Abstract
Background and aims
Carotid sinus hypersensitivity (CSH) is a common cause of fainting and falls in the older adult population and is diagnosed by carotid sinus massage (CSM). Previous work has suggested that age-related stiffening of blood vessels reduces afferent input from the carotid sinus leading to central upregulation of the overall arterial baroreflex response. We examined the differences in arterial stiffness and baroreflex function in older adults at high cardiovascular risk (advanced age, Type 2 diabetes, hypertension and hyperlipidemia) with and without CSH.
Methods
Forty-three older adults (mean age 71.4±0.7) with Type 2 diabetes, hyperlipidemia and hypertension were recruited. After resting supine for 45 minutes prior to the start of data collection, each subject had arterial stiffness measured by pulse wave velocity (PWV, Complior SD), followed by spontaneous baroreflex measures (Baroreflex sensitivity, BRS) and CSM.
Results
Of the 43 subjects tested, 10 subjects met the criteria for CSH (8 pure vasodepressor and 2 mixed CSH). CSH subjects had higher measures of arterial stiffness when compared to normal subjects for both radial PWV (11.5±0.6 vs 9.6±0.4 m/s, p=0.043) and femoral PWV (13.4±0.9 vs 11.0±0.5 m/s, p=0.036). The CSH group demonstrated significantly lower BRS as compared to the normal group (BRS, 6.73±0.58 vs 10.41±0.85 ms/mmHg, p=0.038). These results were unchanged when the analysis was repeated with only the VD subjects.
Conclusions
Older adults with CSH have higher arterial stiffness and reduced arterial baroreflex sensitivity. There was no evidence to support upregulation of the arterial baroreflex in patients with CSH.
Keywords: Arterial baroreflex function, arterial stiffness, carotid sinus hypersensitivity, carotid sinus massage, geriatric medicine
INTRODUCTION
Carotid sinus hypersensitivity (CSH) is an important and frequently ignored etiology for fainting in the older adult population (1). CSH is present in 10% of all healthy older adults in the community, is present in 50% of older adults that present to the emergency department after a fall, and is considered a modifiable risk factor for hip fractures (2). If the carotid sinus is hypersensitive, a patient will respond to a normal upward swing in blood pressure with a temporary stopping of the heart (cardioinhibition, CI) and/or an inappropriate dilatation of the vasculature (vasodepression, VD). CSH is diagnosed by carotid sinus massage (CSM) while monitoring heart rate for cardioinhibition (pause in heart rate lasting longer than 3 seconds) and monitoring systolic blood pressure for vasodepression (a drop of greater than 50 mmHg) (1). CSH is classified into three types: pure vasodepressor, pure cardioinhibitory and mixed CSH (1).
The mechanisms underlying CSH remain uncertain, and previous research has postulated both central and local mechanisms (3). One puzzling aspect of this condition is the seemingly paradoxical fact that increasing age results in an increase in the prevalence of CSH (4) but a decrease in arterial baroreflex sensitivity in the general population (5). This has prompted some investigators to suggest renaming the condition “carotid sinus irritability” (6), but this merely labels as opposed to explain this phenomenon. Animal studies have suggested that age-related stiffening of blood vessels reduces afferent input from the carotid sinus (3) possibly leading to central upregulation of the overall arterial baroreflex response in CSH. An upregulated baroreflex could then result in an overshoot response leading to a fainting spell (7, 8). If the carotid sinus is indeed “irritable” due to central baroreflex upregulation then subjects with an exaggerated response to CSM should have both increased baroreflex sensitivity (since the baroreflex has been upregulated) and increased arterial stiffness, although this has never been formerly examined.
In the current study, we examine the relationship between arterial stiffness and the response (CI and VD) to CSM in subjects with multiple reasons for increased arterial stiffness (advanced age, Type 2 diabetes, hypertension and hyperlipidemia). We hypothesize that subjects demonstrating an exaggerated CSM response will demonstrate both higher levels of arterial stiffness and an elevated arterial baroreflex response.
