Abstract
Objectives: Retinol Binding Protein-4 (RBP-4), the action of which was initially thought to be only the transport of vitamin A, is a major circulating adipocytokine involved in the inflammation. We evaluated the serum RBP-4 levels in children with inflammatory bowel disease (IBD) and correlated them with transthyretin (TTR), inflammation markers, disease activity, and body mass index (BMI).
Design: In 41 children of mean age 11.9 ± 3.6 years (range 5-17.7 y) with IBD (19 with Crohn's disease (CD) and 22 with Ulcerative colitis (UC) serum RBP-4, TTR, Amyloid A (SAA), C-Reactive Protein (CRP), Erythrocyte Sedimentation Rate (ESR), disease activity and BMI were prospectively determined and compared with those of 42 matched controls.
Results: No difference in the RBP-4 and TTR serum levels, between patients and controls as well as between active and remission state of the disease was noticed. A negative correlation of serum RBP-4 with the disease activity, SAA and ESR and a positive correlation with TTR was found, but no significant correlation with CRP or BMI was found. Inflammation markers were significantly increased in patients compared to controls and had a positive correlation with the disease activity.
Conclusions: RBP-4 negatively correlated with disease activity of children with IBD probably indicating a protective anti-inflammatory mechanism of action in addition to transport of vitamin A.
Keywords: retinol binding protein-4, inflammatory bowel disease RBP-4, IBD, children, disease activity index
Introduction
Inflammatory bowel disease (IBD), Crohn's disease (CD) and ulcerative colitis (UC), constitute a multifactorial disorder characterized by a chronic relapsing inflammation of the digestive tract. As multifactorial disorder, IBD is probably caused by a complex interaction of genetic, microbial, immunological and environmental factors1-3. Many studies indicate that cytokines play a key role on inflammation in patients with IBD4.
Adipose tissue is no longer considered merely an energy-storing depot but rather a metabolically active endocrine organ, that secretes many information-carrying molecules, called adipocytokines. They are fat-derived hormones and cytokines with immune-modulating and metabolic properties. There is evidence that adipocytokines are involved in inflammatory and metabolic pathways in humans. Among the adipocytokines, leptin, adiponectin and resistin appear to play an important role5.
Anorexia, malnutrition, altered body composition, and development of mesenteric obesity (accumulation of intra-abdominal white adipose tissue -WAT), are well-known features of IBD, mainly of CD, indicating an important role for WAT-secreted proteins6. Over-expression of adipocytokines such as leptin, adiponectin, and resistin in mesenteric adipose tissue of patients with CD who have been operated has been reported, suggesting that mesenteric adipocytes in IBD may act as immunoregulatory cells7-9. Therefore, it is suggested that adipocytokines may participate in the disease pathogenesis4.
Based on animal and human studies, it is suggested that the soluble form of Retinol Binding Protein-4 (RBP-4) -the action of which was initially thought to be only the transport of retinol (Vitamin A)- is a major circulating adipokine implicated in systemic insulin resistance. RBP-4 is a small, 21kDa protein that circulates bound to transthyretin (TTR, one of the plasma carriers of thyroid hormone) in the form of an 80kDa protein complex that is not easily filtered through the kidneys. Overall, the available evidence indicates the existence of a high degree complementarity between RBP and TTR, the contact areas of which are highly sensitive to conformational changes and amino acid replacements10. TTR is a tryptophan-rich protein, used as one of the nutrition assessment proteins, it acts as an anti-acute phase protein, and its plasma concentration decreases during inflammation and bacterial infection. Moreover, TTR plays important roles in various CNS disorders, diabetes mellitus, and lipid metabolism11. A specific aspect in the determination of the extent of vitamin A deficiency based on reduced RBP-4 levels is the observation that, beside vitamin A deficiency, the inflammation-associated acute phase response (APR) results in decreased plasma RBP-4 levels despite a sufficient vitamin A status12.
C-reactive protein (CRP) and serum amyloid A (SAA) increase in the blood of patients with inflammatory conditions and in IBD13,14. In IBD patients SAA is useful as a marker for disease activity, with a high sensitivity14. The expression of SAA is predominantly in the liver. However, extrahepatic expression of SAA, including adipocytes, has been reported. The association between SAA and leptin suggests an interaction between these two adipokines, which may have implications in inflammatory processes related to obesity and the metabolic syndrome15.
Studies concerning the relationship of RBP-4 with IBD are limited. Since inflammation is the underlying process in IBD, we aimed to evaluate the serum RBP-4 and its binding TTR in children with IBD and correlate them with inflammation markers such as SAA, CRP and erythrocyte sedimentation rate (ESR), disease activity and BMI in order to find out its possible interference with the disease process.
