Abstract
Although the prevalence of a mental disorder, in general, in patients with diabetes mellitus is regarded to be comparable to the general population, an increased prevalence of depressive disorders, often comorbid with anxiety, has been reported in patients with diabetes mellitus.
The co-occurrence of depression in diabetes is attributed to a variety of factors, including the psychological and psychosocial impact of the disease, a potential common genetic susceptibility and common pathophysiological abnormalities involving neuroimmunological and neuroendocrinical pathways, as well as microvascular brain lesions due to diabetes mellitus. However, issues concerning pathogenesis and causality of this high co-occurrence are not fully determined yet. Still, the presence of depression in patients with diabetes mellitus is of vast importance, as it is usually associated with poor disease control, adverse health outcomes and quality of life impairment.
This article aims to provide a comprehensive review of epidemiological findings, clinical considerations and management strategies concerning depression in patients with diabetes mellitus.
Keywords: Diabetes mellitus, depression, epidemiology, glycemic control, complications, mortality, quality of life, treatment
Mental disorders, in general, in patients with diabetes mellitus(DM)
Patients with DM seem not to be at higher risk for a mental disorder in general compared to non-diabetic individuals.
In a cross-sectional population-based study by Kruse et al1 among 141 patients with DM, identified out of a community sample of 4169 individuals, the prevalence of any mental disorder - assessed with the Composite International Diagnostic Interview (CIDI) - was comparable between the patients with DM and the non-diabetic individuals [26.6% vs 26.0%; Odds Ratio(OR)=1.11; Confidence Interval (CI):0.73-1.69]. Notably, after adjusting for age, sex, socioeconomic and family status, no significant difference between the two groups was found, concerning affective, somatoform, substance abuse/dependence disorders; only anxiety disorders were found to be significantly more prevalent in the diabetic group (OR=2.05; CI:1.22-3.43).
Das-Munshi et al2 in another cross-sectional population-based study of 249 patients with diabetes, identified out of a sample of 8580 individuals, reported that the prevalence of any mental disorder - assessed with the Clinical Interview Schedule-Revised (CIS-R) - was 21.6% in the diabetic group vs 16.3% in the non-diabetic group. The crude (unadjusted) odds ratio was non-significant (OR=1.4; CI:1.0-2.0), whereas after adjusting for age, sex and socioeconomical status it became significant (OR=1.5;1.1-2.2; p<0.05). Finally, after adjusting further for impairment in everyday functioning and medical comorbidity, the odds ratio was attenuated again in non-significant levels (OR=1.3; CI:0.9-1.9). The same pattern also apllied to mixed anxiety and depression, whereas the odds ratio concerning depressive, anxiety, comorbid anxiety depressive disorders was not statistically significant throughout all the models applied, adjusting for the confounders mentioned.
Depression Prevalence and relative risk
The prevalence of major depression in patients with DM is mostly estimated around 12% (ranging from 8-18%), while milder types of depression or elevated depressive symptoms, in general, are reported to be present in 15-35%. (Table 1 ) 1-22.
Table 1. Prevalences, odds ratios and risks concerning comorbid depression in diabetes.
