Abstract
Heart rate reduction (HRR) is an important target in the management of patients with chronic stable angina. Most available drugs for HRR, such as β-blockers, have adverse effects, including on cardiac energy substrate metabolism, a well-recognized determinant of cardiac homeostasis. This study aimed at 1) testing whether HRR by ivabradine (IVA) alters substrate metabolism in the healthy normoxic working heart and 2) comparing the effect of IVA with that of the β-blocker metoprolol (METO). This was assessed using our well-established model of ex vivo mouse heart perfusion in the working mode, which enables concomitant evaluation of myocardial contractility and metabolic fluxes using 13C-labeled substrates. Hearts were perfused in the absence (controls; n = 10) or presence of IVA (n = 10, 3 μM) with or without atrial pacing to abolish HRR in the IVA group. IVA significantly reduced HR (35 ± 5%) and increased stroke volume (39 ± 9%) while maintaining similar cardiac output, contractility, power, and efficiency. Effects of IVA on HR and stroke volume were reversed by atrial pacing. At the metabolic level, IVA did not impact on substrate selection to citrate formation, rates of glycolysis, or tissue levels of high-energy phosphates. In contrast, METO, at concentrations up to 40 μM, decreased markedly cardiac function (flow: 25 ± 6%; stroke volume: 30 ± 10%; contractility: 31 ± 9%) as well as glycolysis (2.9-fold) but marginally affected HR. Collectively, these results demonstrate that IVA selectively reduces HR while preserving energy substrate metabolism of normoxic healthy working mouse hearts perfused ex vivo, a model that mimics to some extent the denervated transplanted heart. Our results provide the impetus for testing selective HRR by IVA on cardiac substrate metabolism in pathological models.
Keywords: substrate metabolism, isolated working heart, ivabradine, β-blockers
Heart rate reduction (HRR) is an important target in the management of patients with ischemic heart disease, a major cause of morbidity and mortality in developed countries (for reviews, see Refs. 6 and 45). Given their well-described beneficial effects on cardiovascular outcomes after myocardial infarction and in heart failure, β-blockers remain the first line treatment for many patients with ischemic heart disease (5, 12, 28). However, even new classes of β-blockers exhibit undesirable side effects on cardiac energy substrate metabolism (4, 28), which is now a well-recognized determinant of energy production, redox status, contractile function, ion fluxes, and oxygen consumption as well as hypertrophy development and progression to heart failure (33, 41).
Specifically, acute administration of β-blockers to the isolated perfused heart, which is devoid of neuronal influences, has been shown to affect substrate fluxes through major energy-yielding pathways. The effects include 1) decreased cytosolic glycolytic flux, a process that has been linked to optimal ion pump function, and 2) a shift in mitochondrial substrate selection from long-chain fatty acids (LCFAs) to carbohydrates (CHOs) for oxidation in the Krebs cycle, most likely due to reduction of lipoprotein lipase and/or carnitine palmitoyltransferase I activity (3, 28, 37). Such a shift from LCFA to CHO oxidation may be considered beneficial in the short term, particularly for the ischemic heart due to the greater yield of ATP per unit of O2 consumed from CHO oxidation (i.e., 11–12%); however, potential long-term consequences include lipotoxicity and energy deprivation (33, 41). In addition, long-term β-blocker therapy may also have unwanted systemic metabolic effects, such as worsening of whole body glycemic control (18). These deleterious metabolic effects are of particular concern given that HRR is used for the management of patients with both ischemic heart disease and diabetes (7, 39). In this regard, ivabradine (IVA), which is the focus of the present study, may represent a good alternative for the management of these patients.
Indeed, IVA belongs to a new class of drugs that selectively inhibits the pacemaker current in the sinoatrial node (15), thereby leading to specific HRR (46), without hemodynamic effects on blood pressure, vascular resistance, and cardiac output. Acute IVA administration confers significant benefits in reducing infarct size after ischemia-reperfusion as well as preserving the metabolic energy and redox status in the ex vivo perfused heart subjected to ischemia-reperfusion. In addition, its long-term administration in vivo in pig or rat models of myocardial infarction prevented postmyocardial infarction adverse structural remodeling while preserving contractile function and high-energy phosphate status (22, 30, 32). In an in vivo dog model of exercise, Colin et al. (10) demonstrated a linear relationship between myocardial oxygen consumption (MV̇O2) and the extent of HRR by acute intravenous IVA. Under pacing, all effects were abolished (8, 9, 11), thereby demonstrating that they are due to IVA’s selective modulation of HR, although this concept has recently been challenged (20, 21).
To the best of our knowledge, the effect of IVA-induced HRR on cardiac substrate selection for energy production has not yet been directly assessed. In fact, although both heart rate (HR) and contractility are considered to contribute to MV̇O2, little is known about the impact of HRR per se, independent of contractility, on cardiac energy substrate metabolism (17, 26, 29, 43). Admittedly, this is difficult to assess in vivo due to the existence of complex regulatory mechanisms extrinsic to the heart. Hence, in this study, we have used our well-established model of ex vivo heart perfusion in the working mode, with concomitant evaluation of myocardial contractility and metabolic flux parameters using 13C-labeled CHO (glucose) and a LCFA (oleate) (25) to test the impact of HRR using IVA on cardiac energy substrate metabolism, in the absence of neuronal influences and at fixed values of arterial compliance (i.e., peripheral resistance), preload, and afterload pressures. As a secondary objective, we compared the effect of IVA with that of the β-blocker metoprolol (METO).
MATERIALS AND METHODS
Chemicals
The sources of chemicals, biological products, and 13C-labeled substrates as well as the procedure for the dialysis of albumin have been described previously (48).
Heart Perfusions in Semirecirculating Working Mode
Animal experiments were approved by the local animal care committee in compliance with the guidelines of the Canadian Council on Animal Care. Male C57Bl/6 mice (3 mo old; Charles River Laboratories) were provided with food and water ad libitum. The procedures for heart isolation and its ex vivo perfusion in the working mode as well as measurements of the various functional parameters have been described previously (25).
