Abstract
Lactococcus lactis expresses the homologous glucosaminidases AcmB, AcmC, AcmA and AcmD. The latter two have three C-terminal LysM repeats for peptidoglycan binding. AcmD has much shorter intervening sequences separating the LysM repeats and a lower iso-electric point (4.3) than AcmA (10.3). Under standard laboratory conditions AcmD was mainly secreted into the culture supernatant. An L. lactis acmAacmD double mutant formed longer chains than the acmA single mutant, indicating that AcmD contributes to cell separation. This phenotype could be complemented by plasmid-encoded expression of AcmD in the double mutant. No clear difference in cellular lysis and protein secretion was observed between both mutants. Nevertheless, overexpression of AcmD resulted in increased autolysis when AcmA was present (as in the wild type strain) or when AcmA was added to the culture medium of an AcmA-minus strain. Possibly, AcmD is mainly active within the cell wall, at places where proper conditions are present for its binding and catalytic activity. Various fusion proteins carrying either the three LysM repeats of AcmA or AcmD were used to study and compare their cell wall binding characteristics. Whereas binding of the LysM domain of AcmA took place at pHs ranging from 4 to 8, LysM domain of AcmD seems to bind strongest at pH 4.
Introduction
Peptidoglycan, the major cell wall material of Gram-positive bacteria, is composed of chains of N-acetylmuramic acid and N-acetylglucosamine linked by means of β(1-4) glycosidic bonds. These bonds are cleaved by peptidoglycan hydrolases (PGH) during cell separation and cellular autolysis [8,16,25]. Impairment of cell separation activity of PGHs leads to long chains of cells in the lactic acid bacteria Lactococcus lactis and Streptococcus thermophilus [25,37,40]. Most PGHs are composed of at least two distinct domains, a cell wall-binding domain and a catalytic domain [25]. The cell wall-binding domains assist in adhering the enzymes to the murein layer while the catalytic domains cleave the cell wall. A tight interplay between both domains is essential for optimal PGH activity [42]. The (auto) lysis of the cells of lactic acid bacteria due to the action of its PGHs has been shown to be essential during cheese ripening for the release of intracellular proteins such as peptidases that contribute to flavor development [43].
The Lysin Motif (LysM) is one of the highly conserved cell wall-binding domains in many bacterial PGHs (PF01476) [2,8,25]. Individual LysM domains are formed by 1 to 6 LysM repeats that are connected by short non-homologous amino acid linkers consisting mostly of Ser, Thr, Asp and Pro residues [7,8,33,46-49]. LysM repeats consist of 44 to 65 amino acid residues and have been shown to specifically and non-covalently bind to peptidoglycan and to chitin, a polymer of N-acetylglucosamine [8,33,40,46,48]. Currently eight complete genome sequences of L. lactis are known [1,3,4,12,19,32,38,50]. Blast searches using the LysM domain sequences of the major autolysin AcmA [7] showed that each strain putatively expresses five proteins containing one or more LysM repeats at their C-terminus, except L. lactis CV56, UC509.9, CV56 and IL1403, which lack the gene for a homolog of the putative prophage endolysin (muramidase) Llmg_0851 while a TagH homolog is missing in the strains UC509.9 and SK11 (Table 1). Besides AcmA, a homologous protein named AcmD is putatively expressed in all strains [34]. In all cases the LysM repeats are separated by intervening sequences that are Ser/Thr rich, except in Llmg_0851. The intervening sequence between the two LysM repeats of this protein is as short as those in the LysM domain of AcmD, but they share no homology. The iso-electric points (pIs) of the LysM domains of these proteins vary from 3.8 to 10.3, which may reflect the conditions for strength of their binding to peptidoglycan (Table 1) [8].
Table 1. LysM domain-containing proteins of L. lactis MG1363a.
| Protein | Activity/role | Nr. LysM b | Location c | pI | pI LysM |
|---|---|---|---|---|---|
| AcmA (Llmg_0280) | Glucosaminidase | 3 | Sec | 10.3 | 10.0 |
| AcmD (Llmg_0509) | Glucosaminidased | 3 | Sec | 4.3 | 4.2 |
| Llmg_0851 | Muramidased | 2 | Sec | 6.0 | 6.0 |
| TagH (Llmg_1623) | Teichoic acid export ATP-binding protein | 1 | Mem | 5.5 | 3.8 |
| Llmg_0731 | unknown | 1 | Mem | 6.7 | 7.9 |
a Homologs of all 5 proteins are present in the 8 fully sequenced L. lactis strains, except Llmg_0851, which is absent in strain UC509.9, CV56 and IL1403, and a TagH homolog is missing in UC509.9 and SK11
b All LysM repeats are located at the C-terminus of the indicated proteins
c Sec; Secreted according SignalP predictions, Mem; Membrane inserted according TMHMM predictions
d Endolysin
L. lactis, the paradigm of the lactic acid bacteria, possesses 22 putative PGHs [28] of which four encode N-acetylglucosaminidases namely, AcmA, AcmB, AcmC, and AcmD [25,30,34,43]. They have a highly conserved Glu residue and a tetrad of Tyr, Ala, Thr and Asp amino acid residues forming the active site in the catalytic domain [16]. Mutational analysis of the active site of AcmA showed that Glu94 and Tyr191 are the crucial amino acid residues for catalytic activity of the enzyme [18]. AcmA has been shown to be involved in cell separation and cellular autolysis while AcmB only contributes to cellular autolysis [6,7,17,39,41]. HPLC and mass spectrometry analyses of muropeptides released from B. subtilis peptidoglycan with purified AcmA, AcmB or AcmC showed that all three lactococcal enzymes have N-acetylglucosaminidase specificity [16,17,41]. AcmD was hypothesized to also have N-acetylglucosaminidase activity, but this has not been confirmed experimentally [16]. Here the function of the AcmA homolog AcmD was investigated by analyzing an acmD mutant for cell separation, the effect of AcmD overexpression on autolysis and by examining AcmD substrate binding at different pHs.
Materials and Methods
Bacterial strains, plasmids, growth conditions, and chemicals
The strains and plasmids used in this study are listed in Table 2. L. lactis was grown in M17 broth (Difco, Becton Dickinson, Le Pont de Claix, France) at 30° C as standing cultures or on M17 (1.5% w/v) agar, all of which were supplemented with 0.5% glucose (GM17). For the preparation of electrocompetent cells the media and agar contained 0.5 M sucrose (Acros Organics, Morris Plains, NJ). Erythromycin (Roche Diagnostics GmbH, Mannheim, Germany), chloramphenicol (Sigma Chemicals Co., St. Louis, Mo) and 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) (Sigma Chemicals Co.) were added to concentrations of 5 µg/ml, 5 µg/ml and 0.008%, respectively. Escherichia coli was grown in Tryptone Yeast (TY) extract medium (Difco, Becton Dickinson) at 37° C with vigorous agitation or on TY extract medium solidified with 1.5% (wt/vol) agar and containing 100 µg of erythromycin (Roche Diagnostics GmbH) per ml, when required. For E. coli EC101, 40 µg/ml kanamycin (Roche Diagnostics GmbH) was used and for E. coli MC1061 ampicillin (100 µg/ml) (Sigma, Zwijndrecht, The Netherlands) was used. All chemicals used were of analytical grade and, unless indicated otherwise, obtained from Merck KGaA (Darmstadt, Germany).