METHODS
Subjects
Forty-five older adults (25 males and 20 females, mean age 71.4±0.7) were recruited ranging in age from 65 to 83. All subjects had to be over 65 years of age and were excluded if they had contraindications to CSM (cerebrovascular event within 3 months, myocardial infarction within 3 months, carotid bruit, having a past history of bradyarrhythmia, or having a past history of ventricular arrhythmia). In order to ensure the presence of a large group of subjects with elevated arterial stiffness in our study population, all older subjects were required to have Type 2 diabetes, hypertension and hyperlipidemia. Since Type 2 diabetes, hypertension and hypercholesterolemia are also risk factors for CSH, recruiting subjects with these conditions also allowed us to maximize subject recruitment as well as minimize subject heterogeneity. Hypertension, diabetes and hyperlipidemia were defined by current ADA guidelines (9). Hypertension was defined as taking antihypertensive agents or having an average blood pressure (based on three measurements) with a systolic blood pressure greater than 130 mmHg or a diastolic blood pressure greater than 80 mmHg (9). Subjects were excluded if they took beta-blockers, calcium channel blockers, or any other agent with the potential to influence autonomic function. Two subjects (1 male and 1 female) were excluded, leaving a total of 43 subjects.
This study was approved by the Human Subjects Committee of the University of British Columbia, and all subjects gave written informed consent.
Study design
Each subject attended a single study session which occurred between 7 a.m. and noon for all subjects to avoid bias due to circadian rhythms. Subjects refrained from the consumption of alcohol or caffeine or undergoing vigorous exercise for the 24 hours prior to each session. Subjects did not take any of their medications for 48 hours prior to each study session. Each subject was supine for 45 minutes prior to the start of data collection in order to reach steady state. After this supine rest each subject had arterial stiffness measured by pulse wave velocity (PWV), followed by spontaneous baroreflex measures and CSM. Both the subject and the investigator performing CSM were blinded to all measures.
Data collection and processing
Heart rate was monitored continuously using a 3 lead-electrocardiogram. Blood pressure was monitored using a Finometer (Finapres Medical Systems BV, The Netherlands). The Finometer measures beat-to-beat blood pressure noninvasively using infrared plethysmography through a finger-cuff. Use of the Finometer and infrared plethysmography for monitoring blood pressure changes has been well established as a noninvasive measure of beat-to-beat blood pressure (10), has been extensively validated against intra-arterial blood pressure monitoring in older adults (11) and is validated for the assessment of arterial baroreflex function (12). The Finometer uses waveform filtering, level correction and an additional return-to-flow calibration to reconstruct brachial artery pressures (13). The electrocardiogram and blood pressure signals were sampled at 1000 Hz (Powerlab, AD Instruments) and digitized for later analysis. Using commercially available software, beat-to-beat measures of blood pressure (Beatscope, Finapres Medical Systems BV, The Netherlands) and heart rate (Powerlab, AD Instruments) were calculated. All post-collection analyses of the CSM and arterial baroreflex function were done in a blinded fashion. Prior to all derived measurements, each segment of raw blood pressure and electrocardiogram was manually examined in order to exclude artifacts.
Carotid sinus massage
CSM was performed after 20 minutes supine rest while continuously monitoring heart rate and blood pressure. Landmarks for the carotid sinus consisted of the midpoint between the temporalmandibular joint and the top of the thyroid cartilage (1). As per current guidelines, CSM was performed first on one and then on the other carotid sinus, with a positive on either side indicating hypersensitivity (14). Previous work has shown that CSM is quite reproducible (15), and that the sensitivity increases in the upright position (16). The massage was performed for 5 seconds while the subject was tilted upright on a tilt table at 70 degrees (16) as per previous CSS studies (2). The vasodepressor (VD) and cardioinhibitory (CI) response to CSM was defined as the maximum change in systolic blood pressure and RR interval observed during both massages. We used the standard definition of CSH which is the presence of asystole exceeding 3 seconds or a fall in systolic blood pressure exceeding 50 mmHg during CSM (2). Each CSM was performed by the same investigator, who was blinded to the magnitude of the VD and CI responses, but was obviously not blinded to the presence of any symptoms of syncope (occurred in one subject only).