Patients and methods
Patients
Forty-one children with a confirmed diagnosis of IBD (19 with CD and 22 with UC) according to the ESPGHAN Porto-criteria were included in this study16,17. Approval of the local ethical committee and informed parental concern were obtained. The disease was either active or in remission. Its activity was classified according to Pediatric Crohn's Disease Activity Index (PCDAI) for CD patients (score 0 to 10 inactive disease, 11 to 30 mild disease and above 30 moderate to severe disease)18, while the Pediatric Ulcerative Colitis Activity Index (PUCAI) was used for UC19. Evaluation of disease activity and BMI were performed at the time of serum collection. Serum RBP-4, TTR, SAA and the standard laboratory parameters including red and white blood cell count, hemoglobin, hematocrit, platelet count, total protein, albumin, cholesterol, triglycerides, ESR, CRP, were determined in all patients at a routine clinical and laboratory evaluation. Forty two children were used as controls.
Blood Chemistry
Blood samples were collected in the morning after an overnight fast. Serum concentrations of Transthyretin, SAA and CRP were measured by particle-enhanced immunonephelometric assays (Dade Behring Siemens Healthcare Diagnostics). The intra- and inter-assay coefficients of variation (CVs) were less than 6% and 7% respectively.
Serum RBP-4 concentrations were determined using a sandwich ELISA assay (Immunodiagnostik AG, Behsheim, Germany). Serum samples were diluted so that the absorbance was in the middle of the range of linearity for this assay. The intra-and inter-assay CVs for RBP-4 were 5.0 and 9.7 %, respectively, according to the manufacturer.
Statistical Analysis
Data are expressed as mean ± SD. Differences between groups were analysed using the Student's t-test. The correlation coefficient r between the parameters tested was computed using least squares regression analysis. The p values reported are two tailed. We used the standardized skewness and standardized kurtosis, to determine whether the sample comes from a normal distribution. Values of these statistics outside the range of -2 to +2 indicate significant departures from normality, which would tend to invalidate many of the statistical procedures normally applied to this data. These values were integrated automatically from the program and indicated that the parameters needed to transform in either logarithmic or reciprocal or square root, where needed it. All the statistical analyses were performed using the STATGRAPHICS 5.1 for Windows program (Graphic Software System, STATPOINT TECHNOLOGIES, INC. Warrenton, Virginia, USA).
Results
A total of 41 children with IBD with a mean age 11.9 ± 3.6 years (range 5-17.5 years) participated in the study. Among them 19 suffered from CD and 22 from UC. According to the activity index 25 were in remission and 16 in moderate to severe disease state. Demographic data and mean values (range) of serum RBP-4, ESR, CRP, SAA and BMI are shown on Table 1. Forty two children of mean age 9.9 ± 2.5 years (5.7-14.4 years) were used as controls. No difference of RBP-4, TTR and BMI between patients and controls was found, while the inflammation markers were significantly increased in patients (Table 2).
Table 1. Demographic characteristics and blood chemistry parameters (mean ± SD and ranges in parentheses).
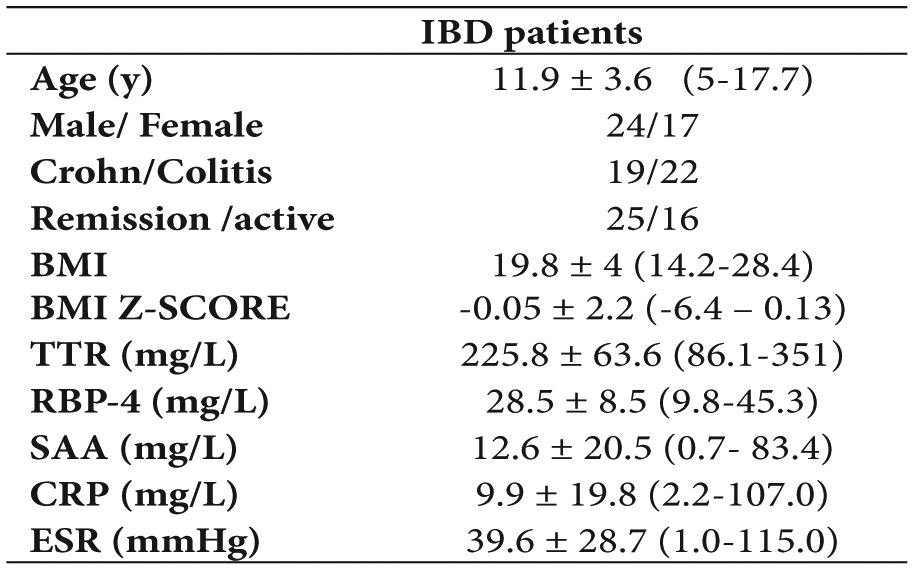
BMI: body mass index, TTR: transthyretin, RBP-4: Retinol Binding Protein-4, SAA: Serum Amyloid A, CRP: C-Reactive Protein, ESR: Erythrocyte Sedimentation Rate.