Table 2. Bacterial strains and plasmids used in this study.
| Strain or plasmid | Characteristic(s) a | Source or reference |
|---|---|---|
| MG1363 | L. lactis subsp. cremoris ; Lac- PrtP-; plasmid-free derivative of NCDO712 | [13] |
| MG1363acmAΔ1 | Derivative of MG1363 carrying a 701-bp SacI-SpeI deletion in acmA | [7] |
| NZ9000 | MG1363pepN::nisRK | [23] |
| NZ9000acmAΔ1 | Derivative of NZ9000 with a 701-bp SacI-SpeI deletion in acmA | [43] |
| NZ9700 | Nisin-producer | [23] |
| LL108 | MG1363 carrying multiple copies of the pWV01 repA gene in the chromosome | [27] |
| IL1403 | L. lactis subsp. lactis ; plasmid-free | [10] |
| IL1403acmA::ISS1 | Insertion of transposon ISSI in acmA | [40] |
| IL1403acmA::ISS1acmD::myc | Derivative of IL1403acmA::ISSI with an in-frame integrated c-myc epitope in acmD | This work |
| EC101 | Kmr; E. coli JM101 with repA from pWV01 integrated in the chromosome | [24] |
| MC1061 | E. coli araD139 Δ(araA-leu)7697 ΔlacX74 galK16 galE15(GalS) λ- e14- mcrA0 relA1 rpsL150(strR) spoT1 mcrB1 hsdR2 | [14] |
| pVE6007 | Cmr, Ts derivative of pWV01 | [31] |
| pORIacmD::myc | Emr, LacZ, pORI280acmD with c-myc epitope inserted into the BamHI site | This work |
| pNG304 | Cmr, pNZ8048 derivative containing the pre-pro sequence of prtP fused to msa2 under control of PnisA | [41] |
| pNG3041 | Cmr, pNZ8048 derivative containing the pre-pro sequence of prtP fused to msa2 and the C-terminal domain of acmA under control of PnisA | [41] |
| pNG3042 | Cmr, pNZ8048 derivative containing the pre-pro encoding sequence of prtP fused to msa2 and the C-terminal domain encoding sequence of acmD under control of PnisA | [5] |
| pBAD | Ampr, carrying arabinose-inducible promoter ParaBAD | [15] |
| pBADcLIC | Ampr, pBAD derivative carrying arabinose promoter and His10 tag | [14] |
| pBADcLIC-GFP | Ampr, pBAD derivative carrying arabinose promoter and gfp fused to the His10 tag encoding sequence | [14] |
| pBADcLIC-GFP-LysMAcmD | Ampr, pBADcLIC-GFP with sequence encoding the 3 LysM repeats of AcmD in SwaI site | This work |
| pBADcLIC-GFP-LysMAcmA | Ampr, pBADcLIC-GFP with sequence encoding the 3 LysM repeats of AcmA in SwaI site | [49] |
PrtP- = proteolytically inactive
Ts = thermosensitive
Ampr, Cmr, Emr = resistance to ampicillin, chloramphenicol and erythromycin, respectively
General DNA techniques and transformation
Molecular cloning techniques were performed essentially as described by Sambrook et al. (23). Genomic DNA of L. lactis was isolated according to the method of Leenhouts et al. [29]. Minipreparations of plasmid DNA from L. lactis were obtained by the alkaline lysis method as described by Seegers et al. [37]. Plasmid DNA was isolated at a large scale using a Nucleobond kit PC 100 (Machery-Nagel, Düren, Germany) as specified by the supplier. Restriction enzymes, T4 DNA ligase, and deoxynucleotides were obtained from Roche Diagnostics GmbH and were used according to the supplier’s instructions. Polymerase chain reactions (PCR) were performed in a Master cycler gradient (Merck KGaA) using Taq DNA polymerase or Expand DNA polymerase according to the instructions of the manufacturer (Roche Diagnostics GmbH). PCR products were purified using the High pure PCR product purification kit and protocol (Roche Diagnostics GmbH). E. coli and L. lactis were transformed by electroporation using a Gene pulser (Bio-Rad Laboratories, Richmond, CA) as described by Zabarovsky and Winburg [51] and Leenhouts and Venema [26], respectively. E. coli MC1061 cells were transformed with the recombinant vector by the heat-shock method [45].
Construction of AcmA and AcmD fusion proteins
The modular architecture of all the constructs used in this study is depicted in Figure 1. Plasmid pNGacmD [41] was digested with the restriction enzyme BamHI for which a recognition site is located in the active site of AcmD. The oligonucleotides pACMDmyc1 (GAT C T A G AACAAAAACTTATTTCAGAAGAAGATCTT, underlined XbaI) and pACMDmyc2 (GATCAA G A T C TTCTTCTGAAATAAGTTTTTGTTCT, underlined XbaI) were annealed at 70oC and cloned into the BamHI site of pNGacmD resulting in the plasmid pNGacmD::myc.
Figure 1. Modular architecture of AcmA, AcmD and the fusion proteins used in this study.
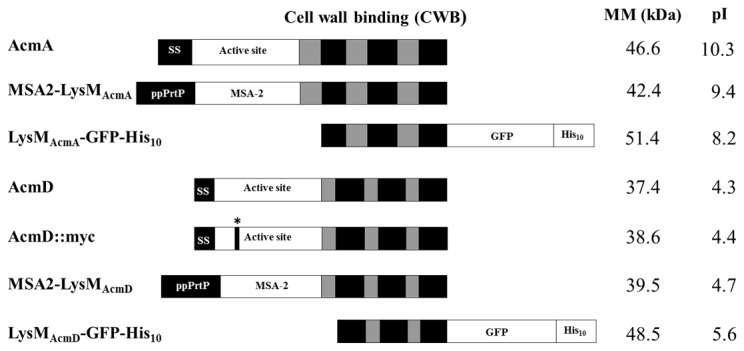
The description of the protein is depicted on the left and their respective molecular masses (MM) (kDa) and pIs are given on the right. Dark and light grey boxes indicate the LysM repeats and their intervening amino acid sequences, respectively. *, site of in-frame myc-tag insertion in the active site of AcmD; MSA-2, Plasmodium falciparum Merozoite Surface Antigen-2; GFP, Green Fluorescence Protein; His10, tag of 10 Histidine residues; SS, Signal Sequence; ppPrtP, pre-pro of prtP.
Primers pACMD2 and pACMD3 (CGG A A T T CAAGGAGGAGAAATATCAGGAGG; underlined EcoRI site) were used to amplify a 603-bp fragment encoding the C-terminal domain of AcmD that contains the three LysM repeats, using chromosomal DNA of L. lactis IL1403 as a template. Upon digestion with EcoRI and HindIII the fragment was cloned into the EcoRI/HindIII sites of pNG3041 thereby replacing the 1162-bp domain encoding the LysM repeats of AcmA. The resulting plasmid was named pNG3042 [5].
The LysM-containing domains of AcmA and AcmD were amplified by PCR using the primers AcmA-LysMcLIC-forward (ATGGGTGGTGGATTTGCTG G A A A T A C T A A T T C T G G T G G C ) and AcmA-LysMcLIC-Reverse (TTGGAAGTATAAATTTTCT T T T A T T C G T A G A T A C T G A C C ) for AcmA and AcmD-LysMcLIC-Forward (ATGGGTGGTGGATTTGCTG T C G G A A C T T A T A A A G T A C A A G ) and AcmD-LysMcLIC-Reverse (TTGGAAGTATAAATTTTCA A T T T T A A T G G T T T G G C C T G G ) for AcmD. Nucleotide sequences of AcmA and AcmD LysM regions are in italic and underlined. LysMAcmA-GFP-His10 and LysMAcmD-GFP-His10 constructs were cloned in pBADcLIC-GFP plasmid containing Green Fluorescent Protein gene from Aequorea victoriae (Clontech, Mountain view, CA, USA), by the Ligation Independent Cloning procedure described by Geertsma et al. [14].
Construction of an acmD insertion mutant of L. lactis IL1403
pNGacmD::myc was cut with SphI and PstI. The fragment carrying acmD::myc was inserted into the SphI /PstI sites of pORI280, an integration vector which lacks the gene encoding the replication initiation protein, repA [27,28]. The resulting plasmid, pORIacmD::myc, was obtained in L. lactis strain LL108, which carries multiple copies of the repA gene on the chromosome [27]. After transformation of L. lactis IL1403acmA::ISS1 with pORIacmD::myc, erythromycin resistant integrants were checked by PCR with the primers: pEM280 (GCCCATATTTTTTCCTCC; annealing in the 5’-end of the erythromycin resistance gene) and pACMD2 (CGCA A G C T TCTGCAGAGCTCTTAGATTCTAATTGTTTGTCCTGG; underlined HindIII, which anneals to the 3’-end of acmD) as was described before [7].
Selection of the second crossover event was done as described by Leenhouts and Venema [26]. A 1040-bp region of acmD was amplified from the chromosomal DNA of selected integrants using the primers pACMD1 (CCTGTCATGAAACAGAAACATAAAT) and pACMD2, which anneal at either end of acmD. The presence of the c-myc epitope in acmD was confirmed by restriction with XbaI, as this site is only present in the DNA coding for this epitope. Although attempts were made to use the same approach to construct a single mutant of acmD in L. lactis IL1403, for unknown reasons this was not successful. Only first step integrants were obtained but excision resulted in all cases in reversion to the wild type.
