Abstract
Objective
Recombinant protective antigen (rPA) is the active pharmaceutical ingredient of a second generation anthrax vaccine undergoing clinical trials both in Korea and the USA. By using the rPA produced from Bacillus brevis pNU212 expression system, correlations of serological immune response to anthrax protection efficacy were analyzed in a guinea pig model.
Methods
Serological responses of rPA anthrax vaccine were investigated in guinea pigs that were given single or two injections (interval of 4 weeks) of various amounts of rPA combined with aluminumhydroxide adjuvant. Guinea pigs were subsequently challenged by the intramuscular injection with 30 half-lethal doses (30LD50) of virulent Bacillus anthracis spores. Serumantibody titerswere determined by anti-PA IgGELISA and the ability of antibodies to neutralize the cytotoxicity of lethal toxin on J774A.1 cell was measured through the toxin neutralizing antibody (TNA) assay.
Results
To examine correlations between survival rate and antibody titers, correlation between neutralizing antibody titers and the extent of protection was determined. Toxin neutralization titers of at least 1176 were sufficient to confer protection against a dose of 30LD50 of virulent anthrax spores of the H9401 strain. Such consistency in the correlation was not observed from those antibody titers determined by ELISA.
Conclusion
Neutralizing-antibody titers can be used as a surrogate marker.
Keywords: anthrax, Bacillus anthracis, Guinea pig, serological correlate
1. Introduction
Gram-positive Bacillus anthracis forms highly stable spores even after excessive UV, heat, or toxic chemical introductions that affect its survival. Exposure to these spores by cutaneous, gastrointestinal, or aerosol routes results in lethal anthrax infection [1]. Poly-D-glutamic acid capsule and anthrax toxin are the two factors that are related to the toxicity of B anthracis in living organism [2]. The expression of poly-D-glutamic acid capsule, coded on pX02 plasmid, is controlled by acpA gene and the capsule itself is in charge of protecting bacteria from the immune system, especially from phagocytosis [2,3]. There are three different protein types of anthrax toxin; protective antigen (PA), lethal factor (LF), and edema factor (EF) [4]. These proteins are coded on pX01 plasmid as pag, lef, and cya genes respectively, and their expression is under the control of atxA gene [3]. Among these anthrax toxins, 83 kDa of PA cleaves into 63 kDa as it attaches to the surface of a host cell, such as a macrophage, and becomes a hydrophobic heptamer [4]. LF or EF binds on this hydrophobic surface to enter the host cell for expressing own toxicity [5]. When the concentration of EF, which is a kind of calmodulin-dependent adenylate cyclase, increases, it raises the concentration of cyclic adenosine monophosphate [6], and, therefore, chloride ions and water molecules flow out of the cell body finally causing edema of the tissue [7]. On the contrary, LF reduces the mitogen-activated protein kinase signal transduction and strengthens the level of cytokinesis tumor necrosis factor-α and interleukin-1β in macrophage cells [8]. It also elevates the amount of oxygen radicals [7] in the host cell resulting in macrophage targeted cytotoxicity, which can cause the actual cell and tissue destruction, and, ultimately, death of the organism.
Interfering with the binding of PA to LF or EF provides effective protection from anthrax infection in immunized animals [9]. Under this concept, the current trend in developing anthrax vaccine ismainly focused onPArather than LF or EF. For example, the anthrax vaccines approved in theUSAandUKare adsorbed formof purified PA obtained from the culture supernatant of nontoxigenic B anthracis [9]. Anthrax Vaccine Adsorbed-Biothrax (AVA-Biothrax, formerly identified as AVA and MDPH-PA; BioPort Corporation, Lansing, MI, USA) is a widely-used anthrax vaccine in the USA which is in a form of aluminum hydroxide-adsorbed highly purified recombinant PA (rPA). However, the need for developing new vaccine is still exist as AVA-Biothrax has some drawbacks such as partial side-effects in some species and inconvenience of multiple-vaccination to achieve a decent level of immunization [10].
In this study, adsorbed form of rPA to adjuvant was tested in a guinea-pig model and their protection rate was determined to set up a reliable surrogate marker of anthrax vaccination. After single or double immunization in 4-week intervals, serum samples from immunized animals were analyzed and compared to the survival rate after virulent B anthracis H9401 spore challenge.