Table 2. Comparison of Retinol Binding Protein-4 (RBP-4) , inflammation markers, transthyretin (TTR) and body mass index (BMI) in children with inflammatory bowel disease (IBD) and controls.
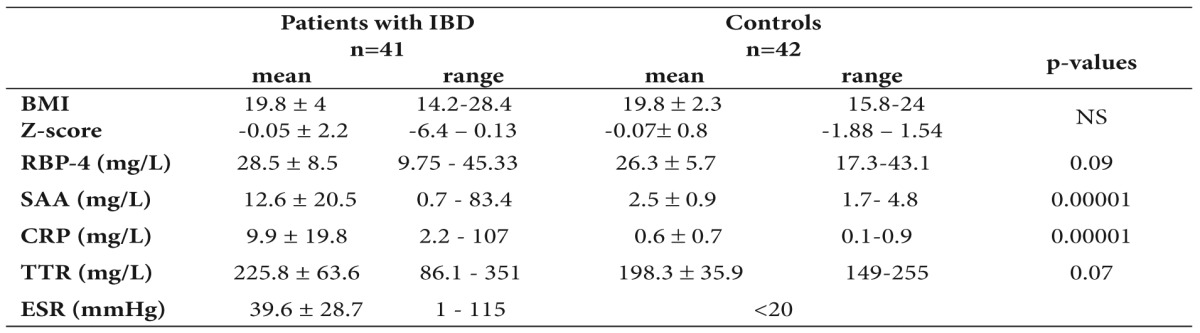
SAA: Serum Amyloid A, CRP: C-Reactive Protein, ESR: Erythrocyte Sedimentation Rate.
A statistically significant increase of inflammation markers was detected between patients in active phase and those in remission, while no significant difference of RBP-4 and TTR values was found between active and remission state of the disease (Table 3). All the above parameters did not differ between CD and UC patients (data not shown).
Table 3. Comparison of Retinol Binding Protein-4 (RBP-4) and inflammation markers in active and remission state of children with inflammatory bowel disease (IBD)(mean values).
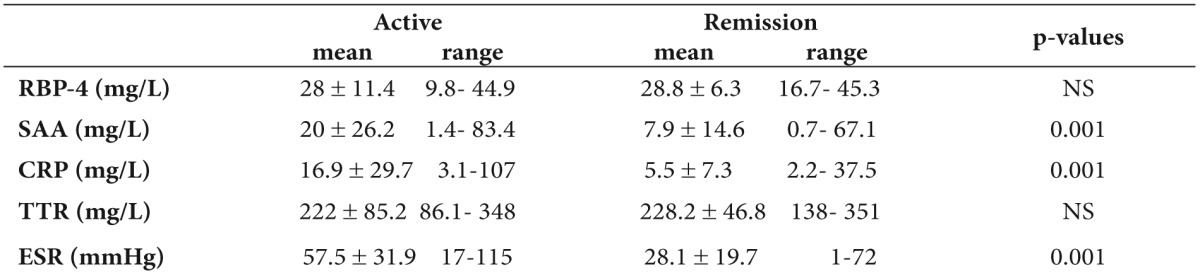
SAA: Serum Amyloid A, CRP: C-Reactive Protein, TTR: transthyretin, ESR: Erythrocyte Sedimentation Rate.