SDS-PAGE, zymography, Western hybridization and in-gel fluorescence detection
AcmA and AcmD activity was detected by a zymogram staining technique using SDS-polyacrylamide (PAA) (12.5%) gels containing 0.15% autoclaved, lyophilized Micrococcus lysodeikticus ATCC 4698 cells (Sigma Chemicals Co.), as described previously [7]. SDS-PAA gels without cells were stained with Coomassie brilliant blue (Bio-Rad Laboratories Inc). For Western hybridizations proteins were transferred from (2D)SDS-PAA gels to polyvinylidene difluoride membranes (Roche Diagnostics GmbH) as described by Towbin et al. [44]. MSA2-LysMAcmA and MSA2-LysMAcmD antigens were detected with a rabbit polyclonal anti-MSA2 antiserum [35] diluted 10000-fold and horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibodies (Pharmacia, Uppsala, Sweden). The c-Myc epitope was detected with 1:5000-diluted monoclonal mouse anti-c-myc antibodies (Amersham Biosciences, Piscataway, NJ) and 1:5000-diluted HRP conjugated anti-mouse antibodies. AcmA was detected with 1:5000-diluted polyclonal rabbit anti-AcmA active site antibodies [42] and 1:5000-diluted HRP conjugated anti-rabbit antibodies (GE Healthcare UK Ltd, Buckinghamshire, England). In all three cases, the Enhanced chemiluminescence detection system and protocol (Amersham Biosciences) was used.
LysMAcmD-GFP-His10 protein samples were subjected to SDS-PAGE with a 15% PAA gel and in-gel GFP fluorescence was visualized using a Gel Documentation System (Bio-Rad Laboratories Inc.). Bands obtained by Western hybridization were semi-quantified using the Quantity One program (Bio-Rad Laboratories B.V., Veenendaal, the Netherlands).
Protein expression, isolation and purification
E. coli MC1061 bearing the desired plasmids (pBADcLIC-GFP-LysMAcmA or pBADcLIC-GFP-LysMAcmD) were grown until an OD600 of 0.6-0.8 and induced with 0.2% arabinose (Merck KGaA) for 2 h. The cells were harvested at 5000 X g for 20 min at 4° C and the cell pellet was resuspended in lysis buffer (50 mM NaH2PO4 pH 8.0, 300 mM NaCl, 20 mM imidazole). Cell extract was obtained by sonication at 4° C using three cycles of 30 s pulses at 70% amplitude with 1 min intervals (Vibra Cell, Sonics & Materials, Newton, CT) followed by centrifugation at 20800 X g for 30 min at 4° C. LysM fusion protein was isolated and purified by Ni-NTA affinity chromatography as recommended by the manufacturer (Qiagen GmbH, Hilden, Germany).
Two-dimensional (2D)-gel electrophoresis
Overnight cultures of L. lactis IL1403acmA::ISS1 and IL1403acmA::ISS1acmD::myc were diluted to an OD600 of 0.05 in fresh GM17 medium and incubated at 30° C until an OD600 of 1.0 was reached. Subsequently, the cultures were centrifuged and proteins in the supernatant fractions were precipitated overnight at 4° C with TCA at a final concentration of 10%. Proteins were collected by centrifugation at 20000 X g for 20 min; the protein pellet was washed three times with acetone, air-dried and dissolved in a solution containing 8 M Urea, 2% CHAPS, 2 mM TBP, 0.2% ampholytes. The amount of protein loaded for 2D-gel electrophoresis was equal to that in 100 ml of supernatant fraction of GM17 cultures with an OD600 of 1.0. Isoelectric focusing (IEF) strips (Bio-Rad Ready Strips IPG; pH 4 to 7, 11 cm) were passively rehydrated overnight with the sample to be analyzed. IEF was performed with a Bio-Rad Protean IEF cell (Bio-Rad). The voltage was step-wise increased from 150 V (0.5 h) to 300 V (1 h) and 600 V (1 h), while the voltage was raised linearly to 8000 V until 25000 Vh. Subsequently, the strips were equilibrated twice for 15 min with 5 ml equilibration buffer (0.05 M Tris-HCl pH 8.8, 6 M Urea, 30% (w/v) glycerol, 2% SDS), the first time containing with 1% DTT, the second time with 4% iodoacetamide. The equilibrated strips were loaded on a standard SDS-(12%) PAA gel and run for 2.5 h at 100 V and 15° C. The proteins in the gel were fixed by washing with a solution of 40% ethanol and 10% acetic acid. Staining was performed overnight with colloidal Coomassie brilliant blue (0.1% CBB G-250, 2% phosphoric acid, 20% ethanol); destaining was with distilled water. PDQuest 2D analysis software (Bio-Rad Laboratories Inc.) was used for comparison between gels and analysis of the 2D gel data.
Bacterial growth, enzyme assays, and microscopy
OD600’s of cultures were measured in a Novaspec II spectrophotometer (Pharmacia Biotech AB, Uppsala, Sweden). To measure cellular lysis of L. lactis, cells were grown in GM17 at 30° C for 50 h. Subsequently, as a measure of the extent of culture lysis, release of intracellular X-prolyl dipeptidyl aminopeptidase (PepX) was measured using the chromogenic substrate Ala–Pro-p-nitroanilid (Bachem Feinchemicalien AG, Bubendorf, Switzerland) as described earlier [42].
The sample/substrate mixture was pipetted into a microtiter plate well, and color development was monitored in a THERMOmax microtiter plate reader (Molecular Devices Corporation, Menlo Oaks, CA) at 405 nm for 145 min at 37° C. The slope of the substrate hydrolysis/color development was calculated for two independent experiments.
Light microscopy pictures of L. lactis were made with a Zeiss microscope (Carl Zeiss, Thornwood, CA) and an Axiovision digital camera (Axion Technologies, Houston, TX). A fluorescence microscope (Zeiss Axiophot) fitted with a digital camera and a green filter was used to view GFP fluorescence.
For electron microscopic analysis, L. lactis MG1363 cells were treated with TCA (as described below) and incubated with MSA2, MSA2-LysMAcmA or MSA2-LysMAcmD as described above for untreated cells. The antibodies against MSA2 were diluted 1:1000 in PBS-containing 0.15 M glycine. Immunogold labeling was performed using Auroprobe 15 nm goat anti-rabbit IgG gold marker (Amersham Biosciences) using preparations of glutaraldehyde-fixed cells on Formvar-carbon-coated nickel grids. The labeled samples were stained with 0.1% uranyl acetate (w/v in water) and examined in a Philips CM10 transmission electron microscope at 100 kV.
To measure the influence of AcmD on cellular lysis, cells of L. lactis NZ9000 (pNGacmD) or NZ9000acmAΔ1 (pNGacmD), both with or without nisin-induced AcmD, were mixed with AcmA and/or AcmD. Cells from 10 ml of culture of L. lactis NZ9000 (pNGacmD) or NZ9000acmAΔ1 (pNGacmD), both either or not induced with nisin at an OD600 of 0.6, were collected by centrifugation at 5000 X g 2 h after induction. The cell pellets were resuspended in 10 ml of the supernatants of the L. lactis MG1363 (which contains both AcmA and AcmD) or L. lactis MG1363acmAΔ1 (which contains AcmD) cultures and incubated at 30° C for 2.5 h (OD600 ~1.8). Subsequently, supernatant samples were collected and used to measure the extent of culture lysis by measuring PepX activity.
Binding of fusion proteins to lactococcal cells
MSA2-LysMAcmA and MSA2-LysMAcmD binding studies were performed by mixing equal amounts of L. lactis cells (the amount of cells present in 1 ml of culture with an OD600 of 1.0) or with L. lactis cells that had been treated with trichloroacetic acid by boiling 25 µl of cell culture in 1 ml of 10% trichloroacetic acid (TCA) with 1 ml of supernatant of a nisin-induced L. lactis NZ9000 acmAΔ1 (pNG3041, producing MSA2-LysMAcmA) or NZ9000 acmAΔ1 (pNG3042, expressing MSA2-LysMAcmD) cultures. The suspensions were incubated at room temperature for 5 min, centrifuged (1 min at 20000 X g) and washed once with M17 broth. The pellets were subsequently resuspended in SDS sample buffer, boiled for 5 min, and subjected to SDS-PAGE.
Purified LysMAcmA-GFP-His10 and LysMAcmD-GFP-His10 purified proteins (3 µM each) were added to L. lactis cells in 50 mM NaH2PO4 (pH 4.0, 6.0 and 8.0), 50 mM NaCl buffer at room temperature and incubated for 30 min. Cells were centrifuged at 20000 X g for 1 min and the pellet was washed three times with the same buffer containing 150 mM NaCl to remove un-specifically bound proteins. Finally the washed pellet was resuspended in the same buffer and observed under a fluorescence microscope. To rule out the possibility of GFP interaction with lactis cells, GFP, purified by Hydrophobic Interaction Chromatography was used as a control.
