Abstract
Understanding how the timing of exposure to endogenous hormones influences cancer development is critical to elucidating disease etiology. Prolactin increases proliferation and cell motility, processes important in later stage tumor development, suggesting that levels proximate (versus distant) to diagnosis may better predict risk. Thus, we calculated relative risks (RR) and 95% confidence intervals (CI) for prolactin levels on samples collected <10 (proximate) versus ≥10 (distant) years before diagnosis in the Nurses’ Health Study (NHS) and NHSII with breast cancer risk, including in a subset of NHS women providing two samples 10 years apart. We measured prolactin via immunoassay in cases diagnosed from 1990–2010 (NHS) and 1999–2009 (NHSII) and matched controls. Overall, 2,468 cases and 4,021 controls had prolactin measured <10 years and 953 cases and 1,339 controls >10 years before diagnosis/reference date. There was an increased risk for higher proximate prolactin levels (RR, >15.7 vs. ≤8.1 ng/mL [i.e. top vs. bottom quartiles]=1.20, 95%CI=1.03–1.40, p-trend=0.005), but not for distant levels (RR=0.97, p-trend=0.94); results were similar among women with two blood samples (p-interaction, proximate versus distant=0.07). The positive association was stronger for ER+ disease (RR=1.28, p-trend=0.003) and postmenopausal women (RR=1.37, p-trend=0.0002). Among postmenopausal women, the association was strongest for ER+ disease (RR=1.52) and lymph node positive cases (RR=1.63). Our data suggest that prolactin levels measured <10 years prior to diagnosis are most strongly associated with postmenopausal breast cancer risk, especially for ER+ tumors and metastatic disease. This corresponds with biologic data that prolactin is etiologically important in tumor promotion.
Keywords: Prolactin, breast cancer, estrogen receptor, postmenopausal
Introduction
Prolactin is an important growth hormone involved in breast development and lactation (1) that has been positively associated with breast cancer risk in two large prospective studies (2,3), although three smaller studies did not observe a significant association (4–6). Further a study of 384 hyperprolactinemia patients did not observe an increased risk of breast cancer (7). Experimental data strongly support a role of prolactin in breast cancer development, particularly by increasing cell proliferation, tumor vascularization, and cell motility, which are important in promoting late-stage carcinogenesis and possibly metastasis (1,8). This suggests that high levels of circulating prolactin may be more strongly associated with more aggressive breast cancer phenotypes or when measured more proximate to diagnosis. However, prior epidemiologic studies had less than 10 years of follow-up after blood collection (2,3) or had small numbers of cases (4–7), precluding a detailed analysis by time between blood collection and diagnosis.
Therefore, we examined the relationship between prolactin and risk of breast cancer for prolactin levels measured <10 versus ≥10 years before breast cancer diagnosis (or reference date for matched controls) in a nested case-control study in the Nurses’ Health Study (NHS) and NHSII, with up to 20 years of follow-up. We then evaluated this association in a subset of NHS women who provided blood samples twice approximately 10 years apart to allow for simultaneous control of prolactin levels at two points in time. In addition, we evaluated potential differences in association by menopausal status, associations by tumor characteristics, and a continuous analysis of the prolactin association by time between blood collection and diagnosis. Compared to our prior analysis (2), this study contains nearly 1,400 more cases and an additional 10 years of follow-up.
Methods
Study Population
The NHS is a prospective cohort study established in 1976 when 121,700 US female registered nurses completed a baseline questionnaire (ages 30–55 years). Similarly, the NHSII was established in 1989 with 116,430 women ages 25–42 years. Women in both cohorts consented to participate and have completed biennial questionnaires since study inception to report exposure status and disease diagnoses.
From 1989–1990, 32,826 NHS women, ages 43–70 years, submitted a heparin blood sample and completed a short questionnaire (9). Participants were mailed a blood collection kit and shipped back the specimen with an ice pack by overnight courier to our laboratory where it was processed and separated into plasma, red blood cell, and white blood cell components. Then, from 2000–2002, we collected a second blood sample from a subset of these women (n=18,743 women, ages 53–80 years and >98% postmenopausal) using the same protocol as in the original collection (10).
In the NHSII, blood samples were collected from 29,611 women (ages 32–54 years) between 1996–1999 (11). Briefly, premenopausal women (n=18,521) who had not taken hormones, been pregnant, or lactated within the prior 6 months provided a blood sample drawn on the 3rd to 5th day of the menstrual cycle (follicular) and another on the 7th to 9th day before the anticipated start date of their next cycle (luteal); these are called timed samples. Plasma collected in the follicular phase was aliquoted by the participant and frozen. All other women (n=11,090) provided a single untimed blood sample. Luteal and untimed samples were shipped and processed similarly to the NHS.
All samples have been stored in liquid nitrogen freezers since collection. Prolactin has shown to be stable in whole blood that remained unprocessed for 24–48 hours (12). Follow-up of the NHS blood cohort was 97% in 2010 and of the NHSII blood cohort was 95% in 2009. These studies were approved by the Committee on the Use of Human Subjects in Research at the Brigham and Women’s Hospital (Boston, MA).
We considered a woman to be premenopausal if (1) she gave timed samples, (2) her periods had not ceased, or (3) she had a hysterectomy with at least one ovary remaining and was 47 years or younger (nonsmokers) or 45 years or younger (smokers). We considered a woman to be postmenopausal if (1) her natural menstrual periods had ceased permanently, (2) she had a bilateral oophorectomy, or (3) she had a hysterectomy with at least one ovary remaining and was 56 years or older (nonsmokers) or 54 years or older (smokers). The remaining women, most of whom had a simple hysterectomy and were 48 to 55 years old, were of unknown menopausal status.
We included cases diagnosed after blood collection but before June 1, 2010 (NHS) or June 1, 2009 (NHSII). We previously published on cases diagnosed through June 1, 2000 in the NHS and June 1, 2003 in the NHSII (2). Cases were matched to one or two controls on menopausal status at baseline and diagnosis (premenopausal, postmenopausal, unknown), age (+/−2 years), month of blood collection (+/−1 month), time of day of blood draw (in two hour increments), fasting status (<8 vs. ≥8 hours), postmenopausal hormone use at blood draw if postmenopausal (yes vs. no), and luteal day of blood draw if providing a timed sample.
Laboratory Assays
Prolactin was measured by microparticle enzyme immunoassay. Samples were assayed at the Clinical Laboratory Research Core at the Massachusetts General Hospital in eleven batches, using the ARCHITECT® chemiluminescence immunoassay system (Abbott Diagnostics, Chicago, IL), except for 164 cases and 245 controls (NHS) assayed by Christopher Longcope, MD (University of Massachusetts Medical Center, Worcester, MA), in three batches, using the IMx System (Abbott Laboratory, Abbott Park, IL). The correlation between the two laboratories was 0.91, and across different batches within the same data set was more than 0.95 (13). The limit of detection was 0.6 ng/mL; no samples were below the limit of detection.