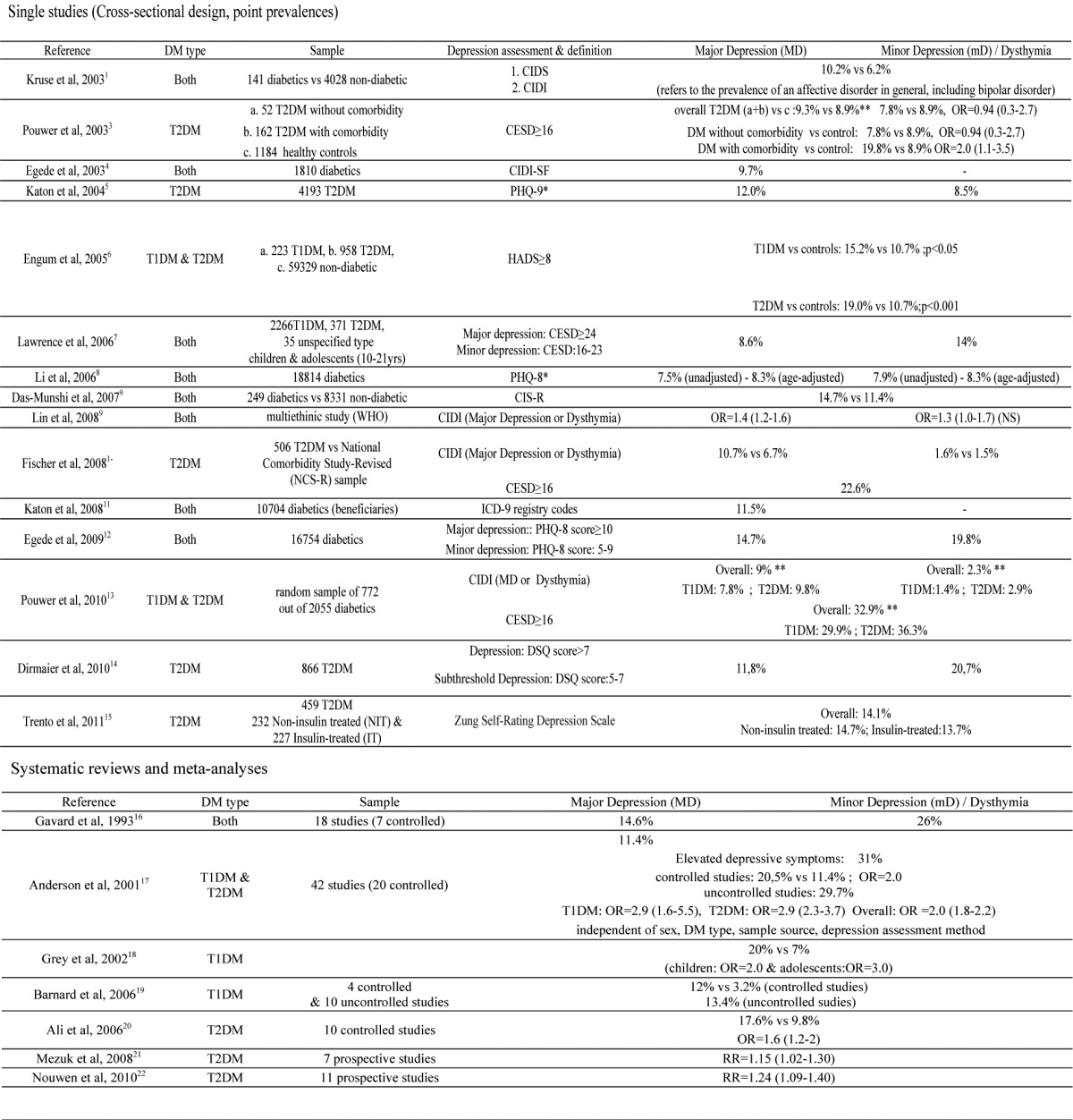
Compared to non-diabetic controls, patients with DM are reported to be about 1.4-3 times as likely to suffer from comorbid depression9, 10, 17 although there have also been some studies - including the two forementioned1, 2– that failed to find any significicant difference in the prevalence of depression (or affective disorders, in general) between diabetic and non-diabetic individuals. Of note, findings of Pouwer et al3 suggest that the presence of medical comorbidity might be a significant factor contributing to the increased prevalence of depression in DM, since depression rates in patients with DM but no other comorbidity were found to be comparable to healthy controls. However, these results need to be replicated in larger control studies, since the subgroup of patients with DM but no other comorbidity was relatively small in comparison to the other two groups of the study.
Concerning causality, the association between diabetes and depression seems to be bidirectional, though the direction depression being a risk factor for the development of DM seems to be stronger. The relative risk for developing T2DM in depressed patients (Depression→Diabetes) is reported as high as 1.621. Conversely, concerning the relative risk for developing depression in patients with DM (Diabetes→Depression), two recent meta-analyses of prospective studies have yielded a relative risk around 1.221, 22.