Perfusion protocols: effect of IVA
The concentrations of IVA have been selected on the basis of previous studies (8, 34) and orientation experiments in which we evaluated the concentration-dependent HRR effects of IVA in our working mouse heart model (n = 10 perfused hearts/dose). Working mouse hearts were perfused for 40 min with a semirecirculating modified Krebs-Henseleit buffer containing physiological concentrations of substrates and hormones (11 mM glucose, 0.8 nM insulin, 50 μM carnitine, 5 nM epinephrine, 1.5 mM lactate, 0.2 mM pyruvate, and 0.4 mM oleate bound to 3% albumin) in the absence or in the presence of 3 μM IVA, with or without atrial pacing to match the HR of controls (n = 10 perfused hearts/group). IVA was added after 10 min of perfusion to enable measurements of functional parameters under basal conditions, prior to IVA addition, in each heart. For any given perfusion, one of the unlabeled substrates was replaced by its corresponding labeled substrate, either [U-13C6]glucose [molar percent enrichment (MPE): 50%; n = 5] or [U-13C18]oleate (MPE: 35%; n = 5), to probe both CHO and LCFA metabolism, respectively. As described previously (25), atrial influent and coronary effluent perfusate samples were collected to assess 1) lactate dehydrogenase release as an index of membrane integrity, 2) PO2, PCO2, pH, and Ca2+, and 2) lactate and pyruvate release rates. At the end of the perfusion, hearts were freeze-clamped with metal tongs cooled in liquid nitrogen, weighed, and stored at −80°C for subsequent analyses.
Effect of METO
In a separate group of ex vivo heart perfusion experiments, we attempted to match the HRR obtained with IVA using the β-blocker METO at concentrations ranging from 4 to 40 μM based on previously published data (31, 38). However, this turned out to be impossible because METO markedly reduced cardiac flows and contractility prior to HR. Consequently, because cardiac function affects substrate metabolism and the specific characterization of the effect of METO on metabolic fluxes, independent of function, was beyond the scope of this study, we conducted only selected metabolic flux measurements in working hearts perfused with 4 (n = 10) or 40 μM METO (n = 3) using [13C6]glucose or [U-13C18]oleate.
Metabolic Flux Measurements
Our previously published studies (25, 48) provide definitions of the 13C terminology and descriptions for the measurements by gas chromatography-mass spectrometry (GC-MS; Agilent 6890N GC coupled to a 5973N MS) and equations for the calculations of 1) flux ratios relevant to substrate selection for energy production through mitochondrial citrate synthesis from the 13C enrichment of the acetyl (carbons 4 and 5) and oxaloacetate (carbons 1, 2, 3, and 6) moiety of citrate and 2) efflux rates of unlabeled lactate and pyruvate reflecting cytosolic glycolysis from exogenous glucose (for perfusions with [U-13C6]glucose).
Myocardial Metabolites
Adenine nucleotides and creatine phosphate were determined in perchloric acid extracts of frozen heart tissue by high-performance liquid chromatography (2).
Statistical Analysis
Data are expressed as means ± SE. Statistical significance was reached at P < 0.05 using a t-test for myocardial high-energy phosphates and ANOVA (1-way or 2-way ANOVA for repeated measurements), followed by the Bonferroni multiple comparison posttest.
RESULTS
Ivabradine Reduces Heart Rate While Increasing Stroke Volume of Perfused Healthy Normoxic Working Mouse Hearts
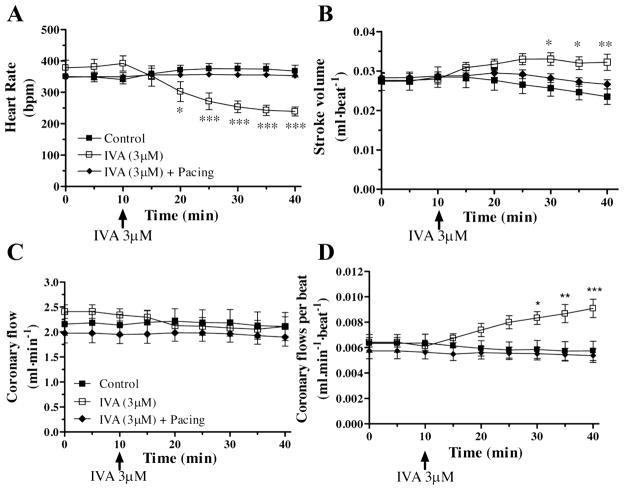
Values for physiologically relevant parameters assessed after 40 min of perfusion of normoxic healthy working mouse hearts in the absence or presence of 3 μM IVA with or without pacing are reported in Fig. 1 and Table 1. Compared with controls, hearts perfused with IVA displayed a reduction in HR of ~35 ± 5% (P < 0.001 at 40 min; Fig. 1A) and an increase in stroke volume (39%; Fig. 1B), whereas coronary flow, developed pressure, and contractility (+dP/dtmax) did not differ significantly (Table 1 and Fig. 1C). When expressed per beat, coronary flow was significantly increased in the IVA-treated group (P < 0.001 vs. controls; Fig. 1D). It is noteworthy that in our study model, which is devoid of neuronal influences, and in which compliance, preload, and afterload pressures are fixed, MV̇O2 expressed per beat is increased significantly in the IVA group (Table 1), concurring with the increased stroke volume (+13.2%) and increased coronary flow (+48%, expressed in ml/beat) and with effective matching of energy demand and supply.
Fig. 1.
Impact of ivabradine (IVA) on heart rate (A), stroke volume (B), coronary flow expressed per minute (C) and coronary flow expressed per beat (D) of isolated working C57Bl/6 mouse hearts perfused in the absence (control) or in the presence of 3 μM IVA with or without atrial pacing. Data are expressed as means ± SE of 9 –12 hearts. *P < 0.05; **P < 0.01; ***P < 0.001 vs. control group.
Table 1.