Results
Comparison of AcmA and AcmD
A comparison between AcmA [GenBank AAK04370] and AcmD [GenBank AAK04639] of L. lactis IL1403 was performed to pinpoint the differences between both proteins. Both have a similar modular structure (Figure 1): a signal sequence is followed by an active site domain (Glucosaminidase family, pfam PF01832) and a C-terminal LysM domain with three LysM repeats (pfam PF01476). The homology between the two proteins is around 58%. AcmD differs from AcmA in the length of the signal sequence (26 versus 57 amino acid residues), the presence of shorter amino acid sequences separating the repeats, while the protein has a pI of 4.3 instead of 10.3 for AcmA (Figure 1 and Table 1). His-tagged purified AcmD showed PGH activity only at pH 4 while AcmA is active at pH 4 to 8 [16,43].
AcmD of L. lactis subsp. lactis strains KF147 [38] and IL1403 [4] are identical while those of strains IO-1 [19] and CV56 [12] are 99% identical. The AcmD proteins of the L. lactis subsp. cremoris A76 [3], UC509.9 [1], MG1363 [50] and SK11 [32] are around 95% identical to IL1403 AcmD, respectively. The differences are mainly located between the active site domain and the LysM domain and in the intervening sequences separating the LysM repeats. No proteins homologous to AcmD that share the same low pI are encoded by the genomes of the other bacteria sequenced to date.
AcmD contributes to cell separation and autolysis
A c-myc epitope was inserted in frame in the active site of AcmD to be able to follow expression and localization of the AcmD fusion protein and to make an acmD mutant by chromosomal integration. The chromosomal acmD gene in IL1403acmA::ISS1 was replaced by acmD::myc via replacement recombination, resulting in an acmAacmD double mutant. However for yet unknown reasons the integration of acmD::myc into the genome of IL1403 failed. The effects of mutation of acmD on protein secretion by and autolysis and cell separation of L. lactis were investigated. Activity of AcmD could not be detected when cell-free extracts of L. lactis NZ9000, L. lactis IL1403, or IL1403acmA::ISS1 all carrying an intact copy of the acmD gene in their chromosomes, were subjected to zymography using cell wall fragments of M . lysodeikticus or L. lactis, even after renaturation of the proteins in the gel at pH 4 (results not shown).
The protein patterns of culture supernatant fractions from the acmA and acmAacmD mutants did not differ significantly, as judged by 2D-gel electrophoresis. Two proteins were present in higher amounts in either the supernatant of the acmA strain or the acmAacmD double mutant while 4 proteins were detected only in the supernatant of the acmA mutant. The main difference was the expected shift in size of the secreted AcmD protein in the double mutant as a consequence of the c-myc epitope (from 34.9 to 36.6 kDa; Figure S1).
Cellular autolysis was examined by following the release of the cytoplasmic peptidase Pep-X into the culture supernatant [9]. No difference in lysis was observed between the acmAacmD double mutant and the acmA single mutant of L. lactis IL1403 (Figure S2).
Nisin-induced overexpression of AcmD has previously been shown to result in increased lysis of L. lactis MG1363 in the presence of AcmA while no additional lysis was obtained for the empty plasmid control [43]. Knowing that AcmD is secreted into the culture supernatant even though no activity could be detected, we investigated whether the contribution of AcmD activity to lysis occurs by peptidoglycan degradation during passage of the protein through the cell wall. Because a single acmD mutant could not be obtained the following approach was used to examine AcmD-mediated lysis: L. lactis strains NZ9000 (pNGacmD) and NZ9000acmAΔ1 (pNGacmD) were grown in the presence of nisin to induce the expression of AcmD. Cell pellets were collected two hours after induction and resuspended in cell free supernatants of L. lactis MG1363, containing both AcmA and AcmD, or L. lactis MG1363acmAΔ1, containing only AcmD. Un-induced cells were used as controls. Using this approach the influence of AcmD when produced from the inside or added from the outside could be separately measured. The cells of the strains NZ9000 (pNGacmD) and NZ9000acmAΔ1 (pNGacmD) released significantly more PepX and thus lysed to a larger extent when AcmD expression was induced (Figure 2, compare un-induced with induced). This was only the case when AcmA activity was present, upon suspension of the cells in the AcmA-containing MG1363 supernatant (Figure 2, see dark grey bars), showing that AcmD contributes to autolysis by its action within the cell wall.
Figure 2. AcmD contributes to cell autolysis.
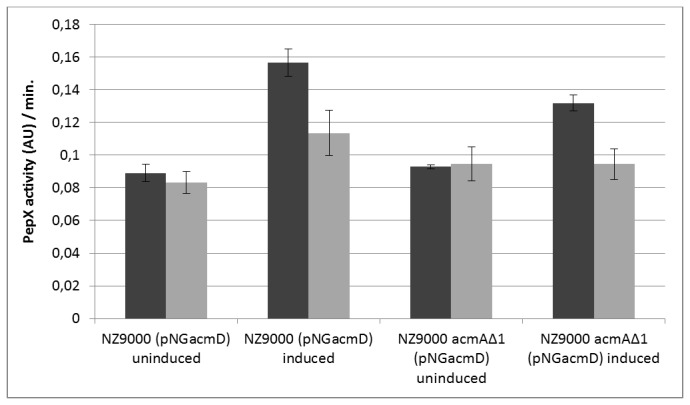
Determination of cell lysis upon induced expression of AcmD. Cultures of L. lactis NZ9000 (pNGacmD) or L. lactis NZ9000acmA∆1 (pNGacmD) were grown until an OD600 of 0.6 was reached. Both cultures were split in two. Expression of AcmD was induced in one half of each culture by the addition of nisin (10 ng/ml), the other halves were left un-induced. The cells of the four cultures were collected after 2 h and mixed with the cell-free culture supernatant of L. lactis MG1363 (dark grey bars) or L. lactis MG1363acmA∆1 (grey bars). After 1 h incubation at 30° C, supernatants were analyzed for PepX activity (in arbitrary units (AU)). The slope of the substrate hydrolysis/color development was determined and is indicated as PepX activity (AU)/min. The average was calculated from two independent experiments.
After overnight growth at 30° C in GM17 broth differences in cell sedimentation were observed between the L. lactis strains IL1403, IL1403acmA::ISS1 and IL1403acmA::ISS1acmD::myc. The wild type IL1403 strain did not sediment whereas the acmA mutant did and the acmAacmD double mutant even more (Figure 3). Light microscopic analysis revealed that the latter strain formed very long chains in comparison to the other two (Figure 3B). The chains formed by the double mutant were calculated to be on average 2 to 3 times longer than those of IL1403acmA::ISS1 (Figure 3D). Further, complementation of AcmD expression in the acmAacmD double mutant resulted in a decrease in the length of the chains to a length resembling that of the acmA single mutant (Figure 3C and D). This data indicates that AcmD, like AcmA, is involved in cell separation.
Figure 3. AcmD of L. lactis is involved in cell separation.
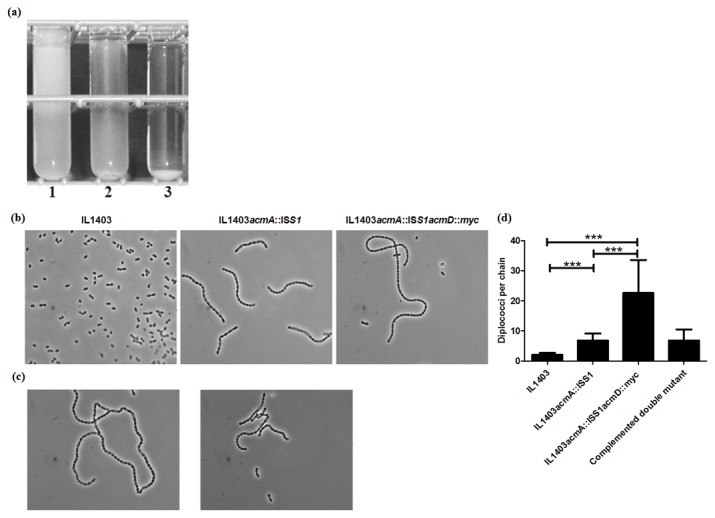
(a) Turbidity and cell sedimentation of L. lactis IL1403 (1), IL1403acmA::ISS1 (2) and IL1403acmA::ISS1acmD::myc (3) after overnight growth at 30° C in GM17 broth. (b) Light microscopic views of L. lactis IL1403, IL1403acmA::ISS1 and IL1403acmA::ISS1acmD::myc after overnight growth at 30° C as standing cultures in GM17 medium. Magnification: 1250x in all three frames; representative views. (c) Phase contrast microscopic views of uninduced (left) and nisin (10 ng/ml)-induced (right) IL1403acmA::ISS1acmD::myc harboring plasmids pNGAcmD (AcmD) and pNZ9530 (nisRK). (d) Quantification of chain length in L. lactis IL1403, IL1403acmA::ISS1, IL1403acmA::ISS1acmD::myc, and nisin (10 ng/ml)-induced IL1403acmA::ISS1acmD::myc (pNGacmD) (the complemented double mutant). Number of diplococci per chain was counted and the mean of 30 chains were depicted. Standard deviation (***) indicates the significance as analyzed by Bonferroni’s multiple comparison test (p<0.05) using one-way ANOVA.