Measures of arterial stiffness
Subject conditions were standardized according to established guidelines: all measures were performed prior to tilt table testing and after 30 minutes supine rest; the environment was quiet and temperature controlled; and all subjects were fasting (17). Arterial stiffness was measured using the Complior device (Artech Medical, Pantin, France), a semi-automated device that uses two pressure transducers (17). The pressure transducers are held in place by velcro straps that allow them be fixed over the skin. Each pressure transducer measures the pulse waveform at each site, allowing one to measure transit time of the pulse wave between the two locations. The transducers are placed over the carotid and femoral arteries for a measure of central arterial stiffness and over the carotid and radial arteries for a measure of peripheral arterial stiffness (17). Pulse wave velocity was calculated from these transducer measures which are digitally recorded (sampling rate 500 Hz).
Arterial baroreflex function
Arterial baroreflex function was measured by the sequence method, which provides a measure of baroreflex sensitivity (BRS, in ms/mmHg) as previously described (18). 20 minutes of electrocardiogram and Finometer data was examined using a custom-written software program (Matlab Release 13, 2008) for progressive increases/decreases in both systolic blood pressure (SBP) and RR-interval (RRI). BRS is defined as the mean slope of the regression lines for all these baroreflex-mediated sequences (+RRI/+SBP or −RRI/−SBP) and is measured in ms/mmHg (19). Parameters used for our spontaneous baroreflex analysis were the inclusion of all baroreflex-mediated sequences of 3 or more beats that had a correlation coefficient greater than 0.80, a threshold of blood pressure change of 1 mmHg, and a threshold for change in RR-interval of 4 milliseconds (19). The BRS (20) has been used previously to examine baroreflex function in older adult subjects and is well validated against traditional measures such as the Oxford method (19), and has high intrasubject reproducibility (20).
Statistical analysis
All data analysis was done in a blinded fashion. Results are expressed as the mean ± standard error. Significant differences with respect to arterial stiffness and arterial baroreflex function between groups (CSH group vs normals) were determined using the 1-way analysis of variance (21). Spearman correlation was performed to determine correlation between any of the parameters (21). A value of p≤0.05 was considered significant (21).
RESULTS
Subject characteristics
Of the 43 subjects tested, 10 subjects met the criteria for CSH (8 pure vasodepressor and 2 mixed) and 33 had normal responses to CSM. Only one of the CSH subjects had symptoms (lost consciousness) during CSM, and this was due to a period of asystole of 4.0 seconds. As shown in Table 1, there was no significant difference between CSH and normal subjects with respect to demographic data, resting heart rate, resting blood pressure, fasting blood sugar, glycosylated hemoglobin or lipid profile (See Table 1).
Table 1.
Subjects’ characteristics (mean ± standard error).
| Measure | All subjects | CSH subjects | Normal subjects | p-value |
|---|---|---|---|---|
| Age (yrs) | 71.4±0.7 | 71.7±1.1 | 72.0±0.9 | 0.37 |
| Weight (kg) | 82.8±2.3 | 81.1±4.3 | 84.1±2.5 | 0.54 |
| Height (cm) | 166.7±1.9 | 166.4±2.1 | 167.0±2.9 | 0.28 |
| Body Mass Index (kg/m2) | 29.5±0.7 | 29.4±1.2 | 29.6±0.8 | 0.91 |
| Blood pressure (mmHg) | ||||
| Systolic | 137±4 | 136±5 | 138±5 | 0.82 |
| Diastolic | 70±2 | 69±3 | 72±2 | 0.40 |
| Mean | 96±2 | 95±4 | 97±3 | 0.65 |
| Heart rate (beats per minute) | 66.2±1.8 | 67.2±3.6 | 65.4±1.7 | 0.63 |
| Fasting blood glucose (mEq) | 7.7±0.3 | 7.8±0.5 | 7.7±0.4 | 0.86 |
| Glycosylated hemoglobin (%) | 6.7±0.2 | 6.9±0.2 | 6.6±0.2 | 0.44 |
| Lipid profile (mmol/L) | ||||
| Total cholesterol | 5.0±0.2 | 4.7±0.3 | 5.1±0.3 | 0.27 |
| LDL cholesterol | 2.6±0.2 | 2.3±0.2 | 2.8±0.2 | 0.16 |
| HDL cholesterol | 1.6±0.1 | 1.5±0.1 | 1.7±0.2 | 0.21 |
A p-value <0.05 was considered significant. CSH: Carotid Sinus Hypersensitivity; LDL: Low-density lipoprotein; HDL: High-density lipoprotein.