Functional and physiological parameters of isolated working C57Bl/6 mouse hearts perfused in the absence (control) or in the presence of IVA with or without atrial pacing
| Treatment Effect (40 min vs. Control) | Control | IVA, 3 μM | IVA (3 μM) + Pacing |
|---|---|---|---|
| HR, beats/min | 368 ± 19 | 239 ± 14*** | 368 ± 19 |
| LVSP, mmHg | 95 ± 4 | 107 ± 5 | 100 ± 3 |
| Min-P, mmHg | −1.09 ± 0.02 | −3.61 ± 0.02 | −4.52 ± 0.02 |
| LVDP, mmHg | 86 ± 4 | 97 ± 6 | 92 ± 4 |
| +dP/dt, mmHg/s | 5,168 ± 381 | 5,446 ± 625 | 5,412 ± 222 |
| −dP/dt, mmHg/s | −3,672 ± 324 | −4,056 ± 418 | −4,112 ± 218 |
| Rate pressure product, mmHg · beats−1 · min−1 | 31,242 ± 2,260 | 22,884 ± 2,303 | 32,432 ± 1,407 |
| Aortic flow, ml/min | 6.32 ± 0.67 | 5.44 ± 0.52 | 7.24 ± 0.48 |
| Aortic flow/beat, ml/beat | 0.018 ± 0.002 | 0.023 ± 0.001 | 0.021 ± 0.001 |
| Coronary flow, ml/min | 2.06 ± 0.26 | 2.11 ± 0.19 | 1.90 ± 0.18 |
| Coronary flow/beat, ml/beat | 0.006 ± 0.001 | 0.009 ± 0.001*** | 0.005 ± 0.001 |
| Cardiac output, ml/min | 8.38 ± 0.77 | 7.55 ± 0.69 | 9.39 ± 0.42 |
| Stroke volume, ml/beat | 0.023 ± 0.002 | 0.032 ± 0.002** | 0.027 ± 0.001 |
| Cardiac power, mW/beat | 0.005 ± 0.001 | 0.007 ± 0.001 | 0.005 ± 0.000 |
| Cardiac efficiency, mW · mmolO2−1 · min−1 · beat−1 | 0.004 ± 0.001 | 0.005 ± 0.000 | 0.005 ± 0.001 |
| MV̇O2, μmol/min | 1.3 ± 0.2 | 1.3 ± 0.1 | 1.3 ± 0.1 |
| MV̇O2/beat, μmol/beat | 0.004 ± 0.000 | 0.006 ± 0.000*** | 0.004 ± 0.000 |
| LDH, mU/min | 24.1 ± 3.5 | 35.3 ± 7.3 | 20.0 ± 3.4 |
Values are expressed as means ± SE of 9–12 hearts for the 35- to 40-min perfusion period. IVA, ivabradine; HR, heart rate; LVSP, left ventricular systolic pressure; min-p, minimum pressure; LVDP, left ventricular developed pressure; MV̇O2, oxygen consumption; LDH, lactate dehydrogenase.
P < 0.01;
P < 0.001 vs. controls.
Pacing of working hearts in the absence of IVA at a physiological rate of 407 ± 13 beats/min, which was slightly but not significantly different from controls (368 ± 19 beats/min), had a marginal impact on the physiological parameters measured (n = 10; data not shown). However, all the aforementioned physiological effects of IVA were reversed by the application of atrial pacing to hearts perfused with IVA to match HR of controls, indicating that any functional changes induced by IVA were a direct consequence of HRR (Fig. 1 and Table 1).
Ivabradine Preserves Energy Substrate Metabolism and Status in Perfused Healthy Normoxic Working Mouse Hearts
Figure 2 depicts metabolic fluxes relevant to energy production, which were assessed in perfused healthy normoxic working mouse hearts. We assessed the effects of IVA on the contributions of oleate and glucose to the generation of mitochondrial acetyl-CoA for citrate synthesis. Acute administration of IVA had no significant impact on the relative contribution of oleate (19.7 ± 1.2% with IVA vs. 19.9 ± 2.3% in controls) or glucose (28.3 ± 3.9% IVA vs. 30.2 ± 1.9% in controls) to acetyl-CoA formation for citrate synthesis. In addition, we found no significant differences between groups for the absolute flux through glycolysis (0.63 ± 0.04 IVA vs. 0.67 ± 0.16 μmol/min in controls). Pacing of working hearts in the presence of IVA (Fig. 2) or in its absence (data not shown) had no significant effects on the contribution of glucose or oleate to acetyl-CoA formation.
Fig. 2.
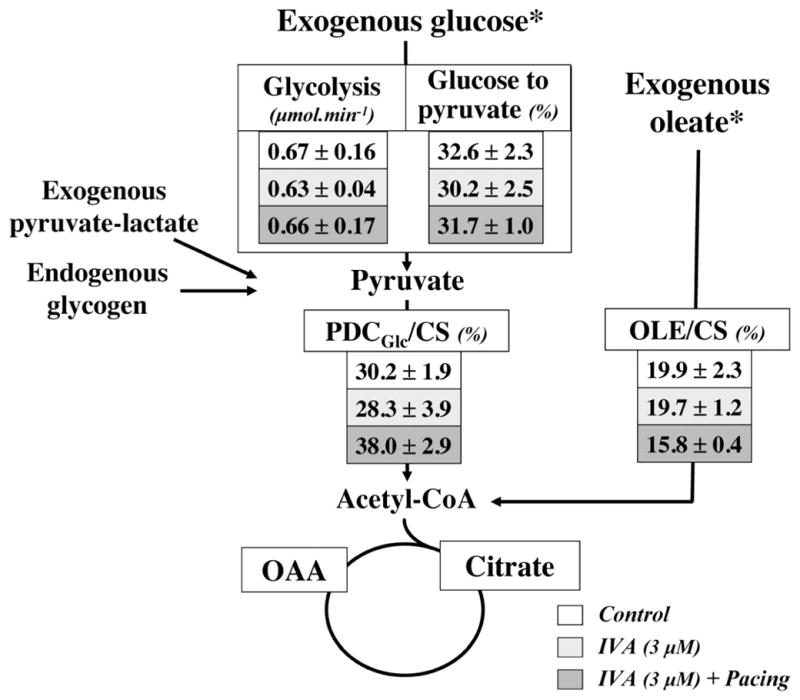
Relative contribution of glucose and oleate to mitochondrial acetyl-CoA formation for citrate synthesis and rates of glycolysis in working C57Bl/6 mouse hearts perfused in the absence (control) or in the presence of IVA with or without atrial pacing. Data are expressed as means ± SE of 4 –5 hearts perfused for 40 min with [U-13C18]oleate or [U-13C6]glucose. The contribution to acetyl-CoA formation of 1) glucose via pyruvate decarboxylation (PDCGlc) and 2) oleate via β-oxidation (OLE) are expressed relative to citrate synthesis (CS). Details about the calculations of these flux ratios and glycolytic rates are in MATERIALS AND METHODS. *Substrates that were labeled with carbon 13. OAA, oxaloacetate.