2. Materials and Methods
2.1. Recombinant PA (rPA) preparation
rPA is purified from culture supernatant of Bacillus brevis 47-5Q, an asporogenic, avirulent, and nontoxigenic strain, which contains a recombinant plasmid encoding the PA component of the anthrax toxin [11]. The host B brevis 47-5Q was cultured form modified PY medium containing proteose peptone (Becton Dickinson, Sparks, MD, USA) 1%, yeast extract (Becton Dickinson) 0.5%, uracil 0.01%, glucose 1%, and 10 mg/ml erythromycin (EM). For the production of protein, it was grown at 30℃ in PY broth (containing proteose peptone 1%, yeast extract 0.5%, uracil 0.01%, glucose 1%, MgSO4·7H2O 0.01%, FeSO4·7H2O 0.01%, MnSO4·H2O 0.001%, ZnSO4·7H2O 0.0001%). Unless otherwise specified, chemicals were obtained from Sigma (St Louis, MO, USA). Biostat-D DCU2 model (72 L, B. Braun Biotech International, Germany) was used as a fermentor for large scale bacteria culture in high concentration. Bacterial supernatant was filtered by absolute filter and flowed through anion exchange and hydrophobic interaction chromatography columns. Obtained protein was tested by SDS-PAGE, western-blot analysis, and anti-PA specific-ELISA.
2.2. Animals
Female Hartley guinea pigs (300 g to 320 g) were obtained from Damul Science, Jungeub City, Korea. The animal procedures were approved by the Institutional Animal Care and Use Committee of Korea National Institute of Health.
2.3. Vaccinations and challenge of guinea pigs
Hartley guinea pigs were inoculated intramuscularly (i.m.) with various concentrations of rPA vaccine preparation in 0.5 mL volumes as a single injection (0 week) or as two injections (0 and 4 weeks). rPA was adsorbed to Alhydrogel (2% Al2O3; Brenntag Biosector, Frederikssund, Denmark) 250 μg of aluminum per injection final concentration, for >1 hour at 4℃ before use. Blood was collected for serum isolation 3 weeks. Guinea pigs were challenged in Week 4 after a single or two injections of vaccine by the i.m. route with a targeted dose of 30 half-lethal doses (30LD50) spore from B. anthracis H9401. The LD50 of H9401 spores in Hartley guinea pigs is 50 ± 2 spores [12]. The survival rates were recorded for 14 days after challenge. Spores used for the challenge were prepared according to Ivins et al [13]. Spores used in the experiment were grown in Leighton and Doi medium [14], purified by centrifugation through 58% Hypaque-76 (Amersham Health, Amersham, Buckinghamshire, UK) and suspended in 0.1% gelatin-PBS and stored at 2℃ to 8℃ until used.
2.4. Serological assay
2.4.1. Anti-PA IgG ELISA
Serum samples from individual guinea pigs were analyzed for PA-specific antibody responses using an enzyme-linked immunosorbent assay (ELISA). Preimmune serum obtained 2 days before vaccination was used as a negative control. The wells of high-binding microplates (Costar; Corning Inc., Corning, NY, USA) were coated with 1 μg/mL of PA in 0.1 M carbonate buffer (pH 9.5) and incubated for overnight at 4℃. The wells were washed with PBS containing 0.05% (v/v) Tween 20 (PBST) and blocked with 3% (v/v) bovine serum albumin in PBST. Serum samples were applied to the first column of each row and serially diluted with two-fold volumes of PBST until the last column of the row and incubated for 1 h at 37℃. PA-specific antibodies were detected using a polyclonal goat anti-guinea pig IgG conjugated to peroxidase (Sigma). After washing with PBST, color was developed using o-phenylenediamine substrate. The reaction was stopped by adding 2N H2SO4, and the absorbance at 492 nm was measured using a Sunrise microplate reader (TECAN Instruments, Grödig, Austria). The endpoint titers were defined as the reciprocal of the highest standard serum dilution that resulted in an absorbance three standard deviations greater than the average absorbance of negative control serum samples at the same dilution.
