28,29 We observed no significant differences in the gene expression of hepatic PPAR-α, LXR-α and SREBP-2 (Data not shown). However, seal-oil-fed BioF1B hamsters showed significantly reduced hepatic SREBP-1c mRNA expression as compared to the fish-oil-fed BioF1B hamsters. Thus, reduced plasma and hepatic lipid levels in seal-oil-fed BioF1B hamsters in our study can be partially explained by suppression of lipogenesis due to lowered SREBP-1c activity.
Earlier studies have also suggested that seal-oil is resistant to oxidants as compared to fish-oil.30 Seal-oil-fed BioF1B hamsters showed significantly lower levels of lipid peroxidation products in both plasma and liver samples as compared to those fed with fish-oil. The changes in plasma TBARS levels were highly pronounced than the changes in liver. The plasma malondialdehyde levels in seal-oil-fed BioF1B hamsters were almost one-third of that observed for fish-oil-fed BioF1B hamsters. The possible explanation for differences in oxidation product levels in response to the two n-3 PUFA rich marine oils could be due to the fact that the long chain n-3 PUFAs in seal-oil are relatively more stable and less prone to lipid peroxidation than in fish-oil.30 The role of long chain n-3 PUFA in fish oil in modulation of oxidative stress is also not conclusive, because EPA has been reported to enhance hepatic anti-oxidant defence in mice,31 whereas long chain n-3 PUFA rich fish-oil has been reported to increase the MDA level, an indicator of lipid peroxidation in rats.32 On the other hand, MUFA enriched diets have been shown to improve the oxidative stability in tissue lipids,33 which may be responsible for reduced oxidative stress in seal-oil-fed hamsters due to higher content of MUFA.
In conclusion, our findings, for the first time, compared the effects of fish-oil vs. seal-oil in BioF1B hamsters, an inbred hamster strain that has low LPL activity. In contrast to dietary fish-oil, seal-oil feeding did not induce milky plasma in the BioF1B hamsters. Seal-oil feeding showed lower lipid levels in plasma and liver, along with reduced plasma and hepatic oxidative stress as compared to fish-oil feeding. Results from our findings suggest that human population with LPL deficiency may have greater health benefits from seal-oil supplementation when compared to fish-oil. Further studies are however warranted to explore the effect of intramolecular distribution of fatty acids in the TG molecule on the regulation of metabolic pathways, and to further understand the molecular mechanisms responsible for the differential response to fish-oil and seal-oil. Studies are also warranted to explore whether genetic polymorphisms in the LPL gene are responsible for this differential effect by using other animal models of LPL deficiency, ie, LPL knockout mice.34
Acknowledgments
Authors would like to thank the Heart and Stroke Foundation of Canada, Canadian Institutes of Health Research and Canada Foundation for Innovation New Opportunities Fund for supporting this research.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Breslow JL. N-3 fatty acid and cardiovascular disease. J Clin Nutr. 2006;83:1477–82. doi: 10.1093/ajcn/83.6.1477S. [DOI] [PubMed] [Google Scholar]
- 2.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197:12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Dyerberg J. Coronary heart disease in Greenland Inuit: a paradox. Implications for western diet patterns. Arctic Med Res. 1989;48:47–54. [PubMed] [Google Scholar]
- 4.Damsgaard CT, Frokiaer H, Andersen AD, Lauritzen L. Fish oil in combination with high or low intakes of linoleic acid lowers plasma triacylglycerols but does not affect other cardiovascular risk markers in healthy men. J Nutr. 2008;138:1061–6. doi: 10.1093/jn/138.6.1061. [DOI] [PubMed] [Google Scholar]
- 5.Hartweg J, Perera R, Montori V, Dinneen S, Neil HA, Farmer A. Omega-3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;23:CD003205. doi: 10.1002/14651858.CD003205.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris WS. Dietary fish oil and blood lipids. Curr Opin Lipidol. 1996;7:3–7. doi: 10.1097/00041433-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Hsu HC, Lee YT, Chen MF. Effect of n-3 fatty acids on the composition and binding properties of lipoproteins in hypertriglyceridemic patients. Am J Clin Nutr. 2000;71:28–35. doi: 10.1093/ajcn/71.1.28. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan DR, Sanders TA, Trayner IM, Thompson GR. Paradoxical elevation of LDL apoprotein B levels in hypertriglyceridaemic patients and normal subjects ingesting fish oil. Atherosclerosis. 1986;61:129–34. doi: 10.1016/0021-9150(86)90072-9. [DOI] [PubMed] [Google Scholar]
- 9.Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980;33:2657–61. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida H, Kumamaru J, Mawatari M, et al. Lymphatic absorption of seal and fish oils and their effect on lipid metabolism and eicosanoid production in rats. Biosci Biotechnol Biochem. 1996;60:1293–8. doi: 10.1271/bbb.60.1293. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida H, Mawatari M, Ikeda I, Imaizumi K, Seto A, Tsuji H. Effect of dietary seal and fish oils on triacylglycerol metabolism in rats. J Nutr Sci Vitaminol (Tokyo) 1999;45:411–21. doi: 10.3177/jnsv.45.411. [DOI] [PubMed] [Google Scholar]