In the NHS, we assayed prolactin levels in the baseline blood samples of all cases (invasive and in situ) and matched controls diagnosed through 2006 and invasive cases and matched controls diagnosed from 2006–2010. Additionally for NHS cases and matched controls diagnosed from 2000–2010, we also assayed their second blood sample if one was available; the blood had to have been collected before disease diagnosis. In the NHSII, we assayed prolactin levels in the baseline blood samples of all cases and matched controls through 2003 and from 2007–2009. For cases (and matched controls) diagnosed from 2003–2007, we assayed only those who were premenopausal or of unknown menopausal status at blood collection (about 85% of cases diagnosed during this time period were postmenopausal at blood draw) for cost saving purposes.
The intraclass correlation (ICC) over three years has been assessed previously among postmenopausal women (ICC=0.53) (14) and among premenopausal women (15); in the latter population, the ICC in the follicular phase was 0.55, in the luteal phase was 0.41, and for the average of the two phases was 0.64. Since there was not a substantial difference in prolactin levels by menstrual phase and the ICC was the highest for the average of the follicular and luteal levels, we used the average levels for NHSII cases and controls with timed samples. Case-control sets, samples from the baseline and follow-up blood collections (NHS), and follicular and luteal samples (NHSII) were assayed together, ordered randomly within a set, and labeled with unique IDs. Case-control sets were randomly ordered within a batch, although NHS and NHSII samples were assayed in separate batches. The mean coefficient of variation from blinded replicate samples across all batches was 7.8% (SD=3.5%).
Statistical Analysis
Outliers (>139 ng/mL, n=10; <0.6, n=1) were excluded (16). We had 2,904 distinct cases and 4,748 distinct controls available for the analysis; of these, 518 cases and 613 controls from the NHS had two prolactin measures from the baseline and follow-up blood collections. Using all the data, there were 2,468 cases and 4,021 controls with prolactin assayed on samples collected <10 years before diagnosis in cases/reference date for controls (this was set to be the same as the diagnosis date of the matched case), and 953 cases and 1,339 controls with prolactin assayed on samples collected ≥10 years before diagnosis/reference date. Mean prolactin concentrations in quality control samples differed slightly by batch, indicating some laboratory drift over time. Therefore, we adjusted prolactin levels for batch according to the methods described by Rosner et al. (17).
Relative risks (RR) and 95% confidence intervals (CI) were determined using unconditional logistic regression comparing quartiles (cut points based on control distribution in the entire data set) of prolactin concentrations for samples collected <10 and ≥10 years before diagnosis (18). The results for unconditional logistic regression were similar to those using conditional logistic regression (data not shown); the former was used to maximize the number of controls in each analysis to increase statistical power. Tests for trend were modeled using quartile medians and assessed using the Wald statistic. When using all samples, we conducted separate models for prolactin levels measured <10 years before diagnosis and ≥10 years before diagnosis. We adjusted for age at blood draw, date of blood draw, fasting status and time of day of blood draw, menopausal status/postmenopausal hormone (PMH) use at blood draw, body mass index (BMI), age at menarche, history of benign breast disease (BBD), family history of breast cancer, age at menopause, average childhood body size at ages 5 and 10, and cohort. We considered separate adjustment for parity, as this may be part of the biologic pathway through which prolactin affects breast cancer (19). We investigated potential effect modification by menopausal status at blood collection by including a multiplicative interaction term between menopausal status and the prolactin quartile median variable, using the Wald test; we used a similar approach to assess potential effect modification by PMH use among postmenopausal women and parity.
Among cases and controls who provided two blood samples, we simultaneously adjusted for prolactin values measured in 1989–1990 and 2000–2002. The intra-class correlation over 10 years calculated in the NHS women who gave two blood samples was 0.39 (95%CI=0.34–0.44); the ICC was similar regardless of menopausal status at the two blood draws (data not shown). We also evaluated the associations of average prolactin levels across the two blood collections, the percent change in prolactin levels over time, and a cross-classification of values (cut point at the median). We examined all women and those women who were postmenopausal at both blood collections. These models were adjusted for age at first blood draw, date of first blood draw, time between first and second blood draw, fasting status and time of day of both blood draws, menopausal status/PMH use at both blood draws, BMI at first blood draw, change in weight between blood draws, age at menarche, history of benign breast disease, family history of breast cancer, age at menopause at second blood draw, and average childhood body size at ages 5 and 10. For the percent change analysis, we additionally adjusted for ln-transformed prolactin levels in 1989–1990 (baseline).
Among postmenopausal women with blood samples collected within 10 years of diagnosis (the only group in which we observed significant association), we further stratified by tumor invasiveness, estrogen receptor (ER) and progesterone receptor (PR) status, Human Epidermal Growth Factor Receptor 2 (HER2) status, histologic type, tumor size, grade, lymph node status, and luminal A (ER+ or PR+, HER2−, and low or intermediate grade) versus luminal B (ER+ or PR+ and either HER2+ or HER2- and high grade) tumors. Tests for heterogeneity were assessed using polytomous unconditional logistic regression (18). We also evaluated associations for triple negative tumors (ER, PR and HER2 negative) and cases who had recurrent breast cancer or died from breast cancer.
To more carefully evaluate whether the effect of prolactin levels on the risk of breast cancer depended on the duration of time between blood draw and cancer diagnosis, we estimated a time-varying hazard ratio (HR) and 95% CI for breast cancer, adjusting for covariates as noted above. We treated time from blood draw to breast cancer diagnosis as the outcome variable, which is observed exactly among cases and right-censored at the last follow-up time (June 1, 2010) for controls. We evaluated postmenopausal women comparing prolactin levels above versus below the median.
All p-values were 2-sided and were considered statistically significant if <0.05. All analyses were conducted using SAS, version 9.1 (SAS Institute Inc., Cary, North Carolina), STATA, version 12.1 (StataCorp. College Station, TX, USA), and R software, version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Among cases and controls whose blood was drawn <10 years before diagnosis (proximate) or 10–20 years before diagnosis (distant), cases were more likely to have a history of benign breast disease and a family history of breast cancer, but lower parity compared to controls (Table 1A). Median prolactin levels were slightly higher in cases with a proximate blood draw than controls (11.7 ng/mL in cases and 11.2 ng/mL in controls), but levels were more similar comparing cases with a distant blood draw versus controls (10.7 vs. 10.5 ng/mL, respectively). Results were similar for women who provided samples at both blood collections (Table 1B).
Table 1.