2.4.2. TNA
The titer of toxin-neutralizing antibody in serum was determined using modifications to the methods of Pitt et al [15] and Little et al [4] by testing the ability of the serum to inhibit the cytotoxicity of the combination of rPA with LF (List Biological Laboratories Inc., Campbell, CA, USA). The assay was carried by exposing J774A.1 murine macrophages (ATCC TIB-67) to PA and LF in the presence or absence of serum. In brief, J774A.1 cells were plated out on 96 well plates (SPL CO. Ltd, Pyeongtaek-si, Korea) and seeded with 106 cells per well in 150 μl volumes 2 hours before testing. Cells were maintained in Dulbecco’s minimal essential medium (DMEM) containing 10% heat-inactivated fetal bovine serum, 4 mM glutamine, and 25 units of penicillin G and 25 μg of streptomycin/ml (DMEM complete). Standards and serial two-fold dilutions of samples were pre-incubated with lethal toxin (LeTx 500 ng of rPA per ml and 100 ng of LF/ml, final concentrations) in a humidified incubator at 37℃, 5% CO2, for 1 hour. Dilutions of serum sample and LeTx were prepared in DMEM complete containing 10 mM HEPES. Medium was removed from wells containing the J774A.1 cells and replaced with 100 μl per well of the sample or standard mixed with LeTx. After a 4-hour incubation at 37℃ in 5% CO2, 25 μl of 3-[4,5- dimethylthiazol-2-yl]2,5-diphenyl-tetrazolium bromide (MTT, Amresco, Solon, OH, USA) was added to each well at a final concentration of 5 mg/ml. After an additional 1-hour incubation at 37℃, the cells were lysed by adding 100 μl extraction buffer (90% isopropanol containing 25 mM HCl and 0.5% [w/v] SDS). Absorbance was then measured at 570 nm with an ELISA reader (Tecan). Each dilution of sample and standard was tested in duplicate. Six wells contained only medium and served as medium control. Six LeTx wells contained only LeTx and served as blanks. The percent neutralization (% control) for each dilution of sample and standard was determined by calculating % control = (sample average – lethal toxin avgerage) / (medium control average – lethal toxin average) × 100. The percent control values were plotted against each respective test dilution using a four-parameter logistic equation algorithm and neutralizing antibody titers were expressed as the reciprocal of the dilution of antiserum that neutralized the cytotoxic activity of lethal toxin on J774A.1 cells at 50% of control value (ED50) using SoftMax Pro 5.3 (Molecular Device, Sunnyvale, CA, USA).
2.5. Statistical analysis
The correlation between of protection and TNA titer or anti-PA IgG titers were acquitted with linear regression analysis was employed. Acceptance criteria include (1) the R2 for the standard curve had to be ≥0.95; (2) the standard curve had to contain at least contiguous standards; and (3) the coefficient of variation (%CV) for the duplicate absorbance readings for standards and samples had to be ≤20%.
3. Results
3.1. Serological correlate in guinea pigs after a single injection of rPA vaccine
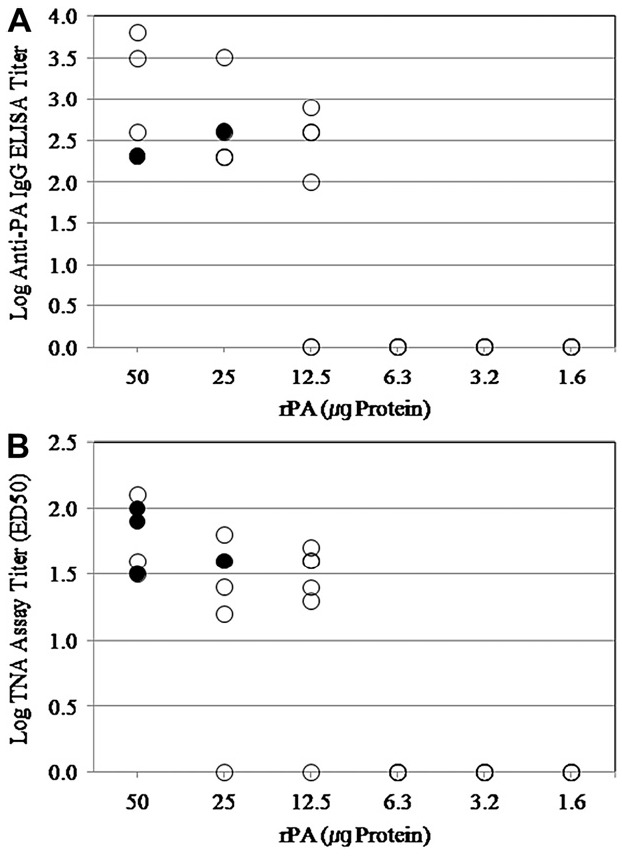
