Abstract
Combination therapy with a dipeptidyl peptidase (DPP)-4 inhibitor and metformin or sulfonylurea results in substantial and additive glucose-lowering effects in patients with type 2 diabetes mellitus (T2DM). However, it is not known whether triple combination therapy with a DPP-4 inhibitor, metformin, and sulfonylurea has greater additive effects or synergic effects. In the present report, we investigated the effect of addition of sitagliptin, the first-in-class DPP-4 inhibitor, to ongoing metformin and sulfonylurea therapy in three female Japanese patients with T2DM who refused insulin therapy. Combined treatment with all three drugs resulted in marked improvements in HbA1c. In the first patient, HbA1c levels decreased from 11.1% to 6.1% after the addition of sitagliptin to metformin 1000 mg, glibenclamide, and miglitol, even though the dose of glibenclamide was decreased. HbA1c levels decreased similarly in the second patient, who was being treated with metformin and glibenclamide, from 7.9% to 6.0% after addition of sitagliptin and an increase in metformin to 2250 mg; this patient ceased glibenclamide because of hypoglycemia and instead was started on low-dose glimepiride. In the third patient, HbA1c levels decreased from 8.6% to 7.1% after addition of glimepiride to ongoing sitagliptin and metformin therapy. All three patients had refused insulin therapy, despite the fact that ongoing combination therapy had failed to achieve satisfactory glycemic control. Based on these results, it is likely that the addition of sitagliptin to metformin and at least a small dose of sulfonylurea may be effective in reducing HbA1c levels without weight gain. This triple combination therapy may prove useful in at least some patients who need initiation of insulin therapy.
Keywords: type 2 diabetes mellitus, oral antidiabetic drug, combination therapy, dipeptidyl peptidase-4 inhibitor, sitagliptin, metformin, sulfonylurea
Introduction
Impaired insulin secretion and insulin resistance both contribute to the pathogenesis of type 2 diabetes mellitus (T2DM). Different oral antidiabetic drugs are used to treat T2DM depending on the pathogenesis in individual patients. For example, α-glucosidase inhibitors are used to treat postprandial hyperglycemia, biguanide or thiazolidinediones are used in cases of insulin resistance, and glinides or sulfonylureas are used in patients with deficient insulin secretion. In Japan, most cases of diabetes start with decreased insulin secretion but, in a small group of patients, particularly obese ones, diabetes may start with insulin resistance.1 In a previous study, we found that sulfonylureas are still the mainstay of treatment of T2DM in Japan,2 with almost 60% of Japanese patients with the disorder being treated with sulfonylurea monotherapy.2
Treatment with a single oral antidiabetic drug sometimes fails to achieve and maintain sufficient glycemic control in patients with T2DM. In our previous study, 14% of patients treated with sulfonylurea monotherapy had HbA1c levels ≥8.0%.2 Therefore, for many patients, combination therapy or initiation of insulin is the next therapeutic stage.3 However, there may be an unwillingness to initiate insulin therapy on the part of both patients and physicians (specifically general practitioners) because of theoretical concerns (eg, hypoglycemia, weight gain, and a belief that insulin has negative metabolic effects) and practical concerns (eg, patient anxiety about insulin, patients’ cognitive abilities, and the complexity of proper insulin use).4
The recent introduction of dipeptidyl peptidase (DPP)-4 inhibitors has implications for combination therapy with metformin or sulfonylurea.5,6 Initial investigations into the combination of a DPP-4 inhibitor and metformin or sulfonylurea reported substantial additive glucose-lowering effects of combination therapy in patients with T2DM.5,6 However, it has not yet been determined whether triple combination therapy with a DPP-4 inhibitor, metformin, and sulfonylurea has further beneficial effects on glycemic control in patients with T2DM or in which patients this type of treatment regimen is the most effective. The combination of a DPP-4 inhibitor and high-dose metformin (>750 mg/day) has been approved for use in Japan since 2010. We have had experience with three T2DM patients in whom HbA1c levels were markedly improved after the addition of sitagliptin, the first-in-class DPP-4 inhibitor, to ongoing sulfonylurea and high-dose metformin treatment. This HbA1c reduction was higher than mean HbA1c levels mentioned in previous reports.5,6 Herein we report on the clinical course of these patients for further consideration of triple combination therapy as a possible oral antidiabetic drug regimen.