CSM vs normal subjects
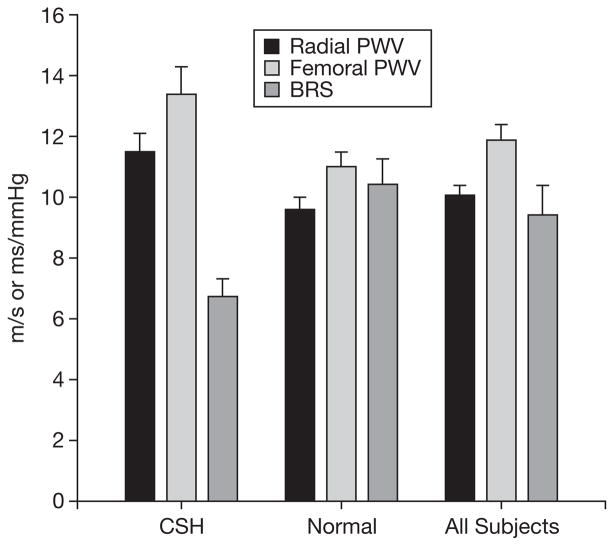
As shown in Figure 1, subjects that met the criteria for CSH had significantly higher measures of arterial stiffness when compared to normal subjects for both radial PWV (11.5±0.6 vs 9.6±0.4 m/s, p=0.043) and femoral PWV (13.4±0.9 vs 11.0±0.5 m/s, p=0.036). These results were essentially unchanged when the analysis was repeated using only the VD subjects for both radial PWV (11.4±0.8 vs 9.3±0.4 m/s, p=0.048) and femoral PWV (12.9±1.0 vs 10.5±0.5 m/s, p=0.041).
Fig. 1.
Measures of arterial stiffness and baroreflex function.
As demonstrated in Figure 1, the CSH group demonstrated significantly lower arterial baroreflex sensitivity as compared to the normal group (BRS, 6.73±0.58 vs 10.41±0.85 ms/mmHg, p=0.038). This was accompanied by a higher number of detectible (a sequence of 3 consecutive upward or downward swings of more than 1 mmHg) swings in blood pressure during 20 minutes supine rest in the CSH group as compared to normals (465±55 vs 348±18 blood pressure swings, p=0.013). These results were essentially unchanged when the analysis was repeated using only the VD subjects for both BRS (7.4±0.3 vs 11.2±0.8 m/s, p=0.048) and blood pressure swings (451±57 vs 338±21 m/s, p=0.037).
Correlations
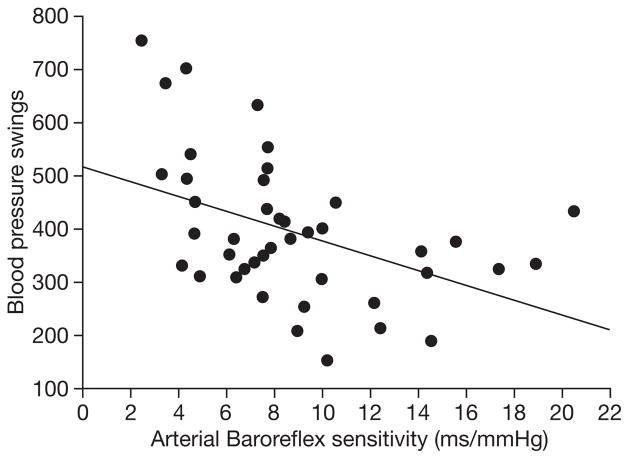
As expected, PWV measured at the radial artery correlated with femoral measures of arterial stiffness (r=0.53, p<0.001). Radial PWV (r=0.21, p=0.19) and femoral PWV (r=0.21, p=0.18) did not significantly correlate with the CI response during CSM. The VD response with CSM did not correlate with either radial PWV (r=0.23, p=0.14) or femoral PWV (r=0.14, p=0.36). BRS showed no significant correlation with the CI response (r=−0.12, p=0.44) or the VD response (r=0.27, p=0.09) during CSM. As shown in Figure 2, BRS showed a significant negative correlation with the number of significant spontaneous swings in blood pressure detected during supine rest (r=−0.42, p=0.01).