Characteristics at blood collection of cases and matched controls, stratified by having blood drawn <10 (proximate) or 10–20 years (distant) before diagnosis or reference date.
|
A. Among all cases and controls (NHS and NHSII) who provided blood samples.
| ||||
|---|---|---|---|---|
| Characteristic | Distant Blood | Proximate Blood | ||
| Cases (n=953) | Controls (n=1339) | Cases (n=2468) | Controls (n=4021) | |
| Mean (SD) | ||||
| Age (yr)1 | 54.2 (7.6) | 54.7 (8.0) | 55.7 (10.0) | 54.3 (10.1) |
| BMI (kg/m2) | 25.6 (4.2) | 25.6 (4.9) | 25.6 (4.9) | 25.8 (5.2) |
| Age at Menopause (yr)2 | 49.5 (3.6) | 49.2 (3.8) | 49.9 (3.4) | 49.7 (3.4) |
| Age at Menarche (yr) | 12.5 (1.4) | 12.6 (1.4) | 12.5 (1.5) | 12.5 (1.4) |
| Average childhood body size3 | 2.4 (1.2) | 2.5 (1.3) | 2.3 (1.2) | 2.5 (1.3) |
| Time from blood draw to diagnosis (yr) | 14.0 (2.8) | NA | 5.1 (3.0) | NA |
| n (%) | ||||
| Postmenopausal1 | 547 (57.4) | 826 (61.7) | 1445 (58.6) | 2004 (49.8) |
| PMH user1,2 | 280 (51.2) | 290 (35.1) | 875 (60.6) | 920 (45.9) |
| History of BBD | 410 (43.0) | 445 (33.2) | 1046 (42.4) | 1271 (31.6) |
| Family history of breast cancer | 135 (14.2) | 162 (12.1) | 439 (17.8) | 452 (11.2) |
| Parous | 848 (89.0) | 1202 (89.8) | 2147 (87.0) | 3519 (87.5) |
| Oral steroid use | 16 (1.7) | 27 (1.2) | 48 (2.0) | 62 (1.5) |
| Antidepressant use | 76 (9.1) | 85 (7.7) | 213 (8.6) | 345 (8.6) |
| NHS1 | 837 (87.8) | 1107 (82.7) | 1795 (72.7) | 2677 (66.6) |
| Median (10th–90th percentile) | ||||
| Prolactin, ng/mL | 10.7 (5.9–21.4) | 10.5 (5.9–22.6) | 11.7 (6.4–22.6) | 11.2 (6.3–22.6) |
|
B. Among women in the NHS who provided two blood samples 10 years apart.
| ||||
|---|---|---|---|---|
| Characteristic | Cases (n=518) | Controls (n=613) | ||
| Distant Blood | Proximate Blood | Distant Blood | Proximate Blood | |
| Mean (SD) | ||||
| Age (yr)1 | 55.4 (6.9) | 66.5 (6.9) | 56.5 (6.8) | 67.5 (6.8) |
| BMI (kg/m2) | 25.6 (4.1) | 26.9 (5.0) | 25.4 (4.7) | 26.4 (5.1) |
| Age at Menopause (yr)2 | 49.6 (3.6) | 50.2 (4.1) | 49.1 (4.1) | 49.6 (4.4) |
| Age at Menarche (yr) | 12.5 (1.4) | 12.6 (1.4) | ||
| Average childhood body size3 | 2.3 (1.2) | 2.5 (1.4) | ||
| Time from blood draw to diagnosis (yr) | 14.7 (2.5) | 3.6 (2.5) | NA | NA |
| n (%) | ||||
| Postmenopausal1 | 322 (62.1) | 509 (98.3) | 420 (68.5) | 604 (98.5) |
| PMH user1,2 | 171 (53.1) | 348 (68.4) | 168 (40.0) | 344 (57.0) |
| History of BBD | 235 (45.4) | 325 (62.7) | 241 (39.3) | 336 (54.8) |
| Family history of breast cancer | 68 (13.1) | 112 (21.6) | 73 (11.9) | 102 (16.6) |
| Oral steroid use | 8 (1.6) | 6 (1.2) | 12 (2.0) | 8 (1.3) |
| Antidepressant use | 18 (3.5) | 66 (12.8) | 22 (3.6) | 67 (11.0) |
| Parous | 473 (91.3) | 572 (93.3) | ||
| Median (10th–90th percentile) | ||||
| Prolactin, ng/mL | 10.6 (5.8–22.0) | 11.7 (6.4–19.9) | 10.3 (5.9–21.0) | 10.4 (6.5–20.9) |
Matching factor, cases who were postmenopausal and not taking hormones or premenopausal (NHS2) had two matched controls, all other cases had one matched control.
Among postmenopausal women
Based on the Stunkard scale of nine body shapes; averaged for ages 5 and 10 years old.
In multivariate analyses, we observed an increased risk for higher proximate prolactin levels (RR, >15.7 vs. ≤8.1 ng/mL [i.e., top vs. bottom quartile]=1.20, 95%CI=1.03–1.40, p-trend=0.005), but not for distant levels (comparable RR=0.97, p-trend=0.94) (Table 2). The positive association for proximate blood levels was only observed in postmenopausal women (comparable RR=1.37, p-trend=0.0002, n=1,445 cases) and not in premenopausal women (comparable RR=1.05, p-trend=0.54, n=1,023 cases), with a p-heterogeneity=0.01. Distant prolactin levels measured during premenopause or postmenopause were not associated with breast cancer risk. Since the majority of premenopausal women were from the NHSII and most postmenopausal women were from the NHS, we considered whether there was heterogeneity by cohort. The p-heterogeneity by cohort was 0.17. Associations for proximate prolactin levels were stronger for ER+ disease; comparing women with >15.7 versus <8.1 ng/mL of prolactin the RR was 1.28 (95%CI=1.07–1.54, p-trend=0.003) for all women and 1.52 (95%CI=1.19–1.93, p-trend=0.0002) for postmenopausal women. No associations were noted for distant prolactin levels and ER+ disease overall or by menopausal status. Adjustment for parity did not alter the results; for example, among all women the RR (95%CI; p-trend) for proximate prolactin comparing >15.7 vs. ≤8.1 ng/mL was 1.18 (1.02–1.39; 0.008), for postmenopausal women was 1.35 (1.10–1.66; <0.001), and for premenopausal women was 1.05 (0.82, 1.34; 0.42). The associations were similar by PMH use among postmenopausal women (p-interaction at proximate blood draw=0.65), by parity (p-interaction=0.31), and after excluding women taking oral steroids or antidepressant medications at blood draw (data not shown).
Table 2.