To determine the efficacy of single vaccination, guinea pigs were given a single injection with different amount of rPA adsorbed in Alhydrogel (250 μg in 0.5 mL PBS). Four weeks after immunization, they were challenged with 30LD50 of B anthracis H9401 spores (Table 1, Figure 1). Their serum samples were collected at 4 weeks after immunization, and were analyzed by anti-PA IgG ELISA and TNA assays to determine their serological titers. However, reasonable levels of antibody titers were not obtained in either assay, hence any significant relationship between antibody titers and the actual animal survival rate was not found. These results also support the clinical study performed by Campbell et al [16], which demonstrated that a single injection could not induce any immunological responses in human volunteers. Taken together with these experimental and clinical evidences, boosting immunization is necessary for anthrax vaccination to obtain effective protection.
Table 1.
Survival against an i.m. challenge and antibody response of guinea pigs after single injection of rPA vaccine
| PA(μg) dose | Survival percentage (alive/total) | Geometric mean ELISA titer (standard error) | Geometric mean ED50 TNA assay titer (standard error)a |
|---|---|---|---|
| 50 | 50 (3/6) | 634.96 (1.89) | 59.01 (1.27) |
| 25 | 16.7 (1/6) | 400.00 (1.55) | 27.41 (1.31) |
| 12.5 | 0 (0/6) | 224.49 (1.45) | 18.66 (1.82) |
| 6.3 | 0 (0/6) | 1.0 (1.00) | 1.0 (1.00) |
| 3.1 | 0 (0/6) | 1.0 (1.00) | 1.0 (1.00) |
| 1.6 | 0 (0/6) | 1.0 (1.00) | 1.0 (1.00) |
| 0 (control) | 0 (0/6) | 1.0 (1.00) | 1.0 (1.00) |
aED50, reciprocal of the dilution that neutralized 50% of in vitro LeTx cytotoxicity.
Guinea pigs received single i.m. injections (0.5 ml) of rPA vaccine containing various concentrations of rPA. Four weeks later, guinea pigs were challenged by the i.m. route with a targeted dose of 30LD50 spores of the B anthracis H9401 strain. Sera were tested in a anti-PA IgG Titer and TNA assay.
Figure 1. Relationship between survivals of guinea pig with a single injection of rPA vaccine after i.m. challenge with B anthracis H9401 spore and Week 3. (A) Anti-PA IgG titer, (B) ED50 TNA assay. Guinea pigs (●) surviving or (○) dying after i.m. injection.
3.2. Serological correlate in guinea pigs after two injections of rPA vaccine
To define the serological correlate in guinea pigs with protection, animals were given different concentrations of serially diluted rPA vaccines with 250 μg of alhydrogel in 0.5 mL PBS and after 4 weeks they received another shot of vaccine. Serum samples derived from these animals at 2 week after last vaccination were analysed by anti-PA IgG titer and TNA assays and their serological titers were compared with the survival rate (Table 2, Figure 2). In
Table 2.
Survival against an i.m. challenge and antibody response of guinea pigs after two injections of rPA vaccine
| PA(μg) dose | Survival percentage (alive/total) | Geometric mean ELISA titer (standard error) | Geometric mean ED50 TNA assay titer (standard error)a |
|---|---|---|---|
| 50 | 100 (9/9) | 43890.9 (1.12) | 4063.8 (1.26) |
| 25 | 100 (9/9) | 25600.0 (1.22) | 3225.4 (1.26) |
| 12.5 | 88.9 (8/9) | 16126.9 (1.29) | 1097.3 (1.35) |
| 6.3 | 80.0 (8/10) | 5198.4 (1.26) | 685.9 (1.18) |
| 3.1 | 33.3 (3/9) | 696.4 (1.72) | 278.6 (1.38) |
| 1.6 | 30.0 (3/10) | 459.5 (1.09) | 113.1 (1.37) |
| 0 (control) | 0.0 (0/10) | 1.0 (1.00) | 1.0 (1.00) |
aED50, reciprocal of the dilution that neutralized 50% of in vitro LeTx cytotoxicity.