Case Series
Patient 1
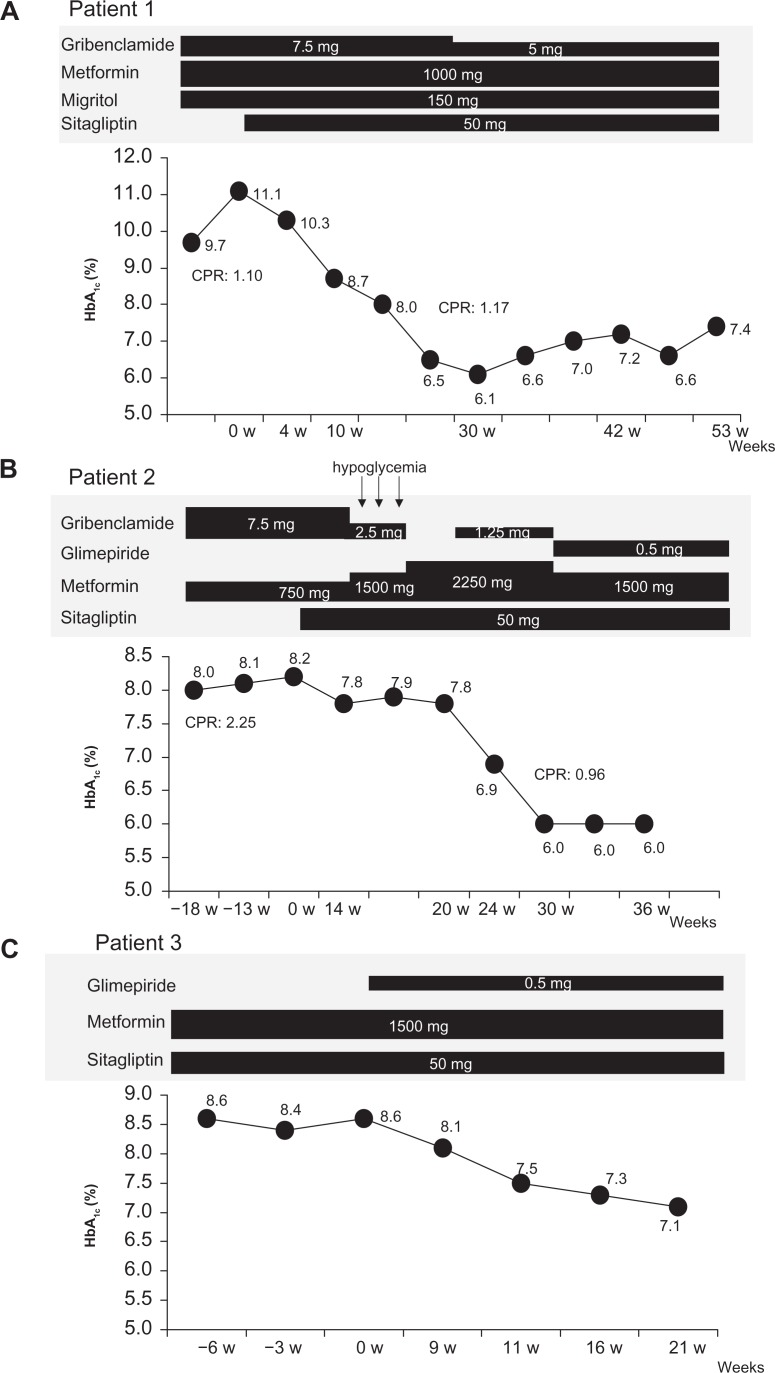
The first patient was a 53-year-old woman who was diagnosed with T2DM at 47 years of age. She had been on 7.5 mg glibenclamide, 1000 mg metformin, and 150 mg miglitol for the previous 3 years, during which time her HbA1c levels never went below 8.9%. The patient was advised to start insulin therapy on many occasions, but she refused because of a fear of injections and hypoglycemia. In December 2009, the patient’s body mass index was 24.4 kg/m2 and she had no diabetic microangiopathy (Table 1). She had 2.5 mg of amlodipine besilate for hypertention. A peripheral blood count revealed that the patient was not anemic, and blood chemistry evaluations indicated normal liver and renal function, including estimated glomerular filtration rate (Table 1). Because the patient’s HbA1c level deteriorated to 11.1% and she still refused to initiate insulin therapy, 50 mg sitagliptin was added to ongoing glibenclamide, metformin, and miglitol. The patient’s HbA1c level decreased gradually, reaching 6.1% over a period of 24 weeks from starting sitagliptin (Fig. 1A). The patient’s fasting plasma glucose improved from 147 mg/dL to 127 mg/dL, whereas there were no significant changes in C-peptide immunoreactivity and body mass index (from 1.10 to 1.17 ng/mL and from 24.4 to 24.6 kg/m2, respectively, Table 1). The patient’s HbA1c levels were maintained between 6.6% and 7.4% over period of 60 weeks after starting sitagliptin, despite a reduction in the daily dose of glibenclamide to 5 mg (Fig. 1A).