Relationship between circulating prolactin levels and risk of total and ER+ breast cancer among all cases and controls (NHS and NHSII), by timing of blood draw in relation to breast cancer diagnosis/reference date and menopausal status at blood collection.
| Prolactin <10 years before diagnosis, proximate (ng/mL) | Prolactin 10–20 years before diagnosis, distant (ng/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤8.1 | >8.1–11.0 | >11.0–15.7 | >15.7 | p-trend1 | ≤8.1 | >8.1–11.0 | >11.0–15.7 | >15.7 | p-trend1 | |
| All Cases | ||||||||||
| n, cases/controls | 545/946 | 555/1004 | 696/1039 | 672/1032 | 0.005 | 272/373 | 226/344 | 224/310 | 231/312 | 0.94 |
| RR2 | 1.00 | 0.98 | 1.16 | 1.20 | 1.00 | 0.92 | 0.96 | 0.97 | ||
| 95% CI | (0.84–1.14) | (1.00–1.35) | (1.03–1.40) | (0.72–1.17) | (0.75–1.23) | (0.75–1.25) | ||||
| Premenopausal3 | ||||||||||
| n, cases/controls | 148/287 | 212/424 | 281/586 | 382/720 | 0.54 | 63/75 | 85/104 | 104/134 | 154/200 | 0.98 |
| RR2 | 1.00 | 1.00 | 0.94 | 1.05 | 1.00 | 1.06 | 1.01 | 1.03 | ||
| 95% CI | (0.77–1.29) | (0.73–1.21) | (0.82–1.33) | (0.67–1.67) | (0.66–1.56) | (0.68–1.56) | ||||
| Postmenopausal3 | ||||||||||
| n, cases/controls | 400/670 | 340/569 | 407/430 | 298/335 | 0.0002 | 213/302 | 137/236 | 116/173 | 81/115 | 0.97 |
| RR2 | 1.00 | 1.00 | 1.48 | 1.37 | 1.00 | 0.86 | 0.96 | 0.93 | ||
| 95% CI | (0.83–1.21) | (1.23–1.80) | (1.11–1.69) | (0.65–1.14) | (0.70–1.30) | (0.66–1.33) | ||||
| ER+ Cases | ||||||||||
| n, cases | 314 | 335 | 413 | 400 | 0.003 | 193 | 152 | 146 | 144 | 0.49 |
| RR2 | 1.00 | 1.02 | 1.22 | 1.28 | 1.00 | 0.90 | 0.92 | 0.89 | ||
| 95% CI | (0.85–1.23) | (1.02–1.46) | (1.07–1.54) | (0.69–1.18) | (0.70–1.21) | (0.66–1.18) | ||||
| Premenopausal3 | ||||||||||
| n, cases | 85 | 108 | 162 | 208 | 0.47 | 43 | 63 | 65 | 87 | 0.21 |
| RR2 | 1.00 | 0.82 | 0.87 | 0.98 | 1.00 | 1.05 | 0.88 | 0.81 | ||
| 95% CI | (0.59–1.13) | (0.64–1.18) | (0.73–1.32) | (0.64–1.75) | (0.54–1.45) | (0.51–1.30) | ||||
| Postmenopausal3 | ||||||||||
| n, cases | 229 | 227 | 251 | 192 | 0.0002 | 150 | 89 | 81 | 57 | 0.81 |
| RR2 | 1.00 | 1.16 | 1.58 | 1.52 | 1.00 | 0.81 | 0.98 | 0.97 | ||
| 95% CI | (0.93–1.45) | (1.26–1.98) | (1.19–1.93) | (0.59–1.12) | (0.70–1.39) | (0.65–1.43) | ||||
p-trend determined using the median values of the quartiles as a continuous term.
Unconditional logistic regression models to calculate relative risk (RR) and 95% confidence intervals (95% CI), adjusting for age at blood draw (continuous), date of blood draw (continuous), fasting status and time of day of blood draw (fasting and morning draw, other), menopausal status/PMH use at blood draw (premenopausal/unknown, postmenopausal not using PMH, postmenopausal using PMH), BMI (continuous), age at menarche (continuous), history of BBD (yes, no), family history of breast cancer (yes, no), age at menopause (continuous), average childhood body size at ages 5 and 10 (continuous), and cohort (NHS, NHSII).
Premenopausal (includes women of unknown menopausal status): 403 cases from the NHS and 620 from the NHSII for the proximate analysis and 306 cases from the NHS and 100 cases from the NHSII for the distant analysis. Postmenopausal: 1392 cases from the NHS and 53 from the NHSII for the proximate analysis and 531 cases from the NHS and 16 cases from the NHSII for the distant analysis. For the proximate analysis, the p-interaction by menopausal status was 0.01 for all cases and 0.02 for ER+ cases; the comparable p-interactions by cohort were 0.17 and 0.05, respectively.
We conducted further analyses among women who provided two blood samples (Table 3). Among all women (n=518 cases and 613 controls), when modeling proximate and distant values simultaneously, we observed an RR=1.40 (95%CI=0.96–2.06, p-trend=0.03) comparing the top versus bottom quartile for proximate blood levels, but no association for distant levels (RR=0.83, p-trend=0.43), (p-interaction, proximate versus distant=0.07). Among 322 cases and 420 controls who were postmenopausal at both blood draws, the RR comparing women with prolactin >15.7 versus ≤8.1 ng/mL was 1.75 (p-trend=0.007) for proximate prolactin and 0.66 (p-trend=0.11) for distant prolactin (p-interaction=0.01). The average of the prolactin levels from the two blood collections was not associated with breast cancer risk. However, there was a suggestive positive association for postmenopausal women with a large positive change in prolactin levels between the two blood draws (RR, >41.6% vs. ≤−27% change=1.53, 95%CI=0.88–2.66, p-trend=0.05). Also, when stratifying prolactin levels from the two blood draws, only women with prolactin levels above the median for the proximate blood had an increased risk. The results were similar, although not statistically significant, when examining ER+ cases (N= 350 total ER+ cases with two blood samples and 226 postmenopausal ER+ cases) (data not shown).
Table 3.