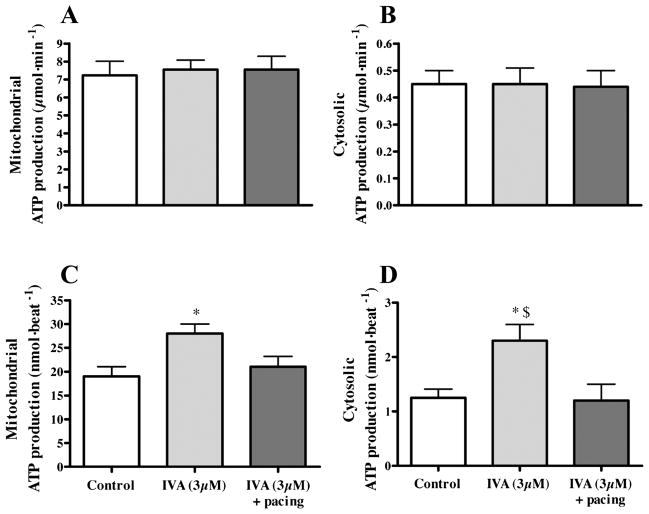
One can estimate the ATP production rate in 1) the mitochondria from the measured MV̇O2 assuming a theoretical ATP/O of 2.83 (25) and 2) the cytosol from the measured glycolytic rates (1 ATP per lactate produced, which typically represents 5–10% of total cellular ATP production). As shown in Fig. 3, mitochondrial (19 ± 2 vs. 28 ± 2 nmol ATP/beat, P < 0.05; Fig. 3, A and C) and cytosolic (1.25 ± 0.16 vs. 2.30 ± 0.30 nmol ATP/beat, P < 0.05; Fig. 3, B and D) ATP production is not modified per minute (Fig. 3, A and B), whereas it is increased per beat (Fig. 3, C and D) in the IVA treated-group, an effect that was abolished by the application of pacing. Substantiating the notion that myocardial energy status is well preserved in working hearts perfused with IVA, we found no difference in the tissue levels of creatine phosphate, ATP, ADP, or AMP compared with the control group (Table 2).
Fig. 3.
Energy production in working C57Bl/6 mouse hearts perfused in the absence (control) or in the presence of IVA with or without atrial pacing. Data are expressed as means ± SE of 4 –5 hearts. Rates of ATP production in cytosol and mitochondria are calculated from the measured rates of 1) glycolysis and 2) MV̇O2, (assuming an ATP/O ratio of 2.83), respectively, and are expressed per minute (A and B) and per beat (C and D). *P < 0.05 vs. control group; $P < 0.05 IVA vs. IVA + pacing group.
Table 2.
Myocardial HEP in working C57Bl/6 mouse hearts perfused for 40 min in the absence (control) or presence of IVA
| HEP, μmol/mg prot | Control | IVA, 3 μM |
|---|---|---|
| Creatine | 216 ± 26 | 237 ± 14 |
| Phosphocreatine | 63.8 ± 10.7 | 80.0 ± 7.5 |
| AMP | 19.6 ± 4.4 | 22.7 ± 3.7 |
| ADP | 29.4 ± 2.3 | 33.8 ± 1.4 |
| ATP | 60.5 ± 10.3 | 71.0 ± 8.6 |
Data are expressed in μmol/mg protein and are means ± SE of 4–5 hearts. HEP, high-energy phosphates.
Altogether, these results demonstrate that in healthy normoxic working hearts the addition of IVA did not affect myocardial substrate selection for energy production or the overall energy status of the heart.
Metoprolol Decreases Contractility, But Not HR, of Perfused Healthy Normoxic Working Mouse Hearts
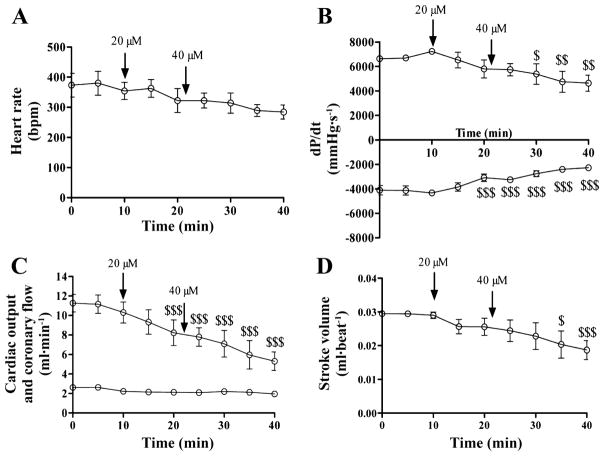
Because IVA belongs to a new class of drugs, its effect was compared with a β-blocker (METO), which is the first-line choice for HRR in the clinic. A comparison of the direct effects of these two drugs on the heart also appeared to be valuable from a mechanistic point of view. We attempted to match HRR induced by IVA at 3 μM with the addition of METO. However, at concentrations up to 40 μM, METO had little impact on HR (~19%; not significant), yet it dramatically reduced contractility, cardiac output, and stroke volume (by 36, 49, and 35% respectively; Fig. 4). At 40 μM METO, hearts displayed unstable function with irregular rate and force of contraction. At the metabolic level, the absolute flux through glycolysis was significantly reduced (3.9-fold; Table 3), an effect that may be a consequence of METO’s effects on cardiac function (38). At 4 μM, a concentration at which METO had only marginal effects on functional parameters compared with controls (data not shown), we observed a small, albeit nonsignificant, decrease in the contribution of exogenous oleate to acetyl-CoA formation or glycolytic rate, whereas the contribution of glucose to pyruvate and acetyl-CoA was unchanged (Table 3).
Fig. 4.
Impact of 20 and 40 μM metoprolol on heart rate (A), contractility (B), cardiac output and coronary flow (C), and stroke volume (D) of isolated working C57Bl/6 mouse hearts. Data are expressed as means ± SE of 3 hearts. $P < 0.05; $$P < 0.01; $$$P < 0.001 vs. t = 10 min.