Table 1.
Patient characteristics and changes in parameters before and after the addition of sitagliptin.
| Patient 1 | Patient 2 | Patient 3 | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Before | After | Before | After | Before | After | |
| Age (years) | 53 | 74 | 71 | |||
| Gender | Female | Female | Female | |||
| Duration of DM (years) | 6 | 21 | 30 | |||
| Complications of DM | None | Microalbuminuria, Neuropathy | None | |||
| eGFR (mL/min/1.73 m2) | 97.9 | 61.3 | 94.2 | |||
| Combined therapy | Glibenclamide (7.5 mg/day) | Glibenclamide (5 mg/day) | Glibenclamide (7.5 mg/day) | Glimepiride (0.5 mg/day) | Metformin (1500 mg/day) | Glimepiride (0.5 mg/day) |
| Metformin (1000 mg/day) | Metformin (1000 mg/day) | Metformin (750 mg/day) | Metformin (1500 mg/day) | Metformin (1500 mg/day) | ||
| Miglitol (150 mg/day) | Miglitol (150 mg/day) | |||||
| BMI (kg/m2) | 24.4 | 24.6 | 24.6 | 24.1 | 19.5 | 19.8 |
| HbA1c (%) | 11.1 | 6.1 | 8.2 | 6.0 | 8.6 | 7.1 |
| FPG (mg/dL) | 147 | 127 | 96 | 94 | 186 | 112 |
| CPR (ng/mL) | 1.10 | 1.17 | 2.25 | 0.96 | – | – |
Note: Value for HbA1c was expressed by NGSP value.
Abbreviations: BMI, body mass index; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; CPR, C-peptide immunoreactivity; HbA1c, glycosylated hemoglobin.
Figure 1.
Change in HbA1c levels in (A) patient 1, (B) patient 2, and (C) patient 3. In patient 1, a 53-year-old woman with type 2 diabetes mellitus, HbA1c levels decreased from 11.1% to 6.1% after addition of sitagliptin to ongoing metformin, glibenclamide, and miglitol therapy, although the dose of glibenclamide was decreased. In patient 2, a 74-year-old woman with type 2 diabetes mellitus being treated with metformin and glibenclamide, HbA1c levels decreased from 8.2% to 6.0% after the addition of sitagliptin to ongoing therapy and increasing metformin up to the maximum dose. In this patient, glibenclamide was ceased because of hypoglycemia and, instead, low-dose glimepiride was added. Patient 3, a 71-year-old woman with type 2 diabetes mellitus treated with metformin and sitagliptin, exhibited a decrease in HbA1c levels from 8.6% to 7.1% after the addition of glimepiride to ongoing therapy.
Abbreviation: CPR, C-peptide immunoreactivity.