Guinea pigs received two i.m. injections at 0 and 4 weeks (0.5 ml each) of rPA vaccine containing various concentrations of rPA. Four weeks after the last injection (Week 8), guinea pigs were challenged by the i.m. route with a targeted dose of 30LD50 spores of the B. anthracis H9401 strain. Sera were tested in a anti-PA IgG Titer and TNA assay.
Figure 2. Relationship between survivals of guinea pig inoculated with two injection of rPA vaccine after i.m. challenge with B. anthracis H9401 spore and Week 7. (A) Anti-PA IgG titer; (B) ED50 TNA assay. Guinea pigs (◆) surviving or (◇) dying after i.m. injection.
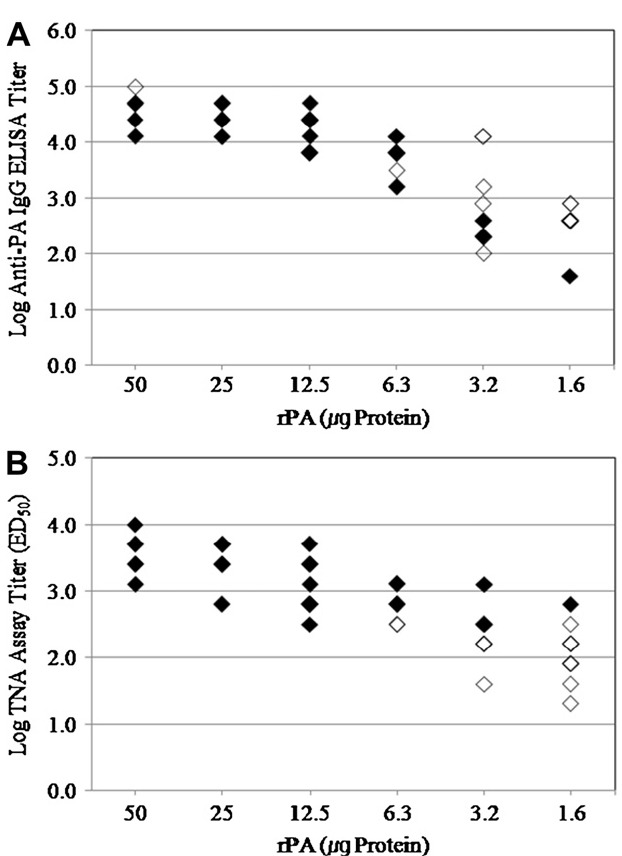
both assays, titers showed significant (p < 0.0001) increase after the second injection and the overall titers were much higher compared to those from single immunization. And increasing tendency of serological titers with higher concentration of rPA and the survival rate was observed (Tables 1 and 2). Furthermore, 100% of animals survived with 50 μg of rPA per dose in 4-week double immunizations, which did not happen with any of the experimental group with single immunization. These
results clearly support the classical immunological concept that the boosting brings higher protection rate to challenge [17].