Table 3.
Metabolic flux parameters assessed in working C57Bl/6 mouse hearts perfused in the presence of METO
| Metabolic Flux Parameters | METO, 4 μM | METO, 40 μM |
|---|---|---|
| Glycolysis, μmol/min | 0.45 ± 0.09 | 0.17 ± 0.07*** |
| Glucose to acetyl-CoA: PDCGlc/CS (%) | 29.2 ± 4.1 | 23.9 ± 8.0 |
| Oleate to acetyl-CoA: OLE/CS (%) | 15.8 ± 2.9 | ND |
Data are expressed as means ± SE of 3–5 hearts. METO, metoprolol; PDCGlc, glucose via pyruvate decarboxylation; OLE, oleate via β-oxidation; CS, citrate synthesis; ND, not determined.
P < 0.001 vs. controls reported in Fig. 2.
DISCUSSION
This study was undertaken to evaluate the metabolic impact of IVA, the first clinical available drug that selectively inhibits the major pacemaker current If in the sinoatrial node and leads to specific HRR. Specifically, we examined substrate fluxes through major energy-producing pathways as well as the energy status using our well-established model of healthy normoxic working mouse hearts perfused ex vivo with 13C-labeled substrates with continuous monitoring of functional parameters (25, 48). It mimics to some extent the conditions prevailing in patients following heart transplant for which IVA was recently proposed to be of some benefit (16). With this model, we assessed the effects of IVA on heart function and metabolism in the absence of confounding external neuronal or hormonal influences and with no change in peripheral resistance (which is fixed by the buffer volume of the arterial compliance chamber). Healthy mouse hearts were perfused under physiologically relevant conditions with respect to substrate supply (mixtures of CHOs and a LCFA bound to albumin) and energy demand (fixed preload of 15 mmHg and afterload of 50 mmHg).
The concentrations of IVA that have been used in many animal investigations in vivo (0.6 mg/kg) and in vitro (3 μM) were aimed at achieving the clinical target of 20% HRR (8, 20). In our ex vivo heart model, we have used 3 μM IVA and achieved a 35% HRR. As a whole, our data concur with previous studies in demonstrating that IVA increases stroke volume, most likely due to prolonged diastole and increased left ventricular filling. Other measured functional parameters were not significantly affected by IVA. The observed effects of IVA on stroke volume and myocardial perfusion were apparently the direct consequence of the effect on HRR, since they were reversed to normal by pacing. The effect of IVA on coronary perfusion may explain some of the benefits of IVA under ischemic conditions (8, 47), since improving cardiomyocyte oxygenation may reduce the risk of functional electro-physiological heterogeneity, a predictor of channel dysfunction and action potential duration (23, 42).
Consistent with the increased stroke volume and myocardial perfusion when expressed per beat, IVA also increased MV̇O2 per beat, whereas it had no impact on MV̇O2 per minute. This is most likely because of the increase in left ventricular cavity volume, due to the prolonged diastole, which, according to the Frank-Starling law, would increase left ventricular wall stress and hence MV̇O2. In contrast, HRR is known to reduce MV̇O2 in heart failure (35). It is important to consider that the isolated denervated heart is perfused with a crystalline buffer, which has a lower oxygen carrying capacity than blood, at fixed pre- and afterload pressures. In this ex vivo model, coronary flow and MV̇O2 vary linearly in response to changes in afterloads. These conditions differ from those that prevail in vivo, where the heart is also controlled by the sympathetic and parasympathetic systems, which play an important role in vivo and might be activated in patients with coronary artery disease and result in enhanced myocardial MV̇O2 prior to IVA administration.
At the metabolic level, our relative flux data obtained using our ex vivo heart perfusion model demonstrate that, at a dose reducing the heart rate by 35% (3 μM), IVA has no impact on substrate selection for energy production, the rate of glycolysis (expressed per minute), or the myocardial levels of high-energy phosphates. It is noteworthy that the ATP production rates in the cytosol (from the measured glycolytic rates) and in the mitochondria [from the measured MV̇O2 assuming a theoretical ATP/O of 2.83 (25)] did not differ between groups when expressed per minute. However, when expressed per beat, these rates were significantly higher for the IVA-treated group (Fig. 3), concurring with our finding of an increased stroke volume and supporting an effective matching of energy supply and demand per beat. In our study, the application of pacing to working heart with IVA abolished all functional effects observed with IVA alone. This finding concurs with that of others obtained in various study models both in vivo and ex vivo (8, 9, 11).
Because IVA belongs to a new class of drugs that reduce HR by a direct action on the sinoatrial node, we wanted to compare its effects with that of a β-blocker, which is the first line choice for HRR. We have attempted to match the effects of IVA to that of the widely used β-blocker METO using concentrations ranging from 4 to 40 μM on the basis of previous studies (13, 49). However, we found only marginal, nonsignificant HRR effects under our experimental conditions, i.e., in the isolated healthy working heart perfused under normoxic conditions. In contrast, METO adversely affected cardiac function, including a large reduction of contractility and cardiac output, as well as irregular beating, demonstrating that β-blockers are important regulators of the myocardium even in our explanted heart model. At the metabolic level, our finding of a significantly reduced glycolytic rate with 40 μM METO concurs with that of Sharma et al. (38). As was pointed out by those authors, because cardiac metabolism is driven by cardiac function, one cannot exclude that METO’s effects on cardiac substrate metabolism may be a consequence of its effects on cardiac function. In support of this notion, we found only marginal, nonsignificant effects of METO both on cardiac function and on metabolic fluxes, including glycolysis as well as oleate and glucose contribution to acetyl-CoA production, when applied at 4 μM METO.
To understand the marked difference between the observed effects of IVA and METO on HR, contractility, and cardiac flow in our ex vivo heart model, it is important to consider that these effects are exerted under basal conditions specifically on the healthy normoxic mouse heart, which is devoid of any external neuronal influences, except for a local release of norephinephrine from endogenous neuronal stores. Although epinephrine is routinely added to the perfusate buffer at a physiological concentration [i.e., 5 nM (25)], it is unlikely that the parasympathetic system, which appears to be relevant for HR control in mice (44), would be optimally active. Nevertheless, IVA remains effective in this ex vivo condition because it exerts its HRR effects by directly acting on the pacemaker current in the sinoatrial node, and more importantly, this occurs without affecting contractility or any of the measured metabolic parameters ex vivo. It is noteworthy that results obtained with IVA represent the first demonstration that pure HRR, i.e., independent of inotropy, does not impact on cardiac energy substrate metabolism under basal conditions.