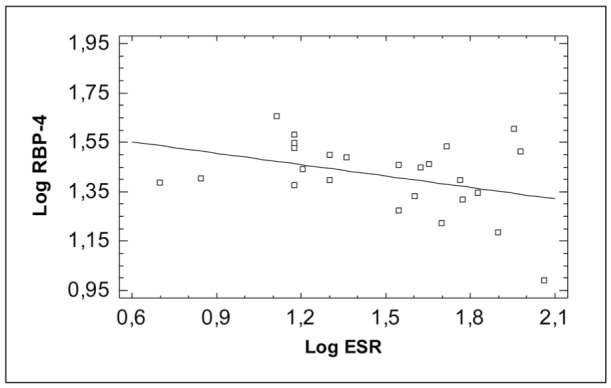
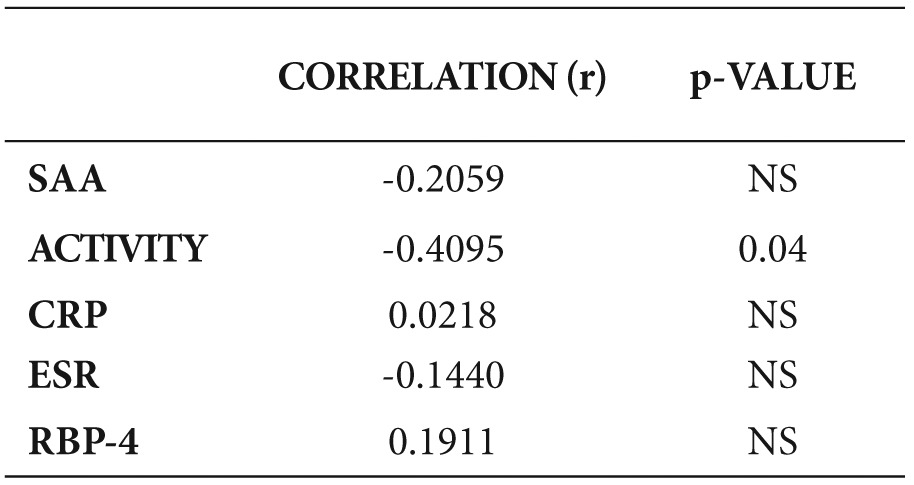
A negative correlation of serum RBP-4 with the disease activity, SAA and ESR and a positive correlation with TTR was detected, but no significant correlation with the other parameters was found (Table 4, Figures 1,2,3).
Table 4. Correlation of Retinol Binding Protein-4 (RBP-4) with different parameters.
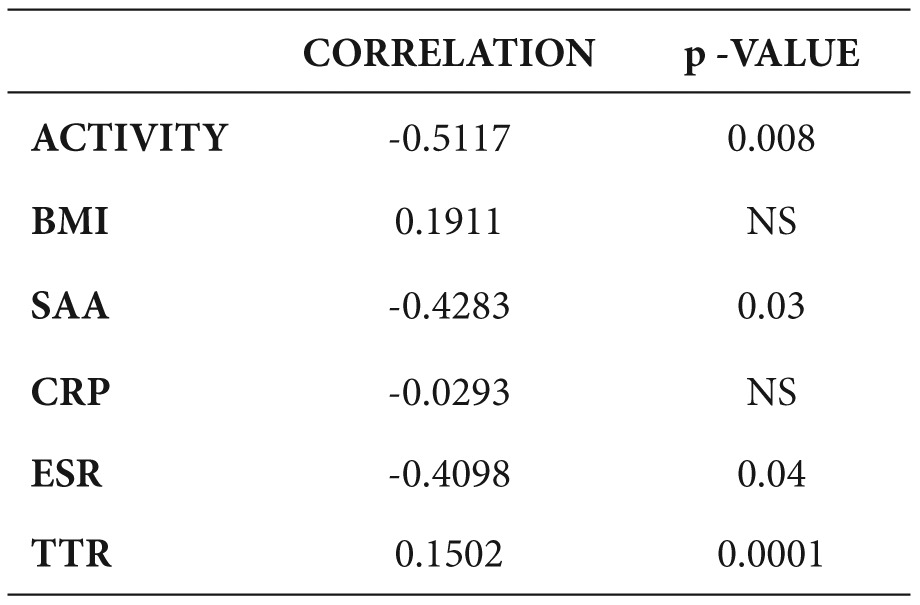
BMI: body mass index, SAA: Serum Amyloid A, CRP: C-Reactive Protein, ESR: Erythrocyte Sedimentation Rate, TTR: transthyretin.
Figure 1. Correlation of Retinol Binding Protein-4 (RBP-4) with activity index.
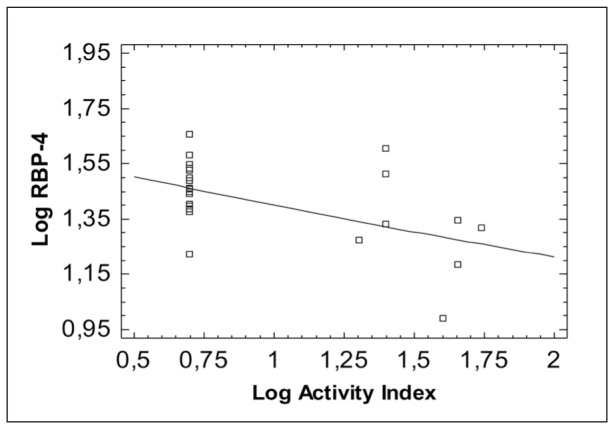
Figure 2. Correlation of Retinol Binding Protein-4 (RBP-4) with Serum Amyloid A (SAA).