LysMAcmD binds to lactococcal cells preferably at low pH
As peptidoglycan binding of AcmD is difficult to examine because its activity is not detectable and antibodies are not available, LysMAcmD (pI=4.2, see column pI LysM in Table 1 and Figure 1) was fused to the MSA2 reporter protein, for which an antibody is available [35]. The fusion protein, MSA2-LysMAcmD, is secreted by fusing the signal sequence of the lactococcal proteinase PrtP to its N-terminal. Western detection revealed that MSA2-LysMAcmD is secreted from L. lactis NZ9000acmAΔ1 (pNG3042) upon nisin induction (results not shown). When a supernatant containing MSA2-LysMAcmD was mixed with L. lactis MG1363 cells poor binding of the fusion protein was observed at pH 6.2 (Figure 4A). A similar result was obtained when L. lactis MG1363 cells were mixed with the supernatant of L. lactis NZ9000acmAΔ1 (pNG3041), containing the MSA2-LysMAcmA fusion protein [41]. Although previously we have shown that MSA2 alone cannot bind lactococcal cells [41] we did observe binding of the MSA2 protein in this study. When the binding of the three proteins was repeated with TCA-pretreated L. lactis cells a substantial increase in binding was observed for the MSA2-LysMAcmA and MSA2-LysMAcmD fusion proteins but not for the MSA2 protein (Figure 4A). To investigate whether the pI of MSA2-LysMAcmD affects cell binding the binding to TCA-pretreated cells was repeated at pHs 3.2 and 6.2. Figure 4B clearly shows that MSA2-LysMAcmD binds 2-fold more at pH 3.2 than at pH 6.2 (compare lanes 1 and 3). No detectable binding of MSA2-LysMAcmA could be observed using electron microscopy (Figure 4C) while strong labeling signals was detected on TCA-treated cells. However, as for MSA2-LysMAcmD, only minor labeling signals were observed (Figure 4C). These results suggest that LysMAcmD has poor cell wall binding properties around neutral pH (pH 7.2) while its binding to the cell wall is increased at a pH closer to its pI, when the net charge of the protein is positive.
Figure 4. Binding of MSA2-LysMAcmA and MSA2-LysMAcmD to L. lactis cells.
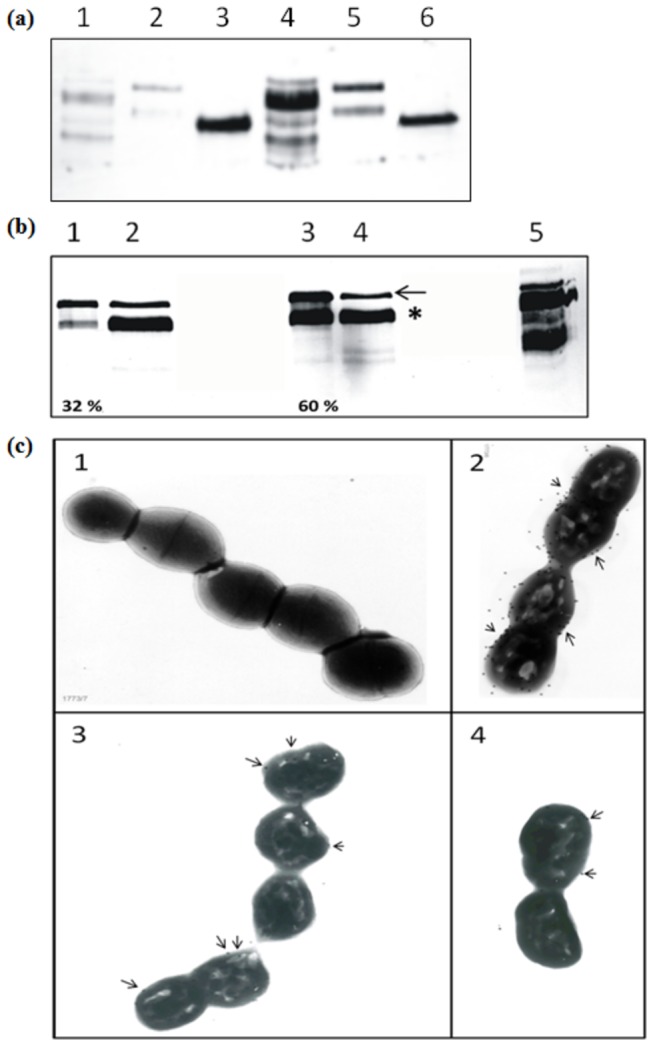
a. Anti-MSA2 antibody-treated Western blot showing binding of MSA2-LysMAcmA, MSA2-LysMAcmD and MSA2 to non-treated (lanes 1, 2 and 3) and TCA-treated (lanes 4, 5 and 6) L. lactis cells, respectively. b. Western blot treated with anti-MSA2 antibody. Lanes 1 and 3 indicate MSA2-LysMAcmD bound to TCA-treated L. lactis cells at pH 6.2 and 3.2, respectively. The unbound protein at pH 6.2 and 3.2 is shown in lanes 2 and 4, respectively. Lane 5, positive control: MSA2-LysMAcmA bound to L. lactis at pH 6.2. Arrow points out Pro-MSA2-LysMAcmD and * indicates mature MSA2-LysMAcmD. The percentage of both MSA2-LysM variants bound to L. lactis cells at pH 6.2 and 3.2 were semi-quantified (as the number of pixels present per mm2 of both bands in lanes 1 or 3) divided by the total signal of the cell and supernatant fractions (number of pixels per mm2 of all bands in lanes 1+2 or 3+4, respectively) is shown at the bottom of lanes 1 and 3. c. Transmission electron microscopic images of L. lactis incubated with MSA2 or MSA2 fusion proteins and subsequently incubated with rabbit anti-MSA2 antibodies and finally decorated with goat anti-rabbit IgG gold marker. Picture 1: non-treated L. lactis cells incubated with MSA2-LysMAcmA. Pictures 2, 3 and 4 show TCA-treated L. lactis cells incubated with MSA2-LysMAcmA, MSA2-LysMAcmD and MSA2 proteins, respectively. Arrows indicate immunogold particles detected on the cells.
LysM domains of AcmD and AcmA bind differently to lactococcal cells
The purified MSA2-LysMAcmA fusion protein binds to the septum and poles of L. lactis cells when added from the outside to these cells [41]. Attempts to repeat these experiments with the MSA2-LysMAcmD protein failed due to side effects of the immunodetection at pH 4. And because the MSA2 protein showed non-specific binding to L. lactis cells another approach was taken. To locate the sites of binding of LysMAcmD on the cell, C-terminal GFP fusions of His10-tagged LysMAcmA (pI=10, see column pI LysM in Table 1) and LysMAcmD were expressed in and purified from E. coli using Ni-NTA. SDS-PAGE with a 15% PAA gel followed by in-gel fluorescence detection showed that the fusion proteins are of the proper size and fluoresce (results not shown and Figure S3). Purified LysMAcmD-GFP-His10 (48.5 kDa; pI=5.6) only binds to L. lactis NZ9000 cells at pH 4, not at pH 6 or pH 8 (Figure 5A and Figure S5). Phase contrast and fluorescence analyses showed that L. lactis does not intrinsically fluorescence under the conditions used (Figure S4A). HIC-purified GFP protein did not bind to the L. lactis cells under any of the conditions tested (Figure S4B and results not shown). Unlike the LysMAcmD fusion protein, LysMAcmA-GFP-His10 (51.4 kDa; pI=8.2) bound to L. lactis cells at pH 4, pH 6 and pH 8 (Figure 5B and C and results not shown).