3.3. Serological titer from double vaccination produces a reliable coefficient to survival rates
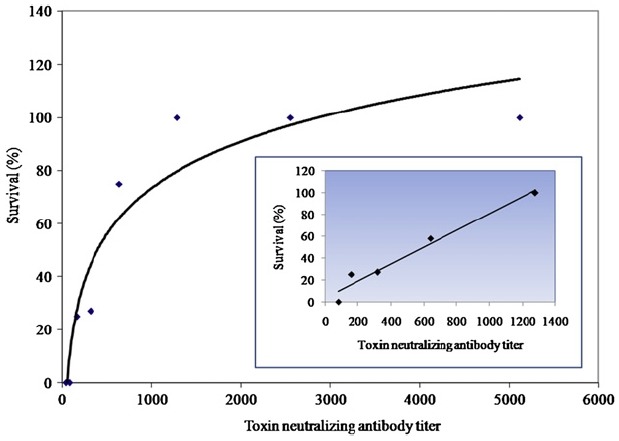
To obtain correlate between serological titers and protection, serological titers of serum samples from individual animals were compared to the survival data and linear regression analysis was performed to obtain R2 values. Among two serological titers, anti-PA IgG titers from the animals of 4 week double immunization schedule did not show any correlation to the survival rate under this experimental condition (R2 = 0.18, data not shown). On the contrary, a high correlation coefficient was obtained from TNA titers with serum samples of double immunizations (R2 = 0.96) (Figure 3). From the linear regression analysis, TNA titers for 50% survival against 30LD50 spore challenge was 551 and for 100% survival was 1176. The correlation coefficient ensured that double immunization schedule with a 4- week interval might be able to give a good indication about vaccine efficacy. This also partially supports the previous finding that asserted admitting TNA as a surrogate marker for testing vaccine efficacy [18].
Figure 3. Correlation between TNA titers and protection after two injections. Relationship between serological titer and protection of guinea pigs with a double vaccination serially diluted of rPA vaccine at a 4-week interval. After 4 weeks after the second vaccination, guinea pigs were challenged i.m with B anthracis H9401 spores. Each point in the group represents the average of duplicate determinations performed with pooled sera derived from 9 or 10 guinea pigs. TNA titers of serum samples from individual animals in each group were compared to the survival data of the animals (inset). Linear regression was performed using data from experimental cohorts with neutralizing-antibody titers of 1176 or less. The calculated R2 value is 0.96.
4. Discussion
The correlation between neutralizing-antibody titers and protection against B anthracis infection in different animal species vaccinated with rPA provides surrogate markers to evaluate the protective efficiency of rPA vaccines in humans [19-27]. Because of the cost associated with conducting such studies with a large enough number of nonhuman primates to achieve statistically significant results, alternative animal models have been examined. McBride et al [26] were unable to identify a correlate of protection in guinea pigs that had received three inoculations of rPA (250, 2.5, 0.25, or 0.025 μg/dose) formulated with Alhydrogel adjuvant at
0, 2, and 4 weeks and challenged at 6 weeks by the aerosol route with the Ames strain of B anthracis. Pitt et al [27] reported that survival of guinea pigs that had been challenged 3 months after being inoculated with two injection of rPA (50, 5, or 0.5 μg/dose) formulated with alhydrogel at 0 and 4 weeks was not dose dependent. Reuveny et al [18] argued against multiple
inoculations and favored a single inoculation of vaccine. Their group demonstrated that their neutralizing antibody titers could serve as an effective correlate of protection for guinea pigs that were challenged intradermally with spores of the Vollum strain spore of B anthracis after a single subcutaneous inoculation of rPA (25, 12.5, 6.3, 3.1, 1.6, or 0.8 μg) adsorbed to Alhydrogel. The anti-PA IgG titers, however, proved to be of limited value in serving as a surrogate marker [18].
Our present study was designed to find correlations between protection level and either anti-PA IgG IgG titers or neutralizing-antibody titers. To eliminate
potential effects of other B anthracis derived components, we have used highly purified rPA adsorbed to alhydrogel as a vaccine. For the first step of establishing a surrogate marker for anthrax vaccine, suitable immunization intervals (4 week) were determined (data not shown). Then we tested the number of vaccinations to obtain adequate level of protection efficacy for testing rPA-alhydrogel vaccine. In the case of the single injection experiment, we obtained just 50% protection with the highest rPA dose and antibody titers were not high enough to guarantee the reproduction of this result, especially in TNA (titers < 100). Because of these reasons, single injection was not the optimal condition to examine vaccine efficacy.
With the information that a single injection is not sufficient to establish serological marker, we performed another set of experiments using two injections with a 4- week interval. In this case, it would obviously obtain higher serological titers in both assays compared to those of single injection schedule. Overall, animals that received two injections could survive more than animals with single injection and serological titers rapidly increased after the second injection. These results clearly support the classical concept that boosting brings higher serological titer and a higher protection rate to immunological challenge [17]. We could obtain relatively high correlation coefficients from anti-PA IgG and neutralizing-antibody titers with serum samples from 4 weeks injection schedule (R2 = 0.18 in anti-PA IgG ELISA, R2 = 0.96 in TNA). These correlation coefficients ensured that a two-injection schedule with a 4 week interval might be able to serum as a good surrogates about vaccine efficacy.