As for β-blockers, to the best of our knowledge, the dose-dependent acute effects of these drugs on cardiac flows and contractility (decreased) vs. HR (unaffected) in isolated healthy hearts perfused ex vivo at physiological pre- and afterload under normoxic conditions have not previously been reported. Previous studies have examined the effect of a selected dose of β-blocker in nonworking Langendorff-perfused hearts under stress conditions (31, 40), except for that of Sharma et al. (38), which was conducted in the working rat heart, but functional parameters were not reported. METO, which was selected because it is one of the most commonly used β-blocker in clinical practice, is a specific β1-adrenergic receptor antagonist. Given the higher ratio of β1- to β2-receptors in the myocardium (~4) than in the sinus node (36), admittedly, one could have probably anticipated that inhibition of β1-receptors, with consequent lowering of cAMP production, would have a greater impact on contractility than HR. Nevertheless, we believe that our observations using METO are valuable from a mechanistic point of view in the specific context of the present study, where its effects are compared with those of IVA. Collectively, our results concur with in vivo studies where IVA, but not β-blockers used at clinically relevant doses, was found to be effective for HRR under basal conditions in healthy animals (1, 10, 19). However, it is noteworthy that both classes of drugs have been shown to be effective at reducing HR in vivo under conditions of increased HR. These include, for example, sympathetic stimulation and exercise as well as pathological conditions under which these drugs are normally prescribed in the clinic (1, 14, 19, 24).
With regard to the potential clinical relevance of this study, the lack of information in the literature about the impact of HRR per se on cardiac energy substrate metabolism created the impetus to characterize the acute effect of pure HRR by IVA in the isolated normoxic working healthy heart prior to its chronic effect being evaluated in a clinically relevant disease model for which potentially confounding factors such as cardiac gene remodeling will need to be considered (33, 41). Despite the limitations of our study model, such as the basal conditions, absence of neuronal influence, and fixed compliance, we believe that this experimental model mimics to some extent the human transplanted heart, a condition for which IVA has been shown to be beneficial for the control of HR (16, 27).
Collectively, our results demonstrate that acute administration of IVA to healthy normoxic working hearts perfused ex vivo with a mixture of substrates mimicking the in situ milieu induces selective HRR, while preserving cardiac substrate selection for energy production, as well as optimal matching of energy supply and demand. Indeed, beyond HRR the only functional changes that were observed following IVA addition (increased stroke volume and coronary flow when expressed per beat) were reversed by pacing, thereby providing evidence that these effects were a direct consequence of HRR. Our results provide the impetus for testing the impact of IVA on energy substrate metabolism in pathological animal models of atherosclerosis or diabetic cardiomyopathy that display metabolic alterations, such as a shift in substrate selection, impaired glycolysis, and/or decreased tissue levels of high-energy phosphates (33, 41).
Acknowledgments
We thank Drs. O. Bouchot and R. Debin for their insightful comments and Dr. Jerzy Kulpa for excellent technical assistance in HPLC analyses. We thank Antoinette Paolitto for secretarial assistance.
GRANTS
This study was supported by an educational grant from Servier (France; to C. Des Rosiers, E. Thorin, and J. C. Tardif) as well as by fellowships from the “Fonds de la recherche en santé du Québec” and from the Fondation Betten-court Schueller (to B. Lauzier) and studentships from the Canadian Institutes of Health Research and the Department of Nutrition, Université de Montréal (to R. Gélinas).
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Abbott JA, Broadstone RV, Ward DL, Pyle RL. Hemodynamic effects of orally administered carvedilol in healthy conscious dogs. Am J Vet Res. 2005;66:637–641. doi: 10.2460/ajvr.2005.66.637. [DOI] [PubMed] [Google Scholar]
- 2.Allard MF, Parsons HL, Saeedi R, Wambolt RB, Brownsey R. AMPK and metabolic adaptation by the heart to pressure overload. Am J Physiol Heart Circ Physiol. 2007;292:H140–H148. doi: 10.1152/ajpheart.00424.2006. [DOI] [PubMed] [Google Scholar]
- 3.An D, Kewalramani G, Qi D, Pulinilkunnil T, Ghosh S, Abrahani A, Wambolt R, Allard M, Innis SM, Rodrigues B. β-Agonist stimulation produces changes in cardiac AMPK and coronary lumen LPL only during increased workload. Am J Physiol Endocrinol Metab. 2005;288:E1120–E1127. doi: 10.1152/ajpendo.00588.2004. [DOI] [PubMed] [Google Scholar]
- 4.Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, Raskin P, Wright JT, Jr, Oakes R, Lukas MA, Anderson KM, Bell DS GEMINI Investigators. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292:2227–2236. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- 5.Bangalore S, Kamalakkannan G, Messerli FH. Beta-blockers: no longer an option for uncomplicated hypertension. Curr Cardiol Rep. 2007;9:441–446. doi: 10.1007/BF02938387. [DOI] [PubMed] [Google Scholar]