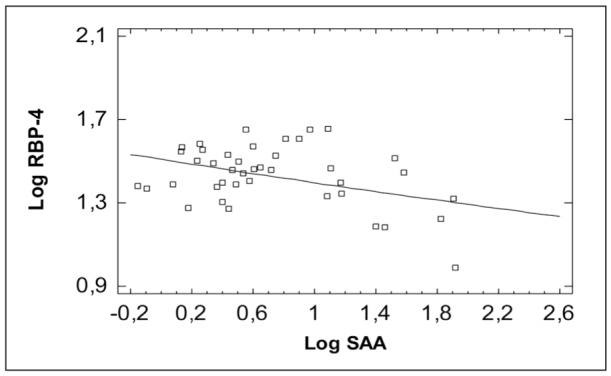
Figure 3. Correlation of Retinol Binding Protein-4 (RBP-4) with Erythrocyte Sedimentation Rate (ESR).
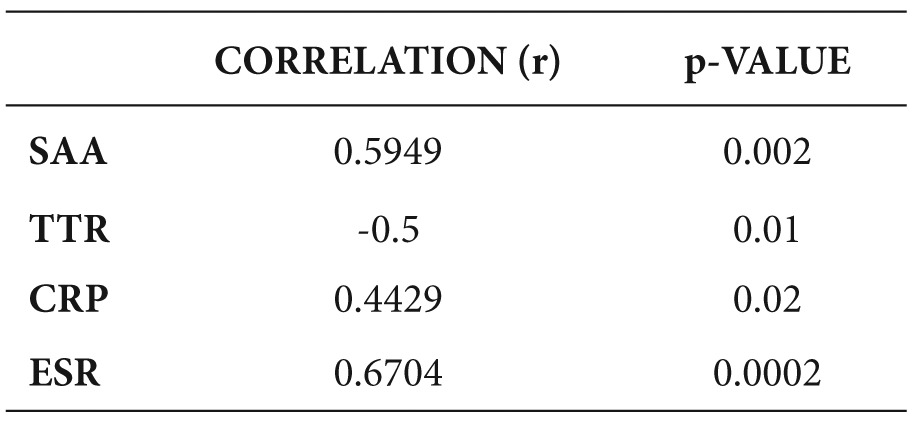
All inflammation markers had a positive correlation with the activity index, except TTR which had a negative correlation (Table 5). BMI was negatively correlated with disease activity (Table 6).
Table 5. Correlation of activity index with inflammatory markers.
SAA: Serum Amyloid A, TTR: transthyretin, CRP: C-Reactive Protein, ESR: Erythrocyte Sedimentation Rate.
Table 6. Correlation of BMI with different parameters.
SAA: Serum Amyloid A, CRP: C-Reactive Protein, ESR: Erythrocyte Sedimentation Rate, RBP-4: Retinol Binding Protein-4.
Discussion
In the present study we determined the serum levels of RBP-4 and its binding protein TTR in children with IBD, compared them with the values of matched controls and correlated with disease activity, serum inflammation markers and BMI.
A negative correlation of RBP-4 and TTR with the disease activity, SAA and ESR in children with IBD was found. Contrary to our findings, in a previous study on patients with IBD, which aimed to evaluate the possible associations of circulating levels of adipokines and glucose homeostasis with the course and characteristics of the disease, RBP-4 was increased in active and inactive disease compared with controls20.
Retinol-binding protein is the specific plasma carrier of retinol, encharged of the vitamin transport from the liver to target cells. Ligand binding influences the RBP affinity for TTR, a homotetrameric protein involved in the RBP/TTR circulating complex, and the secretion rate of RBP. In fact, in vitamin A deficiency the RBP release from the hepatocytes dramatically decreases and the protein accumulates in the cells, until retinol is available again21. In the present study it is unclear whether the negative correlation of RBP-4 with the disease activity could be due to the reduced vitamin A or it indicates the response of RBP-4 to inflammation during active disease.