Our findings support the notion that antibodies involved in neutralization are also involved in protection against the full course of infection. This is in agreement with previous observations in which monoclonal antibodies capable of neutralizing the cytotoxic effect were shown to delay death in guinea pigs [28]. The results indicate that neutralizing-antibody titers can be used as a reliable correlate for protection in guinea pigs. A correlation between protection and neutralization titers was recently observed in a rabbit model [15], suggesting that this phenomenon is not species specific. Application of such markers to human studies therefore appears to be feasible, even though similar experiments in nonhuman primates would be required to gain more confidence.
In conclusion, we have produced a series of experimental data to establish a surrogate marker that can indicate rPA vaccine efficacy. According to our results, the best correlation between serological titers and animal survival rates was obtained with double immunization using a 4-week interval schedule. Under this condition, antibody titers obtained by TNA were high enough to be reproduced and R2 values higher than 0.95, which indicates that TNA can be a reliable experimental technique for estimating animal survival. Therefore, to test the efficacy of an anthrax PA vaccine, two injections in combination of appropriate adjuvant at a 4-week interval and TNA for serological assay are recommended.
Acknowledgments
This study was supported by a grant of the Korea National Institute of Health (No. 4840-300-210-13).
References
- 1.Little SF, Ivins BE, Webster WM, et al. Effect of aluminum hydroxide adjuvant and formaldehyde in the formulation of rPA anthrax vaccine. Vaccine. 2007 Apr;12(15):2771–7. doi: 10.1016/j.vaccine.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 2.Edwards KA, Clancy HA, Baeumner AJ. Bacillus anthracis: toxicology, epidemiology and current rapid-detection methods. Anal Bioanal Chem. 2006 Jan;384(1):73–84. doi: 10.1007/s00216-005-0090-x. [DOI] [PubMed] [Google Scholar]
- 3.Wang SH, Wen JK, Zhou YF, et al. Identification and characterization of Bacillus anthracis by multiplex PCR on DNA chip. Biosens Bioelectron. 2004 Nov;20(4):807–13. doi: 10.1016/j.bios.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Little SF, Ivins BE, Fellows PF, et al. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine. 2004 Jan;22(3-4):422–30. doi: 10.1016/j.vaccine.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Mock M, Fouet A. Anthrax. Annu Rev Microbiol. 2001;55:647–71. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- 6.Bhatnagar R, Batra S. Anthrax toxin. Crit Rev Microbiol. 2001;27(3):167–200. doi: 10.1080/20014091096738. [DOI] [PubMed] [Google Scholar]
- 7.Dixon TC, Meselson M, Guillemin J, Hanna PC. Anthrax. N Engl J Med. 1999 Sep;341(11):815–26. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- 8.Duesbery NS, Webb CP, Leppla SH, et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998 May;280(5364):734–7. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 9.Belton FC, Strange RE. Studies on a protective antigen produced in vitro from Bacillus anthracis: medium and methods of production. Br J Exp Pathol. 1954 Apr;35(2):144–52. [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbull PC, Broster MG, Carman JA, et al. Development of antibodies to protective antigen and lethal factor components of anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Infect Immun. 1986 May;52(2):356–63. doi: 10.1128/iai.52.2.356-363.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhie GE, Park YM, Chun JH, et al. Expression and secretion of the protective antigen of Bacillus anthracis in Bacillus brevis. FEMS Immunol Med Microbiol. 2005 Aug;45(2):331–9. doi: 10.1016/j.femsim.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Lee DY, Chun JH, Ha HJ, et al. Poly-gamma-D-glutamic acid and protective antigen conjugate vaccines induce functional antibodies against the protective antigen and capsule of Bacillus anthracis in guinea-pigs and rabbits. FEMS Immunol Med Microbiol. 2009 Nov;57(2):165–72. doi: 10.1111/j.1574-695X.2009.00595.x. [DOI] [PubMed] [Google Scholar]