Figure 5. Binding of LysMAcmD-GFP-His10 and LysMAcmA-GFP-His10 to L. lactis NZ9000 cells.
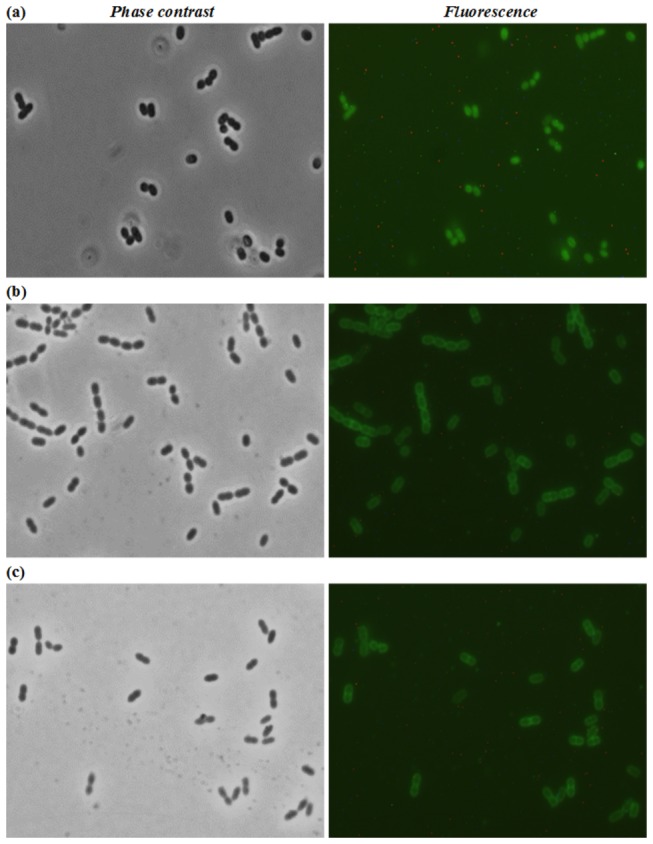
Phase contrast and fluorescence microscopy of L. lactis NZ9000 cells incubated with (a) LysMAcmD-GFP-His10 at pH 4.0 and (b and c) LysMAcmA-GFP-His10 at pH 4.0 and 8.0, respectively. Original magnification: 1250-fold in all frames.
Discussion
In this study we show that the PGH AcmD of L. lactis is involved in cell separation as deletion of acmD results in increased length of the chains of cells. AcmD also contributes to autolysis, but only in the presence of AcmA. The LysM domain of AcmD binds more to the lactococcal cell wall at a pH close to its pI.
Under standard laboratory growth conditions at pH 6 to 7, AcmD is produced and mainly secreted by L. lactis (Figure S1). Peptidoglycan degrading activity could not be detected for the native expressed or overexpressed AcmD enzyme using a zymographic analysis method (results not shown). Earlier, His-tagged purified AcmD protein was shown to be active under renaturation conditions also employed in the present study (pH 4) [16]. A possible explanation for the different results in the two studies could be the very high protein concentration that was used in the earlier study. Although we were not able to show in vitro AcmD activity, a clear phenotypic effect was seen in vivo. The L. lactis acmAacmD mutant formed significantly longer chains (Figure 3B and D) and sedimented more readily than the wild type strain (Figure 3A) showing that AcmD is involved in cell separation, like its homolog AcmA. Complementation of the acmD mutation by plasmid-specified AcmD resulted in a decrease of chain length of the acmAacmD mutant (Figure 3C).
A second phenotypic effect was obtained upon overexpression of AcmD, namely increased autolysis of L. lactis (Figure 2). This effect was only observed when AcmA activity was also present: autolysis was not increased when AcmD was overexpressed in an AcmA-minus background. Although activity and presence in the cell wall of AcmD could not be unequivocally detected, it is possible that AcmD hydrolyses the peptidoglycan at specific sites during its passage through the cell wall. It has been shown that a pH-gradient is present over the cell wall of the Gram-positive model bacterium Bacillus subtilis and that this affects the activity of enzymes such as PGH’s [20]. Possibly, such a pH gradient also exists in the lactococcal cell wall, which would generate a local low pH required for AcmD activity.
The LysMAcmA-GFP fusion protein seems to bind at all pH’s tested to the whole surface of lactococcal cells (Figure 5B and C). Previously, hotspots for binding of the MSA2-LysMAcmA fusion protein were detected by immunofluorescence microscopy [41]. This discrepancy may be caused by differences in the N- (MSA2) and C-terminal (GFP) fusion proteins used in the two studies e.g., the sizes of the proteins used, or by differences in the limits of detection of immunofluorescence and GFP fluorescence. Whereas the GFP fusion proteins can be detected in and (slightly) outside the cell wall, detection of the MSA2 fusion protein depends on proper outer surface exposure of the epitopes that are to be bound by the antibodies, which are too big to enter the cell wall [11]. LysMAcmD-GFP fusion protein (48.5 kDa; pI=5.6) binds to the cell wall at pH 4, when its net charge is slightly positive. At a pH above or below their pI, proteins carry either a net negative or positive charge, respectively, and local pH can greatly influence the characteristics of proteins or their domains. Although AcmA and AcmD share amino acid sequence homology in their active sites and their cell wall-binding domains, a significant difference exists in the pI values of their LysM domains. The observed differences in binding of the LysM domains of AcmA and AcmD (Figure 5) might be due to differences in the LysM domain pI’s and/or the intervening amino acid sequences in these domain. It has been suggested that the low pI of some of the LysM-containing proteins functions in binding of these enzymes to the cell wall at low pH or for positioning of active site domains at the proper location in the peptidoglycan matrix close to the membrane [8]. A more detailed study is needed to determine the specific binding sites in the cell wall peptidoglycan layer for the various types of LysM domains and whether the domains may be used to position different PGHs to different sites in the cell wall.
Supporting Information
Comparison of 2D-gel images of supernatant fractions of L. lactis IL1403acmA::ISS1 and L. lactis IL1403acmA::ISS1acmD::myc. xmlns:xlink="http://www.w3.org/1999/xlink" xmlns:mml="http://www.w3.org/1998/Math/MathML">The amount of protein loaded in both cases was the equivalent of supernatant fraction of 100 ml of a GM17 culture with an optical density at 600 nm of 1.0. The position of the spots of the AcmD and AcmD::myc proteins, identified by Mass-spectroscopic analysis, and their molecular weights and pIs are indicated. Proteins that were more abundant in the supernatant fraction of IL1403acmA::ISS1 (blue) or IL1403acmA::ISS1acmD::myc (red), and those unique in the supernatant of IL1403acmA::ISS1 (yellow) or IL1403acmA::ISS1acmD::myc (green) are indicated. Sizes of the pre-stained molecular mass marker (kDa) are indicated in the middle.
(TIF)
Deletion of acmD does not affect cell lysis during growth. Release of intracellular X-prolyl dipeptidyl aminopeptidase (PepX) from L. lactis IL1403 (♦), IL1403acmA::ISS1 (▲) and IL1403acmA::ISS1acmD::myc (■). Samples were taken at the indicated time points from the bacterial cultures incubated in GM17 broth. Upon removal of the cells by centrifugation the PepX-activity (in arbitrary units) released into the medium due to autolysis was determined using a chromogenic substrate, as described in the Materials and Methods section.
(TIF)
Expression of LysMAcmD-GFP-His10 in E. coli. Coomassie brilliant blue-stained SDS- (15%) PAA-gel (left) and in-gel GFP- fluorescence (right) showing the expression of LysMAcmD-GFP-His10 on SDS- PAGE with a 15% PAA gel. E. coli MC1061 bearing the pBADcLIC-LysMAcmD was grown at 37° C until OD600 of 0.8 and induced with 0.2% arabinose for 2 h (see Materials and Methods section). The cell extracts of control and test samples were loaded on PAA gel for the identification of specific protein band. For the latter figure, the PAA gel is exposed to UV-light prior to coomassie staining for imaging the fluorescent bands. Prestained protein marker lane 1, cell extracts of empty vector control strain, un-induced control and 0.2%-arabinose induced test samples in lanes 2, 3 and 4, respectively. Arrows indicate LysMAcmD-GFP-His10 protein/activity bands.
(TIF)
Negative and autofluorescence controls Phase-contrast and fluorescence microscopy of L. lactis NZ9000 cells incubated at pH 4.0 with HIC-purified GFP (a) and without addition of any recombinant protein (b). Original magnification: 1250-fold in all frames.