Indeed, hypovitaminosis and fat-soluble vitamin deficiency have been reported in adults with IBD. Plasma vitamin A, E and carotenoid concentrations determined in IBD patients were significantly reduced, particularly in those with active disease, with respect to controls22. Busvaros et al in a prospective study estimated the serum levels of vitamins A and E in children and young adults with IBD and the prevalence of hypovitaminosis A or E was 16% in the pediatric IBD population23. Hypovitaminosis was significantly more prevalent in the Crohn's disease patients who had active disease, but in their study RBP-4 was not estimated in order to compare it with our findings. They concluded that children and young adults with active IBD frequently have low serum levels of vitamin A or vitamin E and that the severity of disease activity was a better predictor of risk for hypovitaminosis than the nutritional status. They also stated that further work is necessary to determine whether the hypovitaminosis seen in children with IBD reflects true deficiency. Αs an answer to their concern, other studies showed that plasma levels of retinol are of limited value to evaluate the vitamin A status of an individual because of the homeostatic control of RBP-4 in plasma, as well as the strong influence of inflammation on its plasma levels24,25. In this respect, the negative correlation of RBP-4 with the disease activity in our study is in accordance with the findings by Busvaros et al since vitamin A reflects RBP-4 levels due to tight regulation of vitamin A by synthesis of RBP-423.
RBP-4, as an adipocytokine, has been associated with the pathogenesis of insulin resistance and has gained considerable attention due to its possible link to diabetes, which may be due to the increased inflammatory status in diabetic and obese patients, although several studies have shown controversial results26-29. Additionally, negative30 or positive31,32 correlations of RBP-4 with BMI in obese children and adolescents have been found.
Studies have demonstrated significantly lower serum retinol levels in patients with inflammation-associated acute phase response (APR) or trauma25. The APR leads to transient increase or decrease of plasma concentrations of different proteins due to changed hepatic proteins synthesis, known as positive (e.g. C-reactive protein ) or negative (e.g. RBP-4 and transthyretin) acute phase proteins during inflammation. It has been shown that the inflammation-associated APR results in decreased plasma RBP-4 levels despite a sufficient vitamin A status12. Based on these difficulties, it has been suggested that the determination of the molar ratio RBP-4 to TTR index might help to discriminate between deficiency and an inflammatory induced decrease of RBP-433. Thus, in inflammation retinol, RBP-4 and TTR levels decrease, whereas, in nutritional deficiency, only retinol and RBP-4 are decreased24. During inflammation, both RBP-4 and TTR levels are decreased due to APR, resulting in an unchanged ratio at lower absolute levels24. The negative correlation of both RBP-4 and TTR with the disease activity in the present study is in favour of the interference of inflammation during active disease state. Additionally, an increase of the positive acute phase proteins (SAA and CRP) and ESR was identified in active disease, with a significant negative correlation of RBP-4 with SAA and ESR.
RBP-4 serum levels have been estimated in liver diseases based on its possible interference as an adipocytokine with the inflammation process. An inverse relationship between RBP-4 levels and the degree of liver damage in children with non alcoholic fatty liver disease (NAFLD)34, as well as with the degree of histological lesion in patients with chronic hepatitis C, who had a significant elevation after treatment with interferon and sustained virological response has been found35. Serum RBP-4 has been suggested as a serologic marker for disease severity in patients with chronic liver disease (CLD)36, 37.
In the present study a statistically significant positive correlation of RBP-4 with the TTR was found and both had a negative correlation with the disease activity. Probably both act as anti- acute phase proteins during the severe inflammation in the active IBD. The negative correlation of RBP-4 with SAA, which is the most sensitive acute phase protein during inflammation, is in accordance with this hypothesis.
In conclusion, in the present study an inverse correlation of RBP-4 and TTR with the disease activity, serum amyloid A and ESR was found. Τhe mechanism of this reduction during active inflammation is not clear but the action of RBP-4 could be through its behavior as an adipocytokine, interfering with the inflammation process and oxidative stress. Therefore, RBP-4 as it happens with TTR- can be used not only as nutrition assessment proteins, but possibly may act as anti-acute phase proteins. It is clear that more studies in children are needed concerning RBP-4 in relation with vitamin A intake as well as with the active and remission stages of IBD.
Grant support/funding
Funding was received from Athens University to I.P. The funding source played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Conflict of interest
The authors declare no financial interests and/or conflict of interest related to this study.