- 13.Ivins B, Fellows P, Pitt L, et al. Experimental anthrax vaccines: efficacy of adjuvants combined with protective antigen against an aerosol Bacillus anthracis spore challenge in guinea pigs. Vaccine. 1995;13:1779–84. doi: 10.1016/0264-410x(95)00139-r. [DOI] [PubMed] [Google Scholar]
- 14.Leighton TJ, Doi RH. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 Dec;246(18):3189–95. [PubMed] [Google Scholar]
- 15.Pitt ML, Little SF, Ivins BE, et al. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine. 2001 Sep;19(32):4768–73. doi: 10.1016/s0264-410x(01)00234-1. [DOI] [PubMed] [Google Scholar]
- 16.Campbell JD, Clement KH, Wasserman SS, et al. Safety, reactogenicity and immunogenicity of a recombinant protective antigen anthrax vaccine given to healthy adults. Hum Vaccin. 2007 Sep-Oct;3(5):205–11. doi: 10.4161/hv.3.5.4459. [DOI] [PubMed] [Google Scholar]
- 17.Marcus H, Danieli R, Epstein E, et al. Contribution of immunological memory to protective immunity conferred by a Bacillus anthracis protective antigen-based vaccine. Infect Immun. 2004 Jun;72(6):3471–7. doi: 10.1128/IAI.72.6.3471-3477.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reuveny S, White MD, Adar YY, et al. Search for correlates of protective immunity conferred by anthrax vaccine. Infect Immun. 2001 May;69(5):2888–93. doi: 10.1128/IAI.69.5.2888-2893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnard JP, Friedlander AM. Vaccination against anthrax with attenuated recombinant strains of Bacillus anthracis that produce protective antigen. Infect Immun. 1999 Feb;67(2):562–7. doi: 10.1128/iai.67.2.562-567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen S, Mendelson I, Altboum Z, et al. Attenuated nontoxinogenic and nonencapsulated recombinant Bacillus anthracis spore vaccines protect against anthrax. Infect Immun. 2000 Aug;68(8):4549–58. doi: 10.1128/iai.68.8.4549-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowler K, McBride BW, Turnbull PC, et al. Immune correlates of protection against anthrax. J Appl Microbiol. 1999 Aug;87(2):305. doi: 10.1046/j.1365-2672.1999.00898.x. [DOI] [PubMed] [Google Scholar]
- 22.Gu ML, Leppla SH, Klinman DM. Protection against anthrax toxin by vaccination with a DNA plasmid encoding anthrax protective antigen. Vaccine. 1999 Jan;17(4):340–4. doi: 10.1016/s0264-410x(98)00210-2. [DOI] [PubMed] [Google Scholar]
- 23.Ivins BE, Pitt ML, Fellows PF, et al. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine. 1998 Jul;16(11-12):1141–8. doi: 10.1016/s0264-410x(98)80112-6. [DOI] [PubMed] [Google Scholar]
- 24.Pezard C, Weber MJ, Sirard C, et al. Protective immunity induced by Bacillus anthracis toxin-deficient strains. Infect Immun. 1995 Apr;63(4):1369–72. doi: 10.1128/iai.63.4.1369-1372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little SF, Knudson GB. Comparative efficacy of Bacillus anthracis live spore vaccine and protective antigen vaccine against anthrax in the guinea pig. Infect Immun. 1986 May;52(2):509–12. doi: 10.1128/iai.52.2.509-512.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McBride BW, Mogg A, Telfer JL, et al. Protective efficacy of a recombinant protective antigen against Bacillus anthracis challenge and assessment of immunological markers. Vaccine. 1998 May;16(8):810–7. doi: 10.1016/s0264-410x(97)00268-5. [DOI] [PubMed] [Google Scholar]
- 27.Pitt MLM, Ivins B, Estep JE, et al. Comparative efficacy of a recombinant protective antigen vaccine against inhalation anthrax in guinea pigs, rabbits, and Rhesus monkeys.; In: Proceedings of the 96th Annual Meeting of American Society of Microbiology.; 1996. p. 278. E-70. [Google Scholar]
- 28.Little SF, Ivins BE, Fellows PF, et al. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect Immun. 1997 Dec;65(12):5171–5. doi: 10.1128/iai.65.12.5171-5175.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