(TIF)
Binding of LysMAcmD-GFP-His10 to L. lactis NZ9000 cells at pH 6.0 and 8.0. Phase-contrast and fluorescence microscopy of L. lactis NZ9000 cells incubated with LysMAcmD-GFP-His10 at pH 6.0 (a) and 8.0 (b). Original magnification: 1250-fold in all frames.
(TIF)
Funding Statement
Ganesh Ram R. Visweswaran is funded by the Ubbo-Emmius scholarship from the University of Groningen (www.rug.nl). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ainsworth S, Zomer A, de Jager V, Bottacini F, van Hijum SA et al. (2013) Complete genome of Lactococcus lactis subsp. cremoris UC509.9, host for a model lactococcal P335 bacteriophage. Genome Announc 1(1) doi: e00119-12. 10.1128/genomeA.00119-12. [DOI] [PMC free article] [PubMed]
- 2. Bateman A, Bycroft M (2000) The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J Mol Biol 299: 1113–1119. [DOI] [PubMed] [Google Scholar]
- 3. Bolotin A, Quinquis B, Ehrlich SD, Sorokin A (2012) Complete genome sequence of Lactococcus lactis subsp. cremoris A76. J Bacteriol 194(5): 1241–1242. doi:10.1128/JB.06629-11. PubMed: 22328746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K et al. (2001) The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res 11: 731–753. doi:10.1101/gr.GR -1697R PubMed: 11337471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bosma T, Kanninga R, Neef J, Audouy SAL, van Roosmalen ML et al. (2006) Novel surface display system for proteins on non-genetically modified Gram-positive bacteria. Appl Environ Microbiol 72: 880–889. doi:10.1128/AEM.72.1.880-889.2006. PubMed: 16391130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buist G, Karsens H, Nauta A, van Sinderen D, Venema G et al. (1997) Autolysis of Lactococcus lactis caused by induced overproduction of its major autolysin, AcmA. Appl Environ Microbiol 63: 2722–2728. PubMed: 9212419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buist G, Kok J, Leenhouts KJ, Dabrowska M, Venema G et al. (1995) Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J Bacteriol 177: 1554–1563. PubMed: 7883712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buist G, Steen A, Kok J, Kuipers OP (2008) LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol Microbiol 68: 838–847. doi:10.1111/j.1365-2958.2008.06211.x. PubMed: 18430080. [DOI] [PubMed] [Google Scholar]
- 9. Buist G, Venema G, Kok J (1998) Autolysis of Lactococcus lactis is influenced by proteolysis. J Bacteriol 180: 5947–5953. PubMed: 9811653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chopin A, Chopin MC, Moillo-Batt A, Langella P (1984) Two plasmid-determined restriction and modification systems in Streptococcus lactis . Plasmid 11: 260–263. doi:10.1016/0147-619X(84)90033-7. PubMed: 6087394. [DOI] [PubMed] [Google Scholar]
- 11. Demchick P, Koch AL (1996) The permeability of the wall fabric of Escherichia coli and Bacillus subtilis . J Bacteriol 178: 768-773. PubMed: 8550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao Y, Lu Y, Teng KL, Chen ML, Zheng HJ et al. (2011) Complete genome sequence of Lactococcus lactis subsp. lactis CV56, a probiotic strain isolated from the vaginas of healthy women. J Bacteriol 193: 2886–2887. doi:10.1128/JB.00358-11. PubMed: 21460077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gasson MJ (1983) Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol 154: 1–9. PubMed: 6403500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geertsma ER, Poolman B (2007) High-throughput cloning and expression in recalcitrant bacteria. Nat Methods 4: 705–707. doi:10.1038/nmeth1073. PubMed: 17643108. [DOI] [PubMed] [Google Scholar]
- 15. Guzman LM, Belin D, Carson MJ, Beckwith J (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177(14): 4121–4130. PubMed: 7608087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huard C, Miranda G, Redko Y, Wessner F, Foster SJ et al. (2004) Analysis of the peptidoglycan hydrolase complement of Lactococcus lactis: identification of a third N-acetylglucosaminidase, AcmC. Appl Environ Microbiol 70: 3493–3499. doi:10.1128/AEM.70.6.3493-3499.2004. PubMed: 15184148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huard C, Miranda G, Wessner F, Bolotin A, Hansen J et al. (2003) Characterization of AcmB, an N-acetylglucosaminidase autolysin from Lactococcus lactis . Microbiology 149: 695–705. doi:10.1099/mic.0.25875-0. PubMed: 12634338. [DOI] [PubMed] [Google Scholar]
- 18. Inagaki N, Iguchi A, Yokoyama T, Yokoi KJ, Ono Y et al. (2009) Molecular properties of the glucosaminidase AcmA from Lactococcus lactis MG1363: Mutational and biochemical analyses Molecular properties of the glucosaminidase AcmA from Lactococcus lactis MG1363. Gene 447: 61–71. doi:10.1016/j.gene.2009.08.004. PubMed: 19686822. [DOI] [PubMed] [Google Scholar]
- 19. Kato H, Shiwa Y, Oshima K, Machii M, Araya-Kojima T et al. (2012) Complete genome sequence of Lactococcus lactis IO-1, a lactic acid bacterium that utilizes xylose and produces high levels of L-lactic acid. J Bacteriol 194(8): 2102-2103. doi:10.1128/JB.00074-12. PubMed: 22461545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kemper MA, Urrutia MM, Beveridge TJ, Koch AL, Doyle RJ (1993) Proton motive force may regulate cell wall-associated enzymes of Bacillus subtilis . J Bacteriol 175: 5690–5696. PubMed: 8396121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleerebezem M, Beerthuyzen MM, Vaughan EE, de Vos WM, Kuipers OP (1997) Controlled gene expression systems for lactic acid bacteria: transferable nisin-inducible expression cassettes for Lactococcus, Leuconostoc, and Lactobacillus spp. Appl Environ Microbiol 63: 4581–4584. PubMed: 9361443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuipers OP, Beerthuyzen MM, de Ruyter PG, Luesink EJ, de Vos WM (1995) Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J Biol Chem 270: 27299–27304. doi:10.1074/jbc.270.45.27299. PubMed: 7592991. [DOI] [PubMed] [Google Scholar]
- 23. Kuipers OP, Beerthuyzen MM, Siezen RJ, de Vos WM (1993) Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur J Biochem 216: 281–291. doi:10.1111/j.1432-1033.1993.tb18143.x. PubMed: 7689965. [DOI] [PubMed] [Google Scholar]
- 24. Law J, Buist G, Haandrikman A, Kok J, Venema G et al. (1995) A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J Bacteriol 177: 7011–7018. PubMed: 8522504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Layec S, Decaris B, Leblond-Bourget N (2008) Diversity of Firmicutes peptidoglycan hydrolases and specificities of those involved in daughter cell separation. Res Microbiol 159: 507–515. doi:10.1016/j.resmic.2008.06.008. PubMed: 18656532. [DOI] [PubMed] [Google Scholar]
- 26. Leenhouts KJ, Venema G (1993) Lactococcal plasmid vectors. In Hardy KG, Plasmids, a practical approach. Oxford University Press; pp. 65-94, Oxford, United Kingdom [Google Scholar]
- 27. Leenhouts K, Bolhuis A, Venema G, Kok J (1998) Construction of a food-grade multiple-copy integration system for Lactococcus lactis . Appl Microbiol Biotechnol 49: 417–423. doi:10.1007/s002530051192. PubMed: 9615484. [DOI] [PubMed] [Google Scholar]
- 28. Leenhouts K, Buist G, Kok J (1999) Anchoring of proteins to lactic acid bacteria. Antonie Van Leeuwenhoek 76: 367–376. doi:10.1023/A:1002095802571. PubMed: 10532392. [PubMed] [Google Scholar]
- 29. Leenhouts K, Buist G, Bolhuis A, ten Berge A, Kiel J et al. (1996) A general system for generating unlabelled gene replacements in bacterial chromosomes. Mol Gen Genet 253: 217–224. doi:10.1007/s004380050315. PubMed: 9003306. [DOI] [PubMed] [Google Scholar]
- 30. Lortal S, Chapot-Chartier MP (2005) Role, mechanisms and control of lactic acid bacteria lysis in cheese. Int Dairy J 15: 857–871. doi:10.1016/j.idairyj.2004.08.024. [Google Scholar]