Patient 2
The second patient was a 74-year-old woman who was diagnosed with T2DM at 53 years of age. Although this patient had been receiving treatment with 7.5 mg glibenclamide and 750 mg metformin for several years, her HbA1c levels had never dipped below 8.0%. Her body mass index was 24.6 kg/m2 and she had microalbuminuria and neuropathy (Table 1). She had 10 mg of olmesartan medoxomil for hypertention, 400 mg of bezafibrate for hyper lipidemia, 100 mg of aspirin and 15 microgram of limaprost alfadex for preventuon of cerebral infarction. A peripheral blood count revealed no evidence of anemia, and blood chemistry tests indicated normal liver and renal function, including estimated glomerular filtration rate. Again, it was recommended on many occasions that this patient initiate insulin therapy, but she refused because of a fear of injections and hypoglycemia. Therefore, 50 mg sitagliptin was added to ongoing therapy with 750 mg metformin and 7.5 mg glibenclamide. Over the following month, the patient’s HbA1c levels were reduced by 0.4%, but this effect was less than expected. The dose of metformin was increased to 1500 mg and the patient reported a few episodes of hypoglycemia. The patient herself gradually decreased the daily dose of glibenclamide until she was no longer taking any. The dose of metformin was then increased to 2250 mg instead. However, the patient’s HbA1c level remained at 7.8%. At this point, 1.25 mg glibenclamide was added to ongoing 2250 mg metformin and 50 mg sitagliptin, and the patient’s HbA1c levels improved rapidly, decreasing to 6.0%, where they remained even after 1.25 mg glibenclamide had been changed to 0.5 mg glimepiride and the dose of metformin reduced to 1500 mg (Fig. 1B). The patient did not complain of nausea or anorexia as adverse effects of metformin. The patient’s C-peptide immunoreactivity decreased from 2.25 to 0.96 ng/mL, whereas her fasting plasma glucose and body mass index were almost the same as prior to the addition of sitagliptin (from 96 to 94 mg/dL, from 24.6 to 24.1 kg/m2, respectively, Table 1).
Patient 3
The third patient was a 71-year-old woman who had been diagnosed with T2DM at 41 years of age. This patient’s body mass index was 19.5 kg/m2 and she had no diabetic microangiopathy. She had 5 mg of amlodipine besilate and 40 mg of valsartan for hypertention.
These medication did not change before and after adding sitagliptin. A peripheral blood count revealed no evidence of anemia, and blood chemistry tests indicated normal liver and renal function, including estimated glomerular filtration rate (Table 1). Although this patient was treated with 1500 mg metformin and 50 mg sitagliptin, her HbA1c levels stayed between 8.4% and 8.6%. We added 0.5 mg glimepiride to ongoing therapy, expecting to see a glucose-lowering effect based on our experiences with the previous two patients. Indeed, this patient’s HbA1c levels improved, decreasing to 7.1% at 24 weeks after starting triple combination therapy (Fig. 1C). Her fasting plasma glucose also improved from 186 to 112 mg/dL following the decrease in HbA1c levels, and her body mass index was almost the same as prior to the addition of glimepiride (from 19.5 to 19.8 kg/m2, Table 1).
Discussion
The three cases described herein all had similar difficulties in treatment. Despite optimal combined oral antidiabetic drug therapy, none of the patients achieved satisfactory glycemic control. Furthermore, despite repeated recommendations to initiate insulin therapy, all three patients refused for a variety of reasons (eg, fear of and anxiety regarding injections, inconvenience of injections, concerns regarding hypoglycemia). Although recently in Japan newer therapeutic choices have become available, such as DPP-4 inhibitors and doses >750 mg metformin, in all three cases described herein, we added sitagliptin to ongoing therapy and increased the dose of metformin. In the second case, the addition of sitagliptin did not reduce HbA1c sufficiently; instead, a marked decrease in HbA1c levels was seen after the addition of 1.25 mg glibenclamide and a stepwise increase in the dose of metformin. This suggests that >1000 mg metformin may be necessary but not enough, because addition of sulfonylurea appear remarkable glucose-lowering effect even though decreasing the dose of metformin in parallel. In the first patient, HbA1c levels deteriorated a little following decreasing the dose of sulfonylurea, while her HbA1c levels stayed in a satisfactory range. We considered that this was not denied the effect of the combination therapy and a small dose of sulfonylurea may be preferable for preventing beta-cell function. Based on our experience with the first two patients, we expected that the addition of sulfonylurea to ongoing sitagliptin and >1000 mg metformin would reduce HbA1c levels in the third patient and, indeed, her HbA1c levels improved as expected. This suggests that the adding of sulfonylurea, even in a small dose, may be one of the necessary factors for a remarkable glucose-lowering effect on combination therapy with metformin and sitagliptin.