Fig. 2.
Baroreflex sensitivity and blood pressure swings at rest.
DISCUSSION
We found that subjects that meet the criteria for vasodepressor CSH have greater arterial stiffness (as measured by both radial and femoral PWV) as compared to normal subjects. Contrary to our hypothesis, arterial baroreflex dysfunction was also demonstrated in CSH subjects, as shown by lower BRS and an increased number of blood pressure swings during supine rest. To our knowledge, this is the first demonstration of the co-existence of reduced resting arterial baroreflex sensitivity and increased responses to CSM in this condition.
Cardiovascular risk factors (age, Type 2 diabetes, hyperlipidemia and hypertension) have been associated with both the increased prevalence of CSH (4) and an overall reduction in arterial baroreflex sensitivity (5). Although this seems like a contradiction, some have postulated central upregulation of the arterial baroreflex in response to reduced afferent input from the stiff carotid sinus as a potential explanation (22). Animal studies have shown that age-related stiffening of blood vessels reduces signal input from the carotid sinus (3). This drop in afferent input could potentially result in upregulation of alpha-1 receptors which are located in the medulla oblongata (7, 8). Stimulation of central alpha-1 receptors normally results in a drop in heart rate and vasodilatation (due to increased parasympathetic and decreased sympathetic tone) similar to that seen during a positive CSM (8). An upregulation of alpha-1 receptors in response to an age-related drop in afferent input (23) may be resulting in an overshoot response and consequent syncopal spells (3, 22) in this condition. However, central upregulation as an explanation for CSH was disproven by the results of our study; we demonstrated a reduction in BRS in subjects with CSH, suggesting that other mechanisms are responsible for this condition.
What other mechanisms could explain the co-existence of CSH, increased arterial stiffness and a baseline reduction in BRS? Previous investigations have hypothesized alternative peripheral etiologies for CSH that do not invoke central upregulation of the arterial baroreflex (3). Although histological data has been unable to support any localized damage or alterations to the arterial baroreceptor cells themselves (24), other investigations have demonstrated abnormal sternocleidomastoid electromyographic results in CSH patients (3). The sternocleidomastoid provides proprioceptive input during neck movements and abnormalities in these responses might contribute to CSH pathogenesis. Tea et al. postulated that neck movements might result in extravascular mechanical stimulation of the carotid baroreceptors, requiring appropriate proprioceptive input from the sternocleidomastoid to inhibit the arterial baroreflex. A lack of appropriate proprioceptive input in subjects with CSH could result in abnormally high BRS during neck movements (3), resulting in overshoot responses and syncope. The results of the present study are congruent with these findings, especially if we hypothesize that arterial stiffness is a risk factor for proprioceptive denervation of the sternocleidomastoid. Although the etiology of terminal sternocleidomastoid denervation has never been formally examined, vascular disease has been shown to result in muscle denervation in the setting of peripheral vascular disease (25). If the chronic terminal denervation of the sternocleidomastoid in CSH subjects demonstrated by Tea et al. (3) were due to microvascular damage, this could explain the co-existence of increased arterial stiffness (and a consequent baseline decrease in BRS) and hypersensitivity to CSM. However, these results are not necessarily applicable to the results of our study, since the subjects in Tea et al. had pure cardioinhibitory CSH requiring treatment with a pacemaker, while the subjects in our study mainly demonstrated pure vasodepressor responses to CSM (3).
Limitations
We were not able to demonstrate in all subjects (both normal and CSH) a correlation between measures of arterial stiffness and the response (VD or CI) to CSM. Although CSM is the most well-established technique to screen for this condition (1), it offers no guarantee of reproducible external pressures on the carotid sinus as compared to less common techniques to externally evaluate carotid sinus function (26). Inter-subject variation in external pressure means that this lack of correlation is to be expected due to limitations in the CSM technique itself. We also were only able to comment on the differences in baroreflex sensitivity and arterial stiffness in patients with pure vasodepressor CSM, since only 2 of our subjects met the criteria for the mixed (both CI and VD) and none met the criteria for the pure cardioinhibitory subtype of this condition.