Estimates of depression prevalence vary widely, mainly depending upon depression assessment tools (standardized interviews vs self-report questionnaires), depression classification (discriminating major from minor depression or referring to elevated depressive symptoms - indicative of depression - in general, using specific cut-off values in self-report questionnaires), study designs (controlled vs uncontrolled), sample sizes and diabetes type. As for assessment method bias, in particular, depression is found at a rate of about 2-3 times higher when specific cut-offs in self-report questionnaires are applied compared to major depression being assessed with standardized interviews10, 17.
Depressive symptoms seem to be slightly more prevalent in T2DM compared to T1DM (Table1), though this difference is not regarded to be statistically significant17. Further studies, comparing the prevalence of depression in samples including patients with diabetes of either type, also adjusting for potential confounders, such as age, diabetes duration, treatment regimen, glycemic control, diabetic complications and medical comorbidity are needed.
The clinical course of depression in diabetes
Depression in diabetes is persistent and/or recurrent. In longitudinal and follow-up studies the rates of depression persistance or recurrence have been reported to range widely, between 11.6% and 92%, depending on sample sizes, depression diagnosis criteria and depression classification (major depression or elevated depressive symptoms).
Lustman et al23 followed-up 25 patients who had previously participated in a 8-week depression treatment clinical trial with nortriptyline vs placebo. Persistence or recurrence of depression - assessed with the Diagnostic Interview Schedule (DIS) - was identified in 23 patients (92%), with an average of 4.8 depressive episodes over the 5-year follow-up period. Even after successful initial treatment of depression, recurrence was extremely common (80% of the patients) and rather rapid (58.3% of the patients were relapsed within the first year). However, the percentages reported in that study should be interpreted with caution due to small sample size.
Peyrot24 conducted a follow-up study among 245 patients with DM, who were assesed three times (baseline, after 1-week psychoeducational intervention, and at 6 months). Elevated depressive symptoms [defined as a Center for Epidemiological Studies Depression (CESD) scale score ≥16] were found in 93 patients (38%) at baseline. A percentage of 34.4% of the initially depressed patients remained depressed at all three time points. Predictors of being persistently depressed were lack of high school education, presence of more than 2 complications and treatment other than insulin.
A randomized controlled trial (RCT) in 164 diabetic patients assigned to collaborative care intervention against 165 diabetic patients assigned to usual care, by Katon et al25, revealed that depressive symptoms - assessed with Hopkins Symptoms Checklist 90 (SCL-90) - persisted (persistance was defined as p˂50% decrease in SCL-90 score) in 59.9% of the intervention group compared to 68.3% of the usual care group at the 12-month folow-up.
Fischer et al10 conducted a longitudinal study among 508 patients with type 2 diabetes assessed three times over 18 months (baseline, 9 months and 18months). Major depression - assessed with CIDI - was present in 14.9% of the patients at baseline and in 19.8% at any point during the study. Diagnosis of major depression persisted at all three assessment points in 11.6% of the patients diagnosed with major depression at baseline. Elevated depressive symptoms - defined as CESD scale score≥16 - were present in 15.5% of the patients at baseline and in 34.4% at any point during the study. Elevated depressive symptoms persisted at all three assessment points in 58.1% of the patients with elevated depressive symptoms at baseline. These findings suggest that persistence of depression over time mainly refers to elevated depressive symptoms rather than major depression itself. Of note, younger age and higher comorbidity were independently associated with persistence of major depression over time, whereas younger age, female gender, lower education, higher comorbidity and higher HBA1c values were independently associated with persistence of elevated depressive symptoms.
Katon et al26, in a prospective study among among 2759 diabetic patients who were followed-up for 5 years, found that 83% of the patients with major depression – defined as reporting ≥5 symptoms in Patient Health Questionnaire-9 (PHQ-9), including at least one core sympom of depression, such as depressed mood or anedonia - at follow-up had also been depressed at baseline, while 42.4% of them had also a positive history of depression - based on previous ICD-9 registry codes - within a period of 18 months prior to the study.