Relative risk (95% confidence intervals) between circulating prolactin concentrations and risk of breast cancer among women who gave two blood samples about 10 years apart, by timing of blood draw in relation to breast cancer diagnosis/reference date in the NHS.
| Prolactin concentrations (ng/mL) | |||||
|---|---|---|---|---|---|
| ≤8.1 | >8.1–11.0 | >11.0–15.7 | >15.7 | p-trend1 | |
| Prolactin measured < 10 yr before diagnosis/reference date, proximate | |||||
| n, cases/controls | 118/165 | 108/173 | 166/149 | 125/125 | |
| All women2 | 1.00 | 0.97 (0.68–1.38) | 1.64 (1.16–2.32) | 1.40 (0.96–2.06) | 0.03 |
| Postmenopausal2 | 1.00 | 0.98 (0.63–1.50) | 1.77 (1.14–2.72) | 1.75 (1.07–2.88) | 0.007 |
| Prolactin measured 10–20 yr before diagnosis/reference date, distant | |||||
| n, cases/controls | 150/176 | 123/160 | 124/140 | 120/136 | |
| All women2 | 1.00 | 0.88 (0.62–1.24) | 0.89 (0.62–1.28) | 0.83 (0.56–1.22) | 0.43 |
| Postmenopausal2 | 1.00 | 0.80 (0.54–1.19) | 0.74 (0.48–1.15) | 0.66 (0.39–1.11) | 0.11 |
| Average prolactin levels across the two blood collections | |||||
| n, cases/controls | 98/124 | 135/201 | 172/166 | 112/121 | |
| All women2 | 1.00 | 0.84 (0.59–1.21) | 1.26 (0.88–1.80) | 1.03 (0.69–1.54) | 0.47 |
| Postmenopausal2 | 1.00 | 0.69 (0.46–1.04) | 1.17 (0.77–1.79) | 1.00 (0.59–1.68) | 0.42 |
| Percent change in prolactin levels across the two blood collections | |||||
| ≤−27% | >−27–0.3% | >0.3–41.6% | >41.6% | ||
|
|
|||||
| n, cases/controls | 123/152 | 106/154 | 141/153 | 147/153 | |
| All women2 | 1.00 | 0.94 (0.65–1.37) | 1.32 (0.90–1.95) | 1.30 (0.86–1.97) | 0.13 |
| Postmenopausal2 | 1.00 | 0.89 (0.54–1.49) | 1.49 (0.90–2.48) | 1.53 (0.88–2.66) | 0.05 |
| Cross classification of prolactin levels at first and second blood collections | |||||
| ≤11.0/≤11.0 | ≤11.0/>11.0 | >11.0/≤11.0 | >11.0/>11.0 | ||
|
|
|||||
| n, cases/controls | 149/225 | 124/111 | 77/113 | 167/163 | |
| All women2 | 1.00 | 1.59 (1.12–2.24) | 0.94 (0.64–1.38) | 1.39 (1.01–1.93) | |
| Postmenopausal2 | 1.00 | 1.53 (1.03–2.27) | 0.64 (0.38–1.08) | 1.41 (0.94–2.12) | |
p-trend determined using the median values of the quartiles as a continuous term.
Unconditional logistic regression models to calculate relative risk (RR) and 95% confidence intervals (95% CI), adjusting for age at first blood draw (continuous), date of first blood draw (continuous), time between first and second blood draw (continuous), fasting status and time of day of both blood draws (fasting and morning draw, other), menopausal status/PMH use at both blood draws (premenopausal/unknown, postmenopausal not using PMH, postmenopausal using PMH), BMI at first blood draw (continuous), change in weight between blood draws (continuous), age at menarche (continuous), history of BBD (yes, no), family history of breast cancer (yes, no), age at menopause at second blood draw (continuous), average childhood body size at ages 5 and 10 (continuous). The analysis of postmenopausal women included 321 cases and 419 controls who were postmenopausal at both blood collections.
Model additionally adjusted for continuous ln-transformed prolactin concentrations at first blood draw
Among postmenopausal women in which we had proximate prolactin levels measured, we evaluated associations by breast cancer phenotypes (Table 4). Associations appeared stronger for invasive tumors (RR, >15.7 versus ≤8.1 ng/mL=1.38, p-trend=0.0005) compared to in situ tumors (RR=1.16, p-trend=0.23), although the p-heterogeneity was not statistically significant (p=0.81). Associations were similar for ductal versus lobular tumors, by HER2 status, for luminal A versus B subtypes, tumor size, and grade (p-heterogeneity>0.46). However, associations differed significantly by ER/PR status (p-heterogeneity=0.04). For example, the RR (95%CI) comparing >15.7 versus ≤8.1 ng/mL was 1.53 (1.17–1.98) for ER+/PR+ tumors, 1.61 (0.98–2.65) for ER+/PR- tumors, and 0.88 (0.52–1.47) for ER−/PR− tumors. Prolactin was suggestively more strongly associated with lymph node positive (RR=1.63, 95%CI=1.08–2.44) than lymph node negative (RR=1.33, 95%CI=1.05–1.68) tumors (p-heterogeneity=0.16). Prolactin was not clearly associated with triple negative tumors (n=70 cases, RR, top vs. bottom quartile=0.74, 95%CI=0.33–1.62) or for tumors that recurred or were fatal (n=200 cases, comparable RR=1.30, 95%CI=0.83–2.03), although the number of cases was small (data not shown).
Table 4.
Relationship between proximate prolactin levels and risk of breast cancer among postmenopausal cases and controls (NHS and NHSII), by breast tumor characteristics.
| Prolactin concentrations (ng/mL) | |||||
|---|---|---|---|---|---|
| ≤8.1 | >8.1–11.0 | >11.0–15.7 | >15.7 | p-trend1 | |
| Morphology2 | |||||
| In situ, n | 69 | 46 | 57 | 48 | |
| RR (95%CI)3 | 1.00 | 0.75 (0.50–1.11) | 1.14 (0.77–1.67) | 1.16 (0.77–1.74) | 0.23 |
| Invasive, n | 330 | 293 | 344 | 246 | |
| RR (95%CI)3 | 1.00 | 1.05 (0.86–1.28) | 1.53 (1.25–1.87) | 1.38 (1.11–1.73) | 0.0005 |
| Histology2 | |||||
| Ductal, n | 273 | 233 | 277 | 202 | |
| RR (95%CI)3 | 1.00 | 1.00 (0.81–1.24) | 1.49 (1.21–1.85) | 1.41 (1.12–1.78) | 0.0004 |
| Lobular, n | 35 | 42 | 44 | 32 | |
| RR (95%CI)3 | 1.00 | 1.36 (0.85–2.18) | 1.68 (1.04–2.71) | 1.51 (0.90–2.55) | 0.13 |
| ER/PR Status2 | |||||
| ER+/PR+, n | 182 | 163 | 205 | 156 | |
| RR (95%CI)3 | 1.00 | 1.03 (0.81–1.32) | 1.62 (1.27–2.06) | 1.53 (1.17–1.98) | 0.0002 |