- 31. Maguin E, Duwat P, Hege T, Ehrlich D, Gruss A (1992) New thermosensitive plasmid for gram-positive bacteria. J Bacteriol 174: 5633–5638. PubMed: 1324906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B et al. (2006) Comparative genomics of the lactic acid bacteria. Proc Natl Acad Sci U S A 103: 15611–15616. doi:10.1073/pnas.0607117103. PubMed: 17030793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ohnuma T, Onaga S, Murata K, Taira T, Katoh E (2008) LysM Domains from Pteris ryukyuensis Chitinase-A: A stability study and characterization of the chitin-binding site. J Biol Chem 283: 5178–5187. PubMed: 18083709. [DOI] [PubMed] [Google Scholar]
- 34. Quénée P, Lepage E, Kim WS, Vergnaud G, Gruss A (2005) Minisatellite polymorphism as a tool to distinguish closely related Lactococcus lactis strains. FEMS Microbiol Lett 248: 101–109. doi:10.1016/j.femsle.2005.05.026. PubMed: 15963663. [DOI] [PubMed] [Google Scholar]
- 35. Ramasamy R, Yasawardena S, Kanagaratnam R, Buratti E, Baralle FE et al. (1999) Antibodies to a merozoite surface protein promote multiple invasion of red blood cells by malaria parasites. Parasite Immunol 21: 397–407. doi:10.1046/j.1365-3024.1999.00239.x. PubMed: 10417674. [DOI] [PubMed] [Google Scholar]
- 36. Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 37. Seegers JF, Bron S, Franke CM, Venema G, Kiewiet R (1994) The majority of lactococcal plasmids carry a highly related replicon. Microbiology 140: 1291–1300. doi:10.1099/00221287-140-6-1291. PubMed: 8081493. [DOI] [PubMed] [Google Scholar]
- 38. Siezen RJ, Bayjanov J, Renckens B, Wels M, van Hijum SA et al. (2010) Complete genome sequence of Lactococcus lactis subsp. lactis KF147, a plant-associated lactic acid bacterium. J Bacteriol 192: 2649–2650. doi:10.1128/JB.00276-10. PubMed: 20348266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steen A, Buist G, Horsburgh GJ, Venema G, Kuipers OP et al. (2005) AcmA of Lactococcus lactis is an N-acetylglucosaminidase with an optimal number of LysM domains for proper functioning. FEBS J 272: 2854–2868. doi:10.1111/j.1742-4658.2005.04706.x. PubMed: 15943817. [DOI] [PubMed] [Google Scholar]
- 40. Steen A, Buist G, Kramer NE, Jalving R, Benus GF et al. (2008) Reduced lysis upon growth of Lactococcus lactis on galactose is a consequence of decreased binding of the autolysin AcmA. Appl Environ Microbiol 74: 4671–4679. doi:10.1128/AEM.00103-08. PubMed: 18539791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Steen A, Buist G, Leenhouts KJ, El Khattabi M, Grijpstra F et al. (2003) Cell wall attachment of a widely distributed peptidoglycan binding domain is hindered by cell wall constituents. J Biol Chem 278: 23874–23881. doi:10.1074/jbc.M211055200. PubMed: 12684515. [DOI] [PubMed] [Google Scholar]
- 42. Steen A, Palumbo E, Deghorain M, Cocconcelli PS, Delcour J et al. (2005) Autolysis of Lactococcus lactis is increased upon D-alanine depletion of peptidoglycan and lipoteichoic acids. J Bacteriol 187: 114–124. doi:10.1128/JB.187.1.114-124.2005. PubMed: 15601695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steen A, van Schalkwijk S, Buist G, Twigt M, Szeliga M et al. (2007) Lytr, a phage-derived amidase is most effective in induced lysis of Lactococcus lactis compared with other lactococcal amidases and glucosaminidases. Int Dairy J 17: 926–936. doi:10.1016/j.idairyj.2006.12.007. [Google Scholar]
- 44. Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76: 4350–4354. doi:10.1073/pnas.76.9.4350. PubMed: 388439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Die IM, Bergmans HE, Hoekstra WP (1983) Transformation in Escherichia coli: studies on the role of the heat shock in induction of competence. J Gen Microbiol 129: 663–670. PubMed: 6348205. [DOI] [PubMed] [Google Scholar]
- 46. Visweswaran GRR, Dijkstra BW, Kok J (2010) Two major archaeal pseudomurein endoisopeptidases: PeiW and PeiP. Archaea, 2010: 2010: 480492. PubMed: 21113291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Visweswaran GRR, Dijkstra BW, Kok J (2011) A minimum of three motifs is essential for optimal binding of pseudomurein cell wall-binding domain of Methanothermobacter thermautotrophicus . PLOS ONE 6(6): e21582. doi:10.1371/journal.pone.0021582. PubMed: 21738718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Visweswaran GRR, Dijkstra BW, Kok J (2011) Murein and pseudomurein cell wall binding domains of bacteria and archaea - a comparative view. Appl Microbiol Biotechnol 92: 921–928. doi:10.1007/s00253-011-3637-0. PubMed: 22012341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Visweswaran GR, Dijkstra BW, Kok J (2012) A genetically engineered protein domain binding to bacterial murein, archaeal pseudomurein, and fungal chitin cell wall material. Appl Microbiol Biotechnol 96(3): 729–737. doi:10.1007/s00253-012-3871-0. PubMed: 22262228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wegmann U, O’Connell-Motherway M, Zomer A, Buist G, Shearman C et al. (2007) Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol 189: 3256–3270. doi:10.1128/JB.01768-06. PubMed: 17307855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zabarovsky ER, Winberg G (1990) High efficiency electroporation of ligated DNA into bacteria. Nucleic Acids Res 18: 5912. doi:10.1093/nar/18.19.5912. PubMed: 2216800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of 2D-gel images of supernatant fractions of L. lactis IL1403acmA::ISS1 and L. lactis IL1403acmA::ISS1acmD::myc. xmlns:xlink="http://www.w3.org/1999/xlink" xmlns:mml="http://www.w3.org/1998/Math/MathML">The amount of protein loaded in both cases was the equivalent of supernatant fraction of 100 ml of a GM17 culture with an optical density at 600 nm of 1.0. The position of the spots of the AcmD and AcmD::myc proteins, identified by Mass-spectroscopic analysis, and their molecular weights and pIs are indicated. Proteins that were more abundant in the supernatant fraction of IL1403acmA::ISS1 (blue) or IL1403acmA::ISS1acmD::myc (red), and those unique in the supernatant of IL1403acmA::ISS1 (yellow) or IL1403acmA::ISS1acmD::myc (green) are indicated. Sizes of the pre-stained molecular mass marker (kDa) are indicated in the middle.
(TIF)
Deletion of acmD does not affect cell lysis during growth. Release of intracellular X-prolyl dipeptidyl aminopeptidase (PepX) from L. lactis IL1403 (♦), IL1403acmA::ISS1 (▲) and IL1403acmA::ISS1acmD::myc (■). Samples were taken at the indicated time points from the bacterial cultures incubated in GM17 broth. Upon removal of the cells by centrifugation the PepX-activity (in arbitrary units) released into the medium due to autolysis was determined using a chromogenic substrate, as described in the Materials and Methods section.
(TIF)
Expression of LysMAcmD-GFP-His10 in E. coli. Coomassie brilliant blue-stained SDS- (15%) PAA-gel (left) and in-gel GFP- fluorescence (right) showing the expression of LysMAcmD-GFP-His10 on SDS- PAGE with a 15% PAA gel. E. coli MC1061 bearing the pBADcLIC-LysMAcmD was grown at 37° C until OD600 of 0.8 and induced with 0.2% arabinose for 2 h (see Materials and Methods section). The cell extracts of control and test samples were loaded on PAA gel for the identification of specific protein band. For the latter figure, the PAA gel is exposed to UV-light prior to coomassie staining for imaging the fluorescent bands. Prestained protein marker lane 1, cell extracts of empty vector control strain, un-induced control and 0.2%-arabinose induced test samples in lanes 2, 3 and 4, respectively. Arrows indicate LysMAcmD-GFP-His10 protein/activity bands.
(TIF)
Negative and autofluorescence controls Phase-contrast and fluorescence microscopy of L. lactis NZ9000 cells incubated at pH 4.0 with HIC-purified GFP (a) and without addition of any recombinant protein (b). Original magnification: 1250-fold in all frames.
(TIF)
Binding of LysMAcmD-GFP-His10 to L. lactis NZ9000 cells at pH 6.0 and 8.0. Phase-contrast and fluorescence microscopy of L. lactis NZ9000 cells incubated with LysMAcmD-GFP-His10 at pH 6.0 (a) and 8.0 (b). Original magnification: 1250-fold in all frames.
(TIF)