In conclusion, depression is highly persistant and/or recurrent in DM, even after successful initial treatment. Therefore, patients with a history of a depressive episode ever before should be considered at high risk for relapse, especially under the influence of health-related or psychosocial stressors.
Risk factors for the development of depression in patients with diabetes.
Risk factors associated with the presence of depression in patients with diabetes include female sex, younger age, not having a spouse, poor social support, lower education, low socioeconomic status, poor glycemic control, presence of diabetic complications, presence of medical comorbidity, physical impairment and previous history of depression4-6,26,27.
Pouwer et al3, in a controlled community-based study among 216 patients with T2DM, identified from a sample of 3107 individuals (age range 55-85), evaluated the association of various factors with depression - assessed both with CIDI and CESD scale - , using a 4-layer stepwise linear regression procedure, with demographics (model1; R2=9.0%), clinical characteristics [eye difficulties, cardiovascular disease (CVD), other chronic medical diseases] and medical comorbidity (model2; R2change=2.0%), functional limitations (model3; R2change=3.8%), social support and perceived mastery over the disease (model4; R2change=8.6%) entered successively into the analysis. In the final model (R2=23.4%), they identified the following risk factors being independently associated with depression: functional limitations (beta=0.18, p=0.034), instrumental social support (beta=-0.26, p=0.017) and mastery (beta=-0.26, p=0.001). The female sex appeared significantly associated with depression only in the 1st model. An association between depression and being unmarried emerged as significant in the 2nd and 3rd model but ended up as marginally non-significant (p=0.057) again in the final model. Of note, clinical characteristics were not significantly associated with depression in any of the stepwise regression models.
Egede et al12, in a large-scale cross-sectional study among 16754 patients with DM, examined the differences among the 3 groups in which they divided the sample by depression severity status, according to PHQ-8 questionnaire scores (0-4: no depression, 5-9:minor depression, ≥10: major depression). They identified significant differences among the three groups concerning race (four race groups), sex (females), age (four classes), education (four classes), income (four classes), marital status (married), employment status (employed), insurance (insured), while no significant differences were found concerning health provider and diabetes education. The chi-square analysis performed cannot fully determine the differences found across the subgroups of the variables with more than two classes.
In the prospective study mentioned above by Katon et al26, the following risk factors for major depression were identified: previous history of depression (OR=1.68 CI:1.15-2.45), significant depressive symptoms at baseline (OR=7.70; CI:4.63-12.81 for PHQ-9 score >5, and OR= 2.24; CI:1.30-3.88 for PHQ-9 score >10), elevated diabetes-related symptoms at baseline (OR=1.13; CI:1.05-1.22) and cardiovascular procedures during the study (OR=1.92; CI:1.10-3.3).
As indicated above, the presence of a history of depression is a significant factor that should be adjusted for, when evaluating risk factors associated with the development of depression in DM.
Another factor that has not been sufficiently taken into account is the use of medication with a potential depressiogenic effect, such as certain antihypertensive agents for instance, that are often prescribed in patients with DM and comorbid hypertension.
The impact of depression in diabetes
The presence of depression in DM has been associated with significant negative impact in self-care, glycemic control, health outcomes and quality of life.
Self-care
According to a meta-analysis of 43 studies by Gonzalez et al28, depression was significantly negatively associated with adherence to DM treatment regimen, regarding almost all self-care aspects evaluated [diet, medication, exercise, self-monitoring of blood glucose (SMBG), medical appointments attendance and composite self-care measures] except for diabetic foot care. Nevertheless, the latter behavior was assessed only in two studies. The overall effect size was moderate (r=0.21 CI:0.17-0.25) and it was significantly higher in studies evaluating self-care as a continuous rather than a categorical variable. The effect sizes for a certain self-care behavior were as follows: medical appointments keeping: 0.31 (CI:0.29-0.34), composite measures of self-care: 0.29 (CI:0.23-0.34), diet: 0.18 (CI:0.13-0.22), medication: 0.14 (CI:0.09-0.20), exercise: 0.14 (CI:0.10-0.17), SMBG: 0.10 (CI:0.04-0.16), foot care: 0.07 (CI:-0.08-0.21). The researchers also reported that the type of diabetes did not seem to significantly affect the association between depression and non-adherence and that studies among children or adolescents with diabetes reported larger effects than studies among adults.