References
- 1.Kucharzik T, Maaser C, Lügering A, Kagnoff M, Mayer L, Targan S, et al. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2006;12:1068–1083. doi: 10.1097/01.mib.0000235827.21778.d5. [DOI] [PubMed] [Google Scholar]
- 2.Baron S, Turck D, Leplat C, Merle V, Gower-Rousseau C, Marti R, et al. Enviromental risk factors in paediatric inflammatory bowel diseases: a population based case control study. Gut. 2005;54:357–363. doi: 10.1136/gut.2004.054353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shih DQ, Targan SR, McGovern D. Recent advances in IBD pathogenesis: genetics and immunology. Curr Gastroenterol Rep. 2008;10:568–575. doi: 10.1007/s11894-008-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karmiris K, Koutroubakis IE, Kouroumalis EA. The emerging role of adipocytokines as inflammatory mediators in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:847–855. doi: 10.1097/01.mib.0000178915.54264.8f. [DOI] [PubMed] [Google Scholar]
- 5.Trayhurn P, Wood S. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 6.Desreumaux P, Ernst O, Geboes K, Gambiez L, Berrebi D, Müller-Alouf H, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology. 1999;117:73–81. doi: 10.1016/s0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- 7.Barbier M, Vidal H, Desreumaux P, Dubuquoy L, Bourreille A, Colombel JF, et al. Overexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseases. Gastroenterol Clin Biol. 2003;27:987–991. [PubMed] [Google Scholar]
- 8.Yamamoto K, Kiyohara T, Murayama Y, Kihara S, Okamoto Y, Funahashi T, et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn's disease. Gut. 2005;54:789–796. doi: 10.1136/gut.2004.046516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul G, Schäffler A, Neumeier M, Fürst A, Bataillle F, Buechler C, et al. Profiling adipocytokine secretion from creeping fat in Crohn's disease. Inflamm Bowel Dis. 2006;12:471–477. doi: 10.1097/00054725-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Zanotti G, Berni R. Plasma retinol-binding protein: structure and interactions with retinol, retinoids, and transthyretin. Vitam Horm. 2004;69:271–295. doi: 10.1016/S0083-6729(04)69010-8. [DOI] [PubMed] [Google Scholar]
- 11.Ando Y. Transthyretin: it's miracle function and pathogenesis. Rinsho Byori. 2009;57:228–235. [PubMed] [Google Scholar]
- 12.Rosales FJ, Ritter SJ, Zolfaghari R, Smith JE, Ross AC. Effects of acute inflammation on plasma retinol, retinol-binding protein and its mRNA in the liver and kidneys of vitamin A-sufficient rats. J Lipid Res. 1996;37:962–971. [PubMed] [Google Scholar]
- 13.Vermeire S, Van Assche CGW, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:661–665. doi: 10.1097/00054725-200409000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Niederau C, Backmerhoff F, Schumacher B, Niederau C. Inflammatory mediators and acute phase proteins in patients with Crohn's disease and ulcerative colitis. Hepatogastroenterology. 1997;44:90–107. [PubMed] [Google Scholar]
- 15.Lappalainen T, Kolehmainen M, Schwab U, Pulkkinen L, Laaksonen DE, Rauramaa R, et al. Serum concentrations and expressions of serum amyloid A and leptin in adipose tissue are interrelated: the Genobin Study. Eur J Endocrinol. 2008;158:333–341. doi: 10.1530/EJE-07-0598. [DOI] [PubMed] [Google Scholar]
- 16.IBD working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: recommendation for diagnosis--the Porto criteria. J Ped Gastroenterol Nutr. 2005;41:1–7. doi: 10.1097/01.mpg.0000163736.30261.82. [DOI] [PubMed] [Google Scholar]
- 17.Bousvaros A, Antonioli DA, Colletti RB, Dubinsky MC, Glickman JN, Gold BD, et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn's and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44:653–674. doi: 10.1097/MPG.0b013e31805563f3. [DOI] [PubMed] [Google Scholar]
- 18.Shepanski MA, Markowitz JE, Mamula P, Hurd LB, Baldassano RN. Is an Abbreviated Pediatric Crohn's Disease Activity Index Better Than the Original? J Pediatr Gastroenterol Nutr. 2004;39:68–72. doi: 10.1097/00005176-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, et al. A Prospective Multicenter Study: Development and Validation of a Pediatric Ulcerative Colitis Activity Index. J Pediatr Gastroenterol Nutr. 2006;43:S19–S20. doi: 10.1053/j.gastro.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 20.Valentini L, Wirth EK, Schweizer U, Hengstermann S, Schaper L, Koernicke T, et al. Circulating adipokines and the protective effects of hyperinsulinemia in inflammatory bowel disease. Nutrition. 2009;25:172–181. doi: 10.1016/j.nut.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Bellovino D, Apreda M, Gragnoli S, Massimi M, Gaetani S. Vitamin A transport: in vitro models for the study of RBP secretion. Mol Aspects Med. 2003;24:411–420. doi: 10.1016/s0098-2997(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 22.D'Odorico A, Bortolan S, Cardin R, D'Inca R, Martines D, Ferronato A, et al. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:1289–1294. doi: 10.1080/003655201317097146. [DOI] [PubMed] [Google Scholar]