| ER+/PR−, n | 38 | 53 | 39 | 34 | |
| RR (95%CI)3 | 1.00 | 1.62 (1.04–2.50) | 1.46 (0.91–2.35) | 1.61 (0.98–2.65) | 0.13 |
| ER−/PR−, n | 54 | 36 | 42 | 24 | |
| RR (95%CI)3 | 1.00 | 0.78 (0.50–1.21) | 1.21 (0.77–1.88) | 0.88 (0.52–1.47) | 0.94 |
| Her2 Status2 | |||||
| Her2+, n | 47 | 37 | 45 | 32 | |
| RR (95%CI)3 | 1.00 | 0.86 (0.55–1.36) | 1.31 (0.84–2.04) | 1.19 (0.73–1.94) | 0.28 |
| Her2−, n | 168 | 153 | 189 | 131 | |
| RR (95%CI)3 | 1.00 | 1.06 (0.82–1.36) | 1.57 (1.22–2.03) | 1.34 (1.01–1.77) | 0.01 |
| Luminal A/Luminal B | |||||
| Luminal A, n | 89 | 88 | 100 | 80 | |
| RR (95%CI)3 | 1.00 | 1.09 (0.78–1.50) | 1.46 (1.05–2.03) | 1.42 (1.00–2.02) | 0.03 |
| Luminal B, n | 52 | 42 | 61 | 38 | |
| RR (95%CI)3 | 1.00 | 0.88 (0.57–1.35) | 1.53 (1.02–2.30) | 1.18 (0.75–1.87) | 0.22 |
| Tumor Size | |||||
| Tumor ≤2cm, n | 264 | 232 | 263 | 191 | |
| RR (95%CI)3 | 1.00 | 1.02 (0.82–1.26) | 1.43 (1.15–1.78) | 1.33 (1.05–1.69) | 0.004 |
| Tumor >2cm, n | 78 | 73 | 93 | 61 | |
| RR (95%CI)3 | 1.00 | 1.13 (0.80–1.59) | 1.86 (1.33–2.60) | 1.54 (1.06–2.23) | 0.007 |
| Grade | |||||
| Low-intermediate grade, n | 264 | 232 | 261 | 200 | |
| RR (95%CI)3 | 1.00 | 1.02 (0.83–1.27) | 1.44 (1.16–1.79) | 1.39 (1.10–1.76) | 0.001 |
| High grade, n | 150 | 135 | 156 | 97 | |
| RR (95%CI)3 | 1.00 | 1.08 (0.83–1.41) | 1.58 (1.21–2.06) | 1.27 (0.94–1.71) | 0.04 |
| Lymph node status | |||||
| Lymph node−, n | 271 | 238 | 261 | 192 | |
| RR (95%CI)3 | 1.00 | 1.02 (0.83–1.26) | 1.40 (1.13–1.74) | 1.33 (1.05–1.68) | 0.005 |
| Lymph node+, n | 60 | 55 | 85 | 54 | |
| RR (95%CI)3 | 1.00 | 1.08 (0.73–1.59) | 2.12 (1.47–3.05) | 1.63 (1.08–2.44) | 0.004 |
p-trend determined using the median values of the quartiles as a continuous term.
p-heterogeneity comparing in situ versus invasive=0.81; by ductal versus lobular=0.56; by ER/PR status=0.04; by HER2 status=0.56, by luminal type=0.62, by tumor size=0.49, by grade=0.46, and by lymph node status=0.16
Unconditional logistic regression models to calculate relative risk (RR) and 95% confidence intervals (95% CI), adjusting for age at blood draw (continuous), date of blood draw (continuous), fasting status and time of day of blood draw (fasting and morning, other), PMH use at blood draw (yes, no), BMI at blood draw (continuous), age at menarche (continuous), history of BBD (yes, no), family history of breast cancer (yes, no), age at menopause (continuous), average childhood body size at ages 5 and 10 (continuous), and cohort (NHS, NHSII).
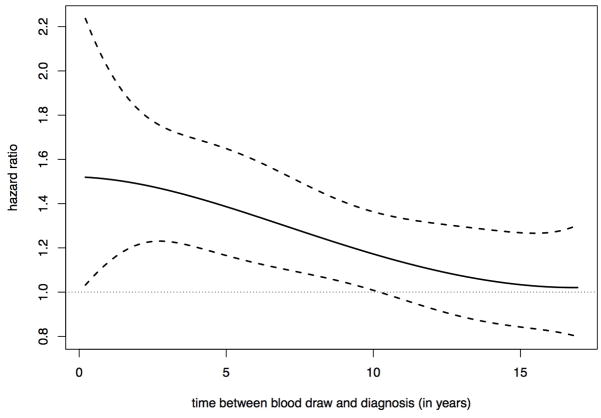
When evaluating the relationship of time between blood draw and diagnosis with risk of breast cancer, comparing postmenopausal women with prolactin levels above versus below the median, we observed that there was a linear decline in the association as time between blood draw and diagnosis increased for total breast cancer (Figure 1). The association became non-statistically significant at approximately 10 years after blood draw, consistent with our prior results. Results were similar for ER+ disease (data not shown).
Figure 1.
Hazard ratio of developing breast cancer among postmenopausal women comparing those above versus below the median prolactin level. The hazard ratio varies over time between blood draw and breast cancer diagnosis. The solid line is the estimated hazard ratio and the two dashed lines are upper and lower bounds of 95% confidence intervals.
Discussion
This is the first study to examine the potential influence of the timing of prolactin exposure in relation to breast cancer diagnosis. This was possible because we have up to 20 years of follow-up, over 2,900 cases, and in a subset of women, two blood samples collected 10 years apart. In this study, we observed that prolactin was significantly positively associated with risk only when measured within 10 years of diagnosis. This association appeared to be restricted to postmenopausal women and was strongest for ER+ disease, as well as for lymph node positive tumors. Importantly, among a subset of women who provided two blood samples about 10 years apart, we observed a similar positive association for proximate prolactin levels in an analysis that simultaneously adjusted for proximate and distant prolactin levels.
Our results generally are consistent with our previous analysis in the NHS and NHSII (2), which included over 1,500 cases with 10 years of follow-up, as well as the largest other prior study (3). In our prior and current analyses, we observed a positive association between prolactin and breast cancer risk that was stronger for ER+ disease, and appeared to attenuate with time between blood collection and diagnosis. However, one key difference emerged between our prior and the current study – in the current study, we did not observe an association among premenopausal women. One possible reason for this difference is that we had than double the number of premenopausal women at blood draw in the present analysis. These newly added cases were more likely to be postmenopausal at diagnosis than in the initial study, although the association was similar by menopausal status at diagnosis (RR, top vs. bottom quartile, premenopausal at blood and diagnosis=1.11 and RR, premenopausal at blood and postmenopausal at diagnosis=0.94). Our expanded analysis strongly suggests that prolactin levels measured during the postmenopausal period are more important in predicting breast cancer risk than premenopausal levels.