Large clinical trials have demonstrated that sitagliptin, the first-in-class DPP-4 inhibitor, provides clinically meaningful reductions in HbA1c, as well as in fasting and postprandial glucose concentrations, and is well tolerated as either monotherapy,7 addon therapy to metformin,8 or in initial combination with metformin5 or sulfonylurea.6 These studies reported an average effect of sitagliptin on HbA1c in the range of −0.45% to −1.9% in combination therapy.5,6,8 In the three patients in the present series, reductions in HbA1c levels ranged from −2% to −5% after the addition of sitagliptin to >1000 mg metformin and sulfonylurea. These effects are greater than those reported in the clinical trials, and we suggest that it may be more of a synergistic effect than an additive effect of three oral antidiabetic drugs. In addition, a previous study has reported that administration of an α-glucosidase inhibitor with sitagliptin to nondiabetic men results in higher plasma levels of active glucagon-like peptide-1 and lower plasma levels of total glucose-dependent insulinotropic polypeptide than those seen following the administration of either an α-glucosidase inhibitor or sitagliptin alone.9 However, as yet, there are no results in the literature of clinical trials with an α-glucosidase inhibitor and sitagliptin.
Metformin has been used to control hyperglycemia in T2DM patients for more than 50 years. Metformin is thought to exert its effect primarily by counteracting insulin resistance to crucial hepatic glucose output and by increasing insulin-stimulated glucose uptake in muscle and fat.10 A recent study showed that metformin did not inhibit plasma DPP-4 activity either in vitro or in vivo.11 DPP-4 inhibitors prevent enzymatic degradation and inactivation of glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide,12 known as “incretins.” Incretins are secreted from enteroendocrine cells postprandially and enhance insulin release and reduce glucagon secretion in a glucose-dependent manner13,14 via binding of glucagon-like peptide-1 receptor.15 Conversely, it is suggested that metformin enhances incretin signaling by increasing plasma levels of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, as well as by increasing the expression of both glucagon-like peptide-1 receptor and glucose-dependent insulinotropic polypeptide receptors in the insulin-containing pancreatic beta-cells.16 In mice, metformin doses that increased plasma glucagon-like peptide-1 and inhibited emptying (75 mg/kg and 150 mg/kg) had no consistent effect on hepatic glucose production; however, at a higher dose (300 mg/kg), metformin significantly reduced hepatic glucose production.16 This finding may be compatible with the observations in the present study that fasting plasma glucose was improved, although C-peptide immunoreactivity was not increased in two patients. In addition, this may be another explanation as to why the addition of a high dose of metformin to ongoing sitagliptin therapy had an effective glucose-lowering effect and improved HbA1c levels in our patients.
On the other hand, a recent study showed that sulfonylurea interacts directly with cAMP-regulated guanine nucleotide exchange factor Epac2, resulting in activation of Rap1, a Ras-related GTPase, in pancreatic beta-cells.17,18 Together with the closure of KATP channels via binding of sulfonylurea to its receptor,19 this may be one of the mechanisms underlying the increased glucose-lowering effect and improved HbA1c levels of sitagliptin in combination with sulfonylurea. Further investigations are necessary to determine whether a small dose of sulfonylurea is sufficient for the additive effects of sitagliptin to be manifested. If the dose of sulfonylurea can be decreased in combination treatments with other oral antidiabetic drugs, beta-cell exhaustion, which is induced by long-term overstimulation of beta-cells with sulfonylurea,20 may be avoided.