- 23.Bousvaros A, Zurakowski D, Duggan C, Law T, Rifai N, Goldberg NE, et al. Vitamins A and E serum levels in children and young adults with inflammatory bowel disease: effect of disease activity. J Pediatr Gastroenterol Nutr. 1998;26:129–135. doi: 10.1097/00005176-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Schweigert FJ. Inflammation-induced changes in the nutritional biomarkers serum retinol and carotenoids. Curr Opin Clin Nutr Metab Care. 2001;4:477–481. doi: 10.1097/00075197-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Stefenson CB, Gildengorin G. Serum retinol, the acute phase response, and the apparent misclassification of vitamin A status in the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2000;72:1170–1178. doi: 10.1093/ajcn/72.5.1170. [DOI] [PubMed] [Google Scholar]
- 26.Olmedilla B, Granado F, Gil-Martinez E, Blanco I, Rojas-Hidalgo E. Reference values for retinol, tocopherol, and main carotenoids in serum of control and insulin-dependent diabetic Spanish subjects. Clin Chem. 1997;43:1066–1071. [PubMed] [Google Scholar]
- 27.Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, et al. Plasma Retinol-Binding Protein-4 Concentrations Are Elevated in Human Subjects With Impaired Glucose Tolerance and Type 2 Diabetes. 2006. Diabetes Care. 2006;29:2457–2461. doi: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- 28.Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T. Retinol binding protein-4 levels and clinical features of type 2 diabetes patients. J Clin Endocrinol Metab. 2007;92:2712–2719. doi: 10.1210/jc.2006-1249. [DOI] [PubMed] [Google Scholar]
- 29.Espe K, Galler A, Raila J, Kiess W, Schweigert FJ. High-normal C-reactive protein levels do not affect the vitamin A transport complex in serum of children and adolescents with type 1 diabetes. Pediatr Res. 2007;62:741–745. doi: 10.1203/PDR.0b013e318158787e. [DOI] [PubMed] [Google Scholar]
- 30.Kanaka-Gantenbein C, Margeli A, Pervanidou P, Sakka S, Mastorakos G, Chrousos GP, et al. Retinol-binding protein 4 and lipocalin-2 in childhood and adolescent obesity: when children are not just "small adults". Clin Chem. 2008;54:1176–1182. doi: 10.1373/clinchem.2007.099002. [DOI] [PubMed] [Google Scholar]
- 31.Qi Q, Yu Z, Ye X, Zhao F, Huang P, Hu FB, et al. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin Endocrinol Metab. 2007;92:4827–4834. doi: 10.1210/jc.2007-1219. [DOI] [PubMed] [Google Scholar]
- 32.Balagopal P, Graham TE, Kahn BB, Altomare A, Funanage V, George D. Reduction of Elevated Serum Retinol Binding Protein in Obese Children by Lifestyle Intervention: Association with Subclinical Inflammation. J Clin Endocrinol Metab. 2007;92:1971–1974. doi: 10.1210/jc.2006-2712. [DOI] [PubMed] [Google Scholar]
- 33.Rosales FJ, Ross AC. Acute inflammation induces hyporetinemia and modifies the plasma and tissue response to vitamin A supplementation in marginally vitamin A-deficient rats. J Nutr. 1998;128:960–966. doi: 10.1093/jn/128.6.960. [DOI] [PubMed] [Google Scholar]
- 34.Nobili V, Alkhouri N, Alisi A, Ottino S, Lopez R, Manco M, et al. Retinol-binding protein 4: a promising circulating marker of liver damage in pediatric nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:575–579. doi: 10.1016/j.cgh.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 35.Iwasa M, Hara N, Miyachi H, Tanaka H, Takeo M, Fujita N, et al. Patients achieving clearance of HCV with interferon therapy recover from decreased retinol-binding protein 4 levels. J Viral Hepat. 2009;16:716–723. doi: 10.1111/j.1365-2893.2009.01119.x. [DOI] [PubMed] [Google Scholar]
- 36.Huang JF, Dai CY, Yu ML, Shin SJ, Hsieh MY, Huang CF, et al. Serum retinol-binding protein 4 is inversely correlated with disease severity of chronic hepatitis C. J Hepatol. 2009;50:471–478. doi: 10.1016/j.jhep.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 37.Kwon JH, Park ST, Kim GD, You CR, Kim JD, Woo HY, et al. The value of serum retinol-binding protein 4 levels for determining disease severity in patients with chronic liver disease. Korean J Hepatol. 2009;15:59–69. doi: 10.3350/kjhep.2009.15.1.59. [DOI] [PubMed] [Google Scholar]