In this study, with up to 20 years of follow-up, we were able to carefully examine the relationship of prolactin with risk of breast cancer by time between blood draw and diagnosis. In categorical and continuous analyses, we observed that prolactin was only associated with breast cancer risk when assessed within 10 years before diagnosis, but no associations were observed for blood sampled 10–20 years before diagnosis. This is in contrast to what was observed for estradiol and testosterone in the same population, in which levels predicted risk for up to 16–20 years (10). In continuous analyses, the prolactin-breast cancer relationship in postmenopausal women decreased linearly as time between blood draw and diagnosis increased. This may be in part because the within person intraclass correlation (ICC) of prolactin in postmenopausal women over three years was about 0.53 (14). Thus as time after blood draw increases, the single blood value measured at baseline may become less reflective of a woman’s more recent exposure. Interestingly, the ICC over ten years was only modestly weaker (ICC=0.39). Another possible reason for the attenuation of the association is that breast tumors can secrete prolactin, possibly increasing circulating levels (20–22), suggesting that prolactin may act as both a tumor and risk marker. However, this likely would only explain a stronger association within the first 2–4 years of follow-up, but we observed a significant association for up to 10 years after blood draw.
Nevertheless, there may be a biologic reason for this attenuation in the association over time. In vitro and in vivo studies strongly support that prolactin is involved in processes related to late stage carcinogenic effects of breast cancer, including increasing cell proliferation and reducing apoptosis (reviewed in (1,8,23,24)), although these also can be important in early carcinogenesis. Further, substantial evidence suggests that prolactin is involved in angiogenesis and cell migration (25–28), which may lead to increased metastases (25,29). Thus it is possible that high circulating prolactin levels are important only after a preclinical lesion has developed and promotes late stage carcinogenesis. This is further supported by our results suggesting that prolactin was more strongly associated with invasive tumors and lymph node positive disease.
As in our prior work, prolactin was only associated with ER+ disease. Biologic data suggest that estradiol and prolactin can act synergistically on cell proliferation (30,31). This is supported by a separate analysis within the NHS among postmenopausal women not using PMH, in which we observed that women with high levels for multiple estrogens, androgens, and prolactin had the highest risk of breast cancer (32). Interestingly, transgenic mouse models with constituent prolactin expression in mammary tumors led to tumors that expressed ER and had characteristics of luminal A phenotypes (33,34), even though ER+ disease is rare in these mouse models. We only observed a significant positive association for prolactin with luminal A, but not luminal B tumors, although the difference was not statistically significant and the number of luminal B tumors was relatively small. Further, ER expression appears to be necessary for prolactin-mediated growth, which acts through ligand-independent ER activation (35). Overall, both biologic and epidemiologic data strongly suggest that prolactin is primarily involved in the etiology of ER+ breast cancer.
This study has several strengths and limitations. Notably, we have nearly 2,900 cases identified over 20 years of follow-up, with excellent epidemiologic data and breast cancer phenotype information. Further, in a subset of cases and controls, we were able to measure prolactin levels twice about 10 years apart to assess the influence of adjusting for proximate and distant prolactin levels simultaneously. There were fewer women with prolactin measured during premenopause; however, we had 80% power to detect an association as strong as that seen in postmenopausal women at a type I error of 0.05. As noted above, prolactin has some within-person variability over time, which likely attenuates our effect estimates. Using the ICC of 0.53 from our prior reproducibility study (13), the RR for total breast cancer comparing the top to bottom quartile of prolactin for postmenopausal women would increase from 1.37 to 1.81 accounting for measurement error (18). Further, the immunoassay for prolactin measures all isoforms, which have differing biologic activities (36,37). Using an assay that considers the biologic activity of prolactin in plasma may provide further insight into the prolactin-breast cancer relationship.
In summary, our study provides further evidence that prolactin is involved in the etiology of breast cancer, particularly among postmenopausal women and for ER+ disease. Risk appears to be increased in postmenopausal women with prolactin concentrations above 11.0 ng/mL, which is well within the normal range (38). Importantly, the association is only observed for prolactin levels measured within 10 years of diagnosis. This is consistent with biologic data that prolactin is important in processes generally thought to be involved in tumor promotion, suggesting that prolactin primarily plays a role in tumor development once a preclinical lesion has been established. Although the risk estimates are lower than those for estradiol or testosterone, prolactin should be considered for inclusion in risk prediction models for postmenopausal women, particularly since it is not strongly correlated with these other hormones. However, prolactin levels would need to be assessed at least every ten years.
Acknowledgments
Financial Support: This project was supported by the National Institutes of Health (R01 CA138580, R01 CA49449, R01 CA67262, R01 CA50385, P01 CA87969). We thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY.
Footnotes
Conflicts: The authors have no conflicts of interest to disclose.
References
- 1.Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tworoger SS, Eliassen AH, Sluss P, Hankinson SE. A prospective study of plasma prolactin concentrations and risk of premenopausal and postmenopausal breast cancer. J Clin Oncol. 2007;25:1482–8. doi: 10.1200/JCO.2006.07.6356. [DOI] [PubMed] [Google Scholar]
- 3.Manjer J, Johansson R, Berglund G, Janzon L, Kaaks R, Agren A, et al. Postmenopausal breast cancer risk in relation to sex steroid hormones, prolactin and SHBG (Sweden) Cancer Causes Control. 2003;14:599–607. doi: 10.1023/a:1025671317220. [DOI] [PubMed] [Google Scholar]
- 4.Wang DY, De Stavola BL, Bulbrook RD, Allen DS, Kwa HG, Fentiman IS, et al. Relationship of blood prolactin levels and the risk of subsequent breast cancer. Int J Epidemiol. 1992;21:214–21. doi: 10.1093/ije/21.2.214. [DOI] [PubMed] [Google Scholar]
- 5.Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE. A prospective study of estradiol and breast cancer in Japanese women. Cancer Epidemiol Biomarkers Prev. 2000;9:575–9. [PubMed] [Google Scholar]