Herein, we report on three Japanese patients with T2DM in whom triple combination therapy with sitagliptin, metformin, and sulfonylurea resulted in a marked improvement in HbA1c levels without weight gain. Further investigations are necessary to determine whether the addition of sitagliptin to metformin and sulfonylurea has better glucose-lowering effects than other combination oral antidiabetic drug therapies, and in which patients this type of treatment regimen is the most effective. This therapeutic regimen may be useful at least in some patients who need initiate insulin therapy and this therapy may less expense than insulin therapy in Japan.
Footnotes
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Yoshinaga H, Kosaka K. Heterogeneous relationship of early insulin response and fasting insulin level with development of non-insulin-dependent diabetes mellitus in non-diabetic Japanese subjects with or without obesity. Diabetes Res Clin Pract. 1999;44:129–36. doi: 10.1016/s0168-8227(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 2.Arai K, Matoba K, Hirao K, et al. Present status of sulfonylurea treatment for type 2 diabetes in Japan: second report of a cross-sectional survey of 15,652 patients. Endocr J. 2010;57:499–507. doi: 10.1507/endocrj.k09e-366. [DOI] [PubMed] [Google Scholar]
- 3.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–12. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 4.Riddle MC. The underuse of insulin in North America. Diabetes Metab Res Rev. 2002;18(Suppl 3):S42–9. doi: 10.1002/dmrr.277. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein BJ, Johnson J, Feinglos MN, Williams-Herman DE, Lunceford JK, Sitagliptin 036 Study Group Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979–87. doi: 10.2337/dc07-0627. [DOI] [PubMed] [Google Scholar]
- 6.Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P, Sitagliptin 035 Study Group Efficiency and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:737–45. doi: 10.1111/j.1463-1326.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 7.Aschner P, Kinpes MS, Lunceford JK, Sanchez M, Michael C, Williams-Herman DE. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–7. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 8.Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficiency and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638–43. doi: 10.2337/dc06-0706. [DOI] [PubMed] [Google Scholar]
- 9.Aoki K, Masuda K, Miyazaki T, Togashi Y, Terauchi Y. Effect of miglitol, sitagliptin or their combination on plasma glucose, insulin and incretin levels in non-diabetic men. Endocr J. 2010;57:667–72. doi: 10.1507/endocrj.k10e-103. [DOI] [PubMed] [Google Scholar]
- 10.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–9. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 11.Migoya EM, Bergeron R, Miller JL, et al. Dipeptidyl peptidase-4 inhibitors administered in combination with metformin results in an additive increase in the plasma concentration of acive GLP-1. Clin Pharmacol Ther. 2010;88:801–8. doi: 10.1038/clpt.2010.184. [DOI] [PubMed] [Google Scholar]
- 12.Drucker DJ, Nauck MA. GLP-1R agonists (incretin mimetics) and DPP-4 inhibitors (incretin enhancers) for the treatment of type 2 diabetes. Lancet. 2006;368:1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 13.Elrick H, Stimmler L, Hlad CJ, Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocinol Metab. 1964;24:1076–82. doi: 10.1210/jcem-24-10-1076. [DOI] [PubMed] [Google Scholar]
- 14.McIntyre N, Holdsworth CD, Turner DS. Intestinal factors in the control of insulin secretion. J Clin Endocrinol Metab. 1965;25:1317–24. doi: 10.1210/jcem-25-10-1317. [DOI] [PubMed] [Google Scholar]
- 15.Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A. 1992;89:8641–5. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maida A, Lamont BJ, Cao X, Drucker DJ. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia. 2011;54:339–49. doi: 10.1007/s00125-010-1937-z. [DOI] [PubMed] [Google Scholar]
- 17.Zhang CL, Katoh M, Shibasaki T, et al. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science. 2009;325:607–10. doi: 10.1126/science.1172256. [DOI] [PubMed] [Google Scholar]
- 18.Shibasaki T, Takahashi H, Miki T, et al. Essential role of Epac2/Rap 1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci U S A. 2007;104:19333–8. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miki T, Nagashima K, Tashiro F, et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998;95:10402–6. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rustenbeck L. Desensitization of insulin secretion. Biochem Pharmacol. 2002;63:1921–35. doi: 10.1016/s0006-2952(02)00996-6. [DOI] [PubMed] [Google Scholar]