Clinical significance
Vasodepressive CSM is a common cause of morbidity and mortality due to falls in the older adult population (1) whose pathogenesis is unknown and, not surprisingly, has no known treatment (27). We have shown that one possible predictor for this condition is an increase in arterial stiffness due to cardiovascular risk factors (age, Type 2 diabetes, hyperlipidemia, hypertension). There are several known pharmacological (28) and lifestyle (29) therapies for decreasing arterial stiffness, but their possible role in treating CSH needs further investigation.
CONCLUSION
Older adults with multiple cardiovascular risk factors (Type 2 diabetes, hyperlipidemia, hypertension and increased age) and vasodepressor CSH simultaneously show increased arterial stiffness and reduced baseline arterial baroreflex sensitivity. There was no evidence to support upregulation of the arterial baroreflex in patients with CSH.
Acknowledgments
This research was supported by the Canadian Institutes of Health Research as well as the Academic Enhancement Fund (Department of Medicine, University of British Columbia).
References
- 1.Kenny RA, Kalaria R, Ballard C. Neurocardiovascular instability in cognitive impairment and dementia. Ann NY Acad Sci. 2002;977:183–95. doi: 10.1111/j.1749-6632.2002.tb04816.x. [DOI] [PubMed] [Google Scholar]
- 2.Kenny RA, Richardson DA, Steen N, et al. Carotid sinus syndrome: a modifiable risk factor for nonaccidental falls in older adults (SAFE PACE) J Am Coll Cardiol. 2001;38:1491–6. doi: 10.1016/s0735-1097(01)01537-6. [DOI] [PubMed] [Google Scholar]
- 3.Tea SH, Mansourati J, L’Heveder G, et al. New insights into the pathophysiology of carotid sinus syndrome. Circulation. 1996;93:1411–6. doi: 10.1161/01.cir.93.7.1411. [DOI] [PubMed] [Google Scholar]
- 4.Lord SR, Clark RD, Webster IW. Physiological factors associated with falls in an elderly population. J Am Geriatr Soc. 1991;39:1194–200. doi: 10.1111/j.1532-5415.1991.tb03574.x. [DOI] [PubMed] [Google Scholar]
- 5.Hunt BE, Farquhar WB, Taylor JA. Does reduced vascular stiffening fully explain preserved cardiovagal baroreflex function in older, physically active men? Circulation. 2001;103:2424–7. doi: 10.1161/01.cir.103.20.2424. [DOI] [PubMed] [Google Scholar]
- 6.Cole CR, Zuckerman J, Levine BD. Carotid sinus “irritability” rather than hypersensitivity: a new name for an old syndrome? Clin Auton Res. 2001;11:109–13. doi: 10.1007/BF02322054. [DOI] [PubMed] [Google Scholar]
- 7.Gillis RA, Gatti PJ, Quest JA. Mechanism of the antihypertensive effect of alpha 2-agonists. J Cardiovasc Pharmacol. 1985;7 (Suppl 8):S38–44. [PubMed] [Google Scholar]
- 8.Ruffolo RR, Jr, Hieble JP. Alpha-adrenoceptors. Pharmacol Ther. 1994;61:1–64. doi: 10.1016/0163-7258(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 9.ADA. Clinical Practice Recommendations 2005. Diabetes Care. 2005;28 (Suppl 1):S1–79. doi: 10.2337/diacare.28.suppl_1.s1. [DOI] [PubMed] [Google Scholar]
- 10.Imholz BP, Wieling W, van Montfrans GA, et al. Fifteen years experience with finger arterial pressure monitoring: assessment of the technology. Cardiovasc Res. 1998;38:605–16. doi: 10.1016/s0008-6363(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 11.Rongen GA, Bos WJ, Lenders JW, et al. Comparison of intra-brachial and finger blood pressure in healthy elderly volunteers. Am J Hypertens. 1995;8:237–48. doi: 10.1016/0895-7061(94)00000-2. [DOI] [PubMed] [Google Scholar]