- 6.Helzlsouer KJ, Alberg AJ, Bush TL, Longcope C, Gordon GB, Comstock GW. A prospective study of endogenous hormones and breast cancer. Cancer Detect Prev. 1994;18:79–85. [PubMed] [Google Scholar]
- 7.Berinder K, Akre O, Granath F, Hulting A-L. Cancer risk in hyperprolactinemia patients: a population-based cohort study. Eur J Endocrinol. 2011;165:209–15. doi: 10.1530/EJE-11-0076. [DOI] [PubMed] [Google Scholar]
- 8.Bernichtein S, Touraine P, Goffin V. New concepts in prolactin biology. J Endocrinol. 2010;206:1–11. doi: 10.1677/JOE-10-0069. [DOI] [PubMed] [Google Scholar]
- 9.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87:1297–302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Tworoger SS, Eliassen AH, Hankinson SE. Postmenopausal plasma sex hormone levels and subsequent risk of breast cancer over 20 years of follow-up. Breast Cancer Res Treat. doi: 10.1007/s10549-012-2391-z. (inpress) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tworoger SS, Sluss P, Hankinson SE. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res. 2006;66:2476–82. doi: 10.1158/0008-5472.CAN-05-3369. [DOI] [PubMed] [Google Scholar]
- 12.Hankinson SE, London SJ, Chute CG, Barbieri RL, Jones L, Kaplan LA, et al. Effect of transport conditions on the stability of biochemical markers in blood. Clinical Chemistry. 1989;35:2313–6. [PubMed] [Google Scholar]
- 13.Tworoger SS, Eliassen AH, Rosner B, Sluss P, Hankinson SE. Plasma prolactin concentrations and risk of postmenopausal breast cancer. Cancer Res. 2004;64:6814–9. doi: 10.1158/0008-5472.CAN-04-1870. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2–3-year period. Cancer Epidemiol Biomarkers Prev. 1995;4:649–54. [PubMed] [Google Scholar]
- 15.Missmer SA, Spiegelman D, Bertone-Johnson ER, Barbieri RL, Pollak MN, Hankinson SE. Reproducibility of plasma steroid hormones, prolactin, and insulin-like growth factor levels among premenopausal women over a 2- to 3-year period. Cancer Epidemiol Biomarkers Prev. 2006;15:972–8. doi: 10.1158/1055-9965.EPI-05-0848. [DOI] [PubMed] [Google Scholar]
- 16.Rosner B. Percentage Points for a Generalized ESD Many-Outier Procedure. Technometrics. 1983;25:165–72. [Google Scholar]
- 17.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167:653–66. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- 18.Rosner BA. Fundamentals of Biostatistics. 6. Belmont, CA: Duxbury Press; 2005. [Google Scholar]
- 19.Eliassen AH, Tworoger SS, Hankinson SE. Reproductive factors and family history of breast cancer in relation to plasma prolactin levels in premenopausal and postmenopausal women. Int J Cancer. 2007;120:1536–41. doi: 10.1002/ijc.22482. [DOI] [PubMed] [Google Scholar]
- 20.Ginsburg E, Vonderhaar BK. Prolactin synthesis and secretion by human breast cancer cells. Cancer Res. 1995;55:2591–5. [PubMed] [Google Scholar]
- 21.Bhatavdekar JM, Patel DD, Shah NG, Vora HH, Suthar TP, Ghosh N, et al. Prolactin as a local growth promoter in patients with breast cancer: GCRI experience. Eur J Surg Oncol. 2000;26:540–7. doi: 10.1053/ejso.2000.0943. [DOI] [PubMed] [Google Scholar]
- 22.Lachelin GC, Yen SC, Alksne JF. Hormonal changes following hypophysectomy in humans. Obstetrics and gynecology. 1977;50:333–9. [PubMed] [Google Scholar]
- 23.Tworoger SS, Hankinson SE. Prolactin and breast cancer risk. Cancer Lett. 2006;243:160–9. doi: 10.1016/j.canlet.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson EM, Hugo ER, Borcherding DC, Ben-Jonathan N. Prolactin in breast and prostate cancer: molecular and genetic perspectives. Discov Med. 2011;11:315–24. [PubMed] [Google Scholar]
- 25.Reuwer AQ, Nowak-Sliwinska P, Mans LA, van der Loos CM, Thüsen von der JH, Twickler MTB, et al. Functional consequences of prolactin signalling in endothelial cells: a potential link with angiogenesis in pathophysiology? J Cell Mol Med. 2012;16:2035–48. doi: 10.1111/j.1582-4934.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aksamitiene E, Achanta S, Kolch W, Kholodenko BN, Hoek JB, Kiyatkin A. Prolactin-stimulated activation of ERK1/2 mitogen-activated protein kinases is controlled by PI3-kinase/Rac/PAK signaling pathway in breast cancer cells. Cell Signal. 2011;23:1794–805. doi: 10.1016/j.cellsig.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maus MV, Reilly SC, Clevenger CV. Prolactin as a chemoattractant for human breast carcinoma. Endocrinology. 1999;140:5447–50. doi: 10.1210/endo.140.11.7245. [DOI] [PubMed] [Google Scholar]
- 28.Struman I, Bentzien F, Lee H, Mainfroid V, D’Angelo G, Goffin V, et al. Opposing actions of intact and N-terminal fragments of the human prolactin/growth hormone family members on angiogenesis: an efficient mechanism for the regulation of angiogenesis. Proc Natl Acad Sci USA. 1999;96:1246–51. doi: 10.1073/pnas.96.4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liby K, Neltner B, Mohamet L, Menchen L, Ben-Jonathan N. Prolactin overexpression by MDA-MB-435 human breast cancer cells accelerates tumor growth. Breast Cancer Res Treat. 2003;79:241–52. doi: 10.1023/a:1023956223037. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen LM, Frederiksen KS, Din N, Galsgaard E, Christensen L, Berchtold MW, et al. Prolactin and oestrogen synergistically regulate gene expression and proliferation of breast cancer cells. Endocr Relat Cancer. 2010;17:809–22. doi: 10.1677/ERC-09-0326. [DOI] [PubMed] [Google Scholar]
- 31.Gutzman JH, Miller KK, Schuler LA. Endogenous human prolactin and not exogenous human prolactin induces estrogen receptor alpha and prolactin receptor expression and increases estrogen responsiveness in breast cancer cells. J Steroid Biochem Mol Biol. 2004;88:69–77. doi: 10.1016/j.jsbmb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Tworoger SS, Rosner BA, Willett WC, Hankinson SE. The combined influence of multiple sex and growth hormones on risk of postmenopausal breast cancer: a nested case-control study. Breast Cancer Res. 2011;13:R99. doi: 10.1186/bcr3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arendt LM, Rugowski DE, Grafwallner-Huseth TA, Garcia-Barchino MJ, Rui H, Schuler LA. Prolactin-induced mouse mammary carcinomas model estrogen resistant luminal breast cancer. Breast Cancer Res. 2011;13:R11. doi: 10.1186/bcr2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler LA. Prolactin induces ERalpha-positive and ERalpha-negative mammary cancer in transgenic mice. Oncogene. 2003;22:4664–74. doi: 10.1038/sj.onc.1206619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González L, Zambrano A, Lazaro-Trueba I, Lopéz E, González JJA, Martín-Pérez J, et al. Activation of the unliganded estrogen receptor by prolactin in breast cancer cells. Oncogene. 2009;28:1298–308. doi: 10.1038/onc.2008.473. [DOI] [PubMed] [Google Scholar]
- 36.Sinha YN. Structural variants of prolactin: occurrence and physiological significance. Endocr Rev. 1995;16:354–69. doi: 10.1210/edrv-16-3-354. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann T, Penel C, Ronin C. Glycosylation of human prolactin regulates hormone bioactivity and metabolic clearance. J Endocrinol Invest. 1993;16:807–16. doi: 10.1007/BF03348932. [DOI] [PubMed] [Google Scholar]
- 38.Yen SC, Barbieri RL. Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 4. Philadelphia, PA: W.B. Saunders; 1999. [Google Scholar]