- 12.Christensen MS, Høy CE. Effects of dietary triacylglycerol structure on triacylglycerols of resultant chylomicrons from fish oil- and seal oil-fed rats. Lipids. 1996;31:341–4. doi: 10.1007/BF02529882. [DOI] [PubMed] [Google Scholar]
- 13.Christensen MS, Høy CE, Redgrave TG. Lymphatic absorption of n-3 polyunsaturated fatty acids from marine oils with different intramolecular fatty acid distributions. Biochim Biophys Acta. 1994;1215:198–204. doi: 10.1016/0005-2760(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 14.de Silva PP, Davis PJ, Cheema SK. Hyperlipidaemic effect of fish oil in Bio F1B hamsters. Br J Nutr. 2004;91:341–9. doi: 10.1079/BJN20031056. [DOI] [PubMed] [Google Scholar]
- 15.de Silva PP, Agarwal-Mawal A, Davis PJ, Cheema SK. The levels of plasma low density lipoprotein are independent of cholesterol ester transfer protein in fish-oil fed F1B hamsters. Nutr Metab (Lond.) 2005;2:8. doi: 10.1186/1743-7075-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheema SK, Cornish ML. Bio F1B hamster: a unique animal model with reduced lipoprotein lipase activity to investigate nutrient mediated regulation of lipoprotein metabolism. Nutr Metab (Lond.) 2007;4:27. doi: 10.1186/1743-7075-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morley N, Kukiss A. Positional specificity of lipoprotein lipase. J Biol Chem. 1972;247:6389–93. [PubMed] [Google Scholar]
- 18.Langlois S, Deeb S, Brunzell JD, Kastelein JJ, Hayden MR. A major insertion accounts for a significant proportion of mutations underlying human lipoprotein lipase deficiency. Proc Natl Acad Sci. 1989;86:948–52. doi: 10.1073/pnas.86.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 20.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 21.Williamson KS, Hensley K, Floyd RA. Fluorometric and colorimetric assessment of thiobarbituric acid-reactive lipid aldehydes in biological matrices. In: Hensley K, Floyd RA, editors. Methods in Pharmacology and Toxicology: Methods in Biological Oxidative Stress. Totowa New Jersey: Humana Press; 2003. [Google Scholar]
- 22.Kowala MC, Nunnari JJ, Durham SK, Nicolosi RJ. Doxazosin and cholestyramine similarly decrease fatty streak formation in the aortic arch of hyperlipidemic hamsters. Atherosclerosis. 1991;91:35–49. doi: 10.1016/0021-9150(91)90185-6. [DOI] [PubMed] [Google Scholar]
- 23.McAteer MA, Grimsditch DC, Vidgeon-Hart M, Benson GM, Salter AM. Dietary cholesterol reduces lipoprotein lipase activity in the atherosclerosis-susceptible Bio F(1)B hamster. Br J Nutr. 2003;89:341–50. doi: 10.1079/BJN2002802. [DOI] [PubMed] [Google Scholar]
- 24.Ackman RG. Some possible effects on lipid biochemistry of differences in the distribution on glycerol of long-chain n-3 fatty acids in the fats of marine fish and marine mammals. Atherosclerosis. 1988;70:171–3. doi: 10.1016/0021-9150(88)90112-8. [DOI] [PubMed] [Google Scholar]
- 25.Christensen MS, Mortimer BC, Høy CE, Redgrave TG. Clearance of chylomicrons following fish oil and seal oil feeding. Nutrition Research. 1995;15:359–68. [Google Scholar]
- 26.Waterman E, Lockwood B. Active components and clinical applications of olive oil. Altern Med Rev. 2007;12:331–42. [PubMed] [Google Scholar]
- 27.Pérez-Jiménez F, Ruano J, Perez-Martinez P, Lopez-Segura F, Lopez-Miranda J. The influence of olive oil on human health: not a question of fat alone. Mol Nutr Food Res. 2007;51:1199–208. doi: 10.1002/mnfr.200600273. [DOI] [PubMed] [Google Scholar]
- 28.Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006;86:465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- 29.Wójcicka G, Jamroz-Winiewska A, Horoszewicz K, Betowski J. Liver X receptors (LXRs). Part I: structure, function, regulation of activity, and role in lipid metabolism. Postepy Hig Med Dosw. 2007;61:736–59. [PubMed] [Google Scholar]
- 30.Xiao W, Wang L, Davis PJ, Liu H. Microemulsion of seal oil markedly enhances the transfer of a hydrophobic radiopharmaceutical into acetylated low density lipoprotein. Lipids. 1999;34:503–9. doi: 10.1007/s11745-999-0391-7. [DOI] [PubMed] [Google Scholar]
- 31.Demoz A, Willumsen N, Berge RK. Eicosapentaenoic acid at hypotriglyceridemic dose enhances the hepatic antioxidant defence in mice. Lipids. 1992;27:968–71. doi: 10.1007/BF02535573. [DOI] [PubMed] [Google Scholar]
- 32.Mete N, Isik B, Erdinc L, Gurkan F. The effect of fish oil on liver and plasma MDA and antioxidant status of rats. Tr J of Medical Sciences. 1999;29:1–6. [Google Scholar]
- 33.Daza A, Rey AI, Ruiz J, Lopez-Bote CJ. Effects of feeding in free-range conditions or in confinement with different dietary MUFA/PUFA ratios and a-tocopheryl acetate, on antioxidants accumulation and oxidative stability in Iberian pigs. Meat Sci. 2005;69:151–63. doi: 10.1016/j.meatsci.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Levak-Frank S, Hofmann W, Weinstock PH, et al. Induced mutant mouse lines that express lipoprotein lipase in cardiac muscle, but not in skeletal muscle and adipose tissue, have normal plasma triglyceride and high-density lipoprotein-cholesterol levels. Proc Natl Acad Sci U S A. 1999;96:3165–70. doi: 10.1073/pnas.96.6.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]