- 12.Omboni S, Parati G, Frattola A, et al. Spectral and sequence analysis of finger blood pressure variability. Comparison with analysis of intra-arterial recordings. Hypertension. 1993;22:26–33. doi: 10.1161/01.hyp.22.1.26. [DOI] [PubMed] [Google Scholar]
- 13.Guelen I, Westerhof BE, Van Der Sar GL, et al. Finometer, finger pressure measurements with the possibility to reconstruct brachial pressure. Blood Press Monit. 2003;8:27–30. doi: 10.1097/00126097-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Kumar NP, Thomas A, Mudd P, et al. The usefulness of carotid sinus massage in different patient groups. Age Ageing. 2003;32:666–9. doi: 10.1093/ageing/afg114. [DOI] [PubMed] [Google Scholar]
- 15.Richardson DA, Bexton R, Shaw FE, et al. How reproducible is the cardioinhibitory response to carotid sinus massage in fallers? Europace. 2002;4:361–4. doi: 10.1053/eupc.2002.0264. [DOI] [PubMed] [Google Scholar]
- 16.Parry SW, Richardson DA, O’Shea D, et al. Diagnosis of carotid sinus hypersensitivity in older adults: carotid sinus massage in the upright position is essential. Heart. 2000;83:22–3. doi: 10.1136/heart.83.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent S, Kingwell B, Bank A, et al. Clinical applications of arterial stiffness: therapeutics and pharmacology. Am J Hypertens. 2002;15:453–8. doi: 10.1016/s0895-7061(01)02329-9. [DOI] [PubMed] [Google Scholar]
- 18.Madden KM, Tedder G, Lockhart C, et al. Oral glucose tolerance test reduces arterial baroreflex sensitivity in older adults. Can J Physiol Pharmacol. 2008;86:71–7. doi: 10.1139/y07-126. [DOI] [PubMed] [Google Scholar]
- 19.Bertinieri G, di Rienzo M, Cavallazzi A, et al. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl. 1985;3:S79–81. [PubMed] [Google Scholar]
- 20.Johnson P, Shore A, Potter J, et al. Baroreflex sensitivity measured by spectral and sequence analysis in cerebrovascular disease: methodological considerations. Clin Auton Res. 2006;16:270–5. doi: 10.1007/s10286-006-0351-6. [DOI] [PubMed] [Google Scholar]
- 21.Dawson-Saunders B, Trapp RG. Basic and clinical biostatistics. Toronto: Prentice Hall of Canada; 1994. p. 343. [Google Scholar]
- 22.O’Mahony D. Pathophysiology of carotid sinus hypersensitivity in elderly patients. Lancet. 1995;346:950–2. doi: 10.1016/s0140-6736(95)91563-x. [DOI] [PubMed] [Google Scholar]
- 23.Raskind MA, Peskind ER, Holmes C, et al. Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer’s disease. Biol Psychiatry. 1999;46:756–65. doi: 10.1016/s0006-3223(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 24.Smith HL, Moerrsch FD. Further study on the hypersensitive carotid reflex. Mayo Clin Proc. 1935;11:380–3. [Google Scholar]
- 25.England JD, Regensteiner JG, Ringel SP, et al. Muscle denervation in peripheral arterial disease. Neurology. 1992;42:994–9. doi: 10.1212/wnl.42.5.994. [DOI] [PubMed] [Google Scholar]
- 26.Kardos A, Rau H, Greenlee MW, et al. Comparison of two mechanical carotid baroreceptor stimulation techniques. Acta Physiol Hung. 1995;83:21–33. [PubMed] [Google Scholar]
- 27.Brignole M, Alboni P, Benditt DG, et al. Guidelines on management (diagnosis and treatment) of syncope - update 2004. Europace. 2004;6:467–537. doi: 10.1016/j.eupc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 28.McEniery CM. Novel therapeutic strategies for reducing arterial stiffness. Br J Pharmacol. 2006;148:881–3. doi: 10.1038/sj.bjp.0706805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka H, Dinenno FA, Monahan KD, et al. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–5. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]