- 6.Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–894. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 7.Borer JS, Tardif JC. Efficacy of ivabradine, a selective I(f) inhibitor, in patients with chronic stable angina pectoris and diabetes mellitus. Am J Cardiol. 2010;105:29–35. doi: 10.1016/j.amjcard.2009.08.642. [DOI] [PubMed] [Google Scholar]
- 8.Ceconi C, Cargnoni A, Francolini G, Parinello G, Ferrari R. Heart rate reduction with ivabradine improves energy metabolism and mechanical function of isolated ischaemic rabbit heart. Cardiovasc Res. 2009;84:72–82. doi: 10.1093/cvr/cvp158. [DOI] [PubMed] [Google Scholar]
- 9.Colin P, Ghaleh B, Hittinger L, Monnet X, Slama M, Giudicelli JF, Berdeaux A. Differential effects of heart rate reduction and β-blockade on left ventricular relaxation during exercise. Am J Physiol Heart Circ Physiol. 2002;282:H672–H679. doi: 10.1152/ajpheart.00547.2001. [DOI] [PubMed] [Google Scholar]
- 10.Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exp Ther. 2004;308:236–240. doi: 10.1124/jpet.103.059717. [DOI] [PubMed] [Google Scholar]
- 11.Colin P, Ghaleh B, Monnet X, Su J, Hittinger L, Giudicelli JF, Berdeaux A. Contributions of heart rate and contractility to myocardial oxygen balance during exercise. Am J Physiol Heart Circ Physiol. 2003;284:H676–H682. doi: 10.1152/ajpheart.00564.2002. [DOI] [PubMed] [Google Scholar]
- 12.Cruickshank JM. Are we misunderstanding beta-blockers. Int J Cardiol. 2007;120:10–27. doi: 10.1016/j.ijcard.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 13.Di Verniero C, Hocht C, Opezzo JA, Taira CA. Changes in the in vitro pharmacodynamic properties of metoprolol in atria isolated from spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2007;34:161–165. doi: 10.1111/j.1440-1681.2007.04566.x. [DOI] [PubMed] [Google Scholar]
- 14.Di Verniero CA, Silberman EA, Mayer MA, Opezzo JA, Taira CA, Hocht C. In vitro and in vivo pharmacodynamic properties of metoprolol in fructose-fed hypertensive rats. J Cardiovasc Pharmacol. 2008;51:532–541. doi: 10.1097/FJC.0b013e3181730306. [DOI] [PubMed] [Google Scholar]
- 15.DiFrancesco D, Borer JS. The funny current: cellular basis for the control of heart rate. Drugs. 2007;67(Suppl 2):15–24. doi: 10.2165/00003495-200767002-00003. [DOI] [PubMed] [Google Scholar]
- 16.Doesch AO, Ammon K, Konstandin M, Celik S, Kristen A, Frankenstein L, Buss S, Hardt S, Sack FU, Katus HA, Dengler TJ. Heart rate reduction for 12 mo with ivabradine reduces left ventricular mass in cardiac allograft recipients. Transplantation. 2009;88:835–841. doi: 10.1097/TP.0b013e3181b4e0f5. [DOI] [PubMed] [Google Scholar]
- 17.Elbeery JR, Lucke JC, Feneley MP, Maier GW, Owen CH, Lilly RE, Savitt MA, Hickey MS, Gall SA, Jr, Davis JW. Mechanical determinants of myocardial oxygen consumption in conscious dogs. Am J Physiol Heart Circ Physiol. 1995;269:H609–H620. doi: 10.1152/ajpheart.1995.269.2.H609. [DOI] [PubMed] [Google Scholar]
- 18.Fardoun RZ. Carvedilol versus cardioselective beta-blockers for the treatment of hypertension in patients with type 2 diabetes mellitus. Pharmacotherapy. 2006;26:1491–1500. doi: 10.1592/phco.26.10.1491. [DOI] [PubMed] [Google Scholar]
- 19.Gordon SG, Arsenault WG, Longnecker M, Boothe DM, Miller MW, Chalkley J. Pharmacodynamics of carvedilol in conscious, healthy dogs. J Vet Intern Med. 2006;20:297–304. doi: 10.1892/0891-6640(2006)20[297:pocich]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Heusch G. Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: benefit from selective bradycardic agents. Br J Pharmacol. 2008;153:1589–1601. doi: 10.1038/sj.bjp.0707673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heusch G. Pleiotropic action(s) of the bradycardic agent ivabradine: cardiovascular protection beyond heart rate reduction. Br J Pharmacol. 2008;155:970–971. doi: 10.1038/bjp.2008.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heusch G, Skyschally A, Gres P, van Caster P, Schilawa D, Schulz R. Improvement of regional myocardial blood flow and function and reduction of infarct size with ivabradine: protection beyond heart rate reduction. Eur Heart J. 2008;29:2265–2275. doi: 10.1093/eurheartj/ehn337. [DOI] [PubMed] [Google Scholar]
- 23.Indolfi C, Guth BD, Miyazaki S, Miura T, Schulz R, Ross J., Jr Heart rate reduction improves myocardial ischemia in swine: role of interventricular blood flow redistribution. Am J Physiol Heart Circ Physiol. 1991;261:H910–H917. doi: 10.1152/ajpheart.1991.261.3.H910. [DOI] [PubMed] [Google Scholar]
- 24.Kalaycioglu S, Sinci V, Imren Y, Oz E. Metoprolol prevents ischemia-reperfusion injury by reducing lipid peroxidation. Jpn Circ J. 1999;63:718–721. doi: 10.1253/jcj.63.718. [DOI] [PubMed] [Google Scholar]
- 25.Khairallah M, Labarthe F, Bouchard B, Danialou G, Petrof BJ, Des Rosiers C. Profiling substrate fluxes in the isolated working mouse heart using 13C-labeled substrates: focusing on the origin and fate of pyruvate and citrate carbons. Am J Physiol Heart Circ Physiol. 2004;286:H1461–H1470. doi: 10.1152/ajpheart.00942.2003. [DOI] [PubMed] [Google Scholar]
- 26.Korvald C, Elvenes OP, Ytrebo LM, Sorlie DG, Myrmel T. Oxygen-wasting effect of inotropy in the “virtual work model”. Am J Physiol Heart Circ Physiol. 1999;276:H1339–H1345. doi: 10.1152/ajpheart.1999.276.4.H1339. [DOI] [PubMed] [Google Scholar]
- 27.Lage-Gallé E, Romero-Rodríguez N, Nevado-Portero J, Guisado-Rasco A, Sobrino-Márquez M, Machuca MG, Fernández-Quero M, Campos-Pareja A, Ballesteros-Pradas S, Martínez-Martínez A. Safety and effectiveness of ivabradine after cardiac transplantation. Transplant Proc. 2010;42:3191–3192. doi: 10.1016/j.transproceed.2010.05.125. [DOI] [PubMed] [Google Scholar]
- 28.Lama PJ. Systemic adverse effects of beta-adrenergic blockers: an evidence-based assessment. Am J Ophthalmol. 2002;134:749–760. doi: 10.1016/s0002-9394(02)01699-9. [DOI] [PubMed] [Google Scholar]
- 29.Lucke JC, Elbeery JR, Koutlas TC, Gall SA, Jr, D’Amico TA, Maier GW, Rankin JS, Glower DD. Effects of cardiac glycosides on myocardial function and energetics in conscious dogs. Am J Physiol Heart Circ Physiol. 1994;267:H2042–H2049. doi: 10.1152/ajpheart.1994.267.5.H2042. [DOI] [PubMed] [Google Scholar]
- 30.Milliez P, Messaoudi S, Nehme J, Rodriguez C, Samuel JL, Delcayre C. Beneficial effects of delayed ivabradine treatment on cardiac anatomical and electrical remodeling in rat severe chronic heart failure. Am J Physiol Heart Circ Physiol. 2009;296:H435–H441. doi: 10.1152/ajpheart.00591.2008. [DOI] [PubMed] [Google Scholar]
- 31.Momose M, Reder S, Raffel DM, Watzlowik P, Wester HJ, Nguyen N, Elsinga PH, Bengel FM, Remien J, Schwaiger M. Evaluation of cardiac beta-adrenoreceptors in the isolated perfused rat heart using (S)-11C-CGP12388. J Nucl Med. 2004;45:471–477. [PubMed] [Google Scholar]
- 32.Mulder P, Barbier S, Chagraoui A, Richard V, Henry JP, Lallemand F, Renet S, Lerebours G, Mahlberg-Gaudin F, Thuillez C. Long-term heart rate reduction induced by the selective I(f) current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109:1674–1679. doi: 10.1161/01.CIR.0000118464.48959.1C. [DOI] [PubMed] [Google Scholar]
- 33.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 34.Padua-Filho WC, Brasil DP, Neves HJ, Gomes OM, Bocchi EA. Effects of metoprolol and amiodarone combination on heart rate, myocardial contractility and coronary flow: Study in isolated perfused rat hearts. Exp Clin Cardiol. 2004;9:133–137. [PMC free article] [PubMed] [Google Scholar]
- 35.Palatini P. Elevated heart rate: a “new” cardiovascular risk factor? Prog Cardiovasc Dis. 2009;52:1–5. doi: 10.1016/j.pcad.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP, Jr, Barsh GS, Bernstein D, Kobilka BK. Targeted disruption of the mouse beta1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci USA. 1996;93:7375–7380. doi: 10.1073/pnas.93.14.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma V, Dhillon P, Parsons H, Allard MF, McNeill JH. Metoprolol represses PGC1alpha-mediated carnitine palmitoyltransferase-1B expression in the diabetic heart. Eur J Pharmacol. 2009;607:156–166. doi: 10.1016/j.ejphar.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Sharma V, Dhillon P, Wambolt R, Parsons H, Brownsey R, Allard MF, McNeill JH. Metoprolol improves cardiac function and modulates cardiac metabolism in the streptozotocin-diabetic rat. Am J Physiol Heart Circ Physiol. 2008;294:H1609–H1620. doi: 10.1152/ajpheart.00949.2007. [DOI] [PubMed] [Google Scholar]
- 39.Shigetoh Y, Adachi H, Yamagishi S, Enomoto M, Fukami A, Otsuka M, Kumagae S, Furuki K, Nanjo Y, Imaizumi T. Higher heart rate may predispose to obesity and diabetes mellitus: 20-year prospective study in a general population. Am J Hypertens. 2009;22:151–155. doi: 10.1038/ajh.2008.331. [DOI] [PubMed] [Google Scholar]
- 40.Skobel E, Dannmann O, Reffelmann T, Böhm V, Weber C, Hanrath P, Uretsky BF, Scharwz ER. Carvedilol protects myocardial cytoskeleton during hypoxia in the rat heart. J Appl Res. 2005;5:378–386. [Google Scholar]
- 41.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 42.Stoll M, Quentin M, Molojavyi A, Thamer V, Decking UK. Spatial heterogeneity of myocardial perfusion predicts local potassium channel expression and action potential duration. Cardiovasc Res. 2008;77:489–496. doi: 10.1093/cvr/cvm060. [DOI] [PubMed] [Google Scholar]
- 43.Suga H. Minimal oxygen consumption and optimal contractility of the heart: theoretical approach to principle of physiological control of contractility. Bull Math Biol. 1979;41:139–150. doi: 10.1007/BF02460874. [DOI] [PubMed] [Google Scholar]
- 44.Swoap SJ, Li C, Wess J, Parsons AD, Williams TD, Overton JM. Vagal tone dominates autonomic control of mouse heart rate at thermo-neutrality. Am J Physiol Heart Circ Physiol. 2008;294:H1581–H1588. doi: 10.1152/ajpheart.01000.2007. [DOI] [PubMed] [Google Scholar]
- 45.Tardif JC. Heart rate as a treatable cardiovascular risk factor. Br Med Bull. 2009;90:71–84. doi: 10.1093/bmb/ldp016. [DOI] [PubMed] [Google Scholar]
- 46.Thollon C, Cambarrat C, Vian J, Prost JF, Peglion JL, Vilaine JP. Electrophysiological effects of S 16257, a novel sinoatrial node modulator, on rabbit and guinea-pig cardiac preparations: comparison with UL-FS 49. Br J Pharmacol. 1994;112:37–42. doi: 10.1111/j.1476-5381.1994.tb13025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaillant F, Timour Q, Descotes J, Manati W, Belhani D, Bui-Xuan B, Tabib A, Bricca G, Chevalier P. Ivabradine induces an increase in ventricular fibrillation threshold during acute myocardial ischemia: an experimental study. J Cardiovasc Pharmacol. 2008;52:548–554. doi: 10.1097/FJC.0b013e3181913df4. [DOI] [PubMed] [Google Scholar]
- 48.Vincent G, Khairallah M, Bouchard B, Des Rosiers C. Metabolic phenotyping of the diseased rat heart using 13C-substrates and ex vivo perfusion in the working mode. Mol Cell Biochem. 2003;242:89–99. [PubMed] [Google Scholar]
- 49.Wang P, Zaragoza C, Holman W. Sodium-hydrogen exchange inhibition and beta-blockade additively decrease infarct size. Ann Thorac Surg. 2007;83:1121–1127. doi: 10.1016/j.athoracsur.2006.10.039. [DOI] [PubMed] [Google Scholar]