It is worth mentioning the results of two prospective studies; Gonzalez et al29 followed-up 128 patients with DM for 9 months. They concluded that, after adjusting for baseline self-care – assessed with Diabetes Self-Care Activities (SDSCA) questionnaire -, patients with higher depressive symptoms – assessed with Harvard Department of Psychiatry/National Depression Screening Day Scale (HANDS) - showed lower adherence to general diet recommendations (beta=-0.17, p=0.007) and specific dietary behaviors such as fruits and vegetables consumption (beta=-0.18, p=0.004) and spacing of carbohydrates (beta=-0.23, p=0.001), less exercise and poorer foot care at follow-up. Concerning SMBG, baseline HANDS score predicted lower SMBG in the initial model, but this association remained no longer significant after adjusting for baseline SMBG. As for medication adherence, each one-point increase in baseline HANDS was associated with a 1.08-fold increase in the odds for non-adherence (OR=1.08, CI:1.01-1.16). Furthermore, increases in HANDS scores over time also predicted poorer adherence concerning diet in general (beta=-0.21, p=0.001), spacing carbohydrates (beta=-0.16, p=0.017) high-fat foods consumption (beta=0.15, p=0.036) and exercise (beta=-0.14, p=0.036).
Katon et al30 in a prospective study among 4117 patients with diabetes, found that major depression - defined as reporting ≥5 symptoms in PHQ-9 questionnaire, including at least 1 core symptom of depression, such as depressed mood or anedonia - was associated with an increased likelihood of poor adherence to medication concerning control of DM (OR=1.98; CI:1.31-29.8; pp<0.001), hypertension (OR=2.06; CI:1.47-2.88; pp<0.001) and Low-Density Lipoproteins (LDL (OR=2.43; CI:1.19-4.97; pp<0.01).
Glycemic control
Depression has generally been regarded to be associated with poor glycemic control in both types of diabetes, with a small to moderate effect size though, as reported in the only meta-analysis performed so far (Lustman et al)31 and several other studies, either cross-sectional or longitudinal. However, findings are not consistent across literature, since a significant amount of studies argue against such an association, particularly concerning type 2 diabetes.
The subject will be further presented focusing on each type of DM.
Type 1 Diabetes Mellitus
A significant association between HBA1c and depressive symptoms has been reported in several cross-sectional studies32,33,34,13. There are also some studies reporting that depressive symptoms prospectively predicted HBA1c35,36. Finally, fewer cross-sectional studies either found a significant correlation in univariate but not multivariate analysis37 or found no significant association at all38.
The subject will be further presented focusing on each type of DM.
Type 2 Diabetes Mellitus:
Some studies revealed a significantly higher mean HBA1c in the group of depressed vs non-depressed patients in a predominantly T2DM sample30 and a sample of T2DM39 patients or that depressive symptoms severity was independently associated with HBA1c, in a predominantly T2DM sample40. There are also some prospective studies reporting that mean HBA1c difference over time was higher in depressed patients41 or that baseline clinical - but not subclinical - depression predicted poor glycemic control (defined as HBA1c≥7,0%) at follow-up independent of baseline HBA1c14.
On the contrary, several cross-sectional studies either have not found any association13,33,38,42,43 or found an association only in univariate but not multivariate analysis44. In addition, there are also some prospective studies where the association found cross-sectionally failed to be replicated longitudinaly44 or the association of baseline depression and HBA1c at follow-up remained no longer significant after controlling for baseline HBA1c or diabetes clinical characteristics45,46.
Conclusively, the association between depression and glycemic control reported in previous research seems less doubtful in the case of T1DM compared to T2DM, where the findings have been contradictory, since a significant part of the literature argues against such an association.
Inconsistencies in research findings on this specific subject could be attributed to various methodological issues. First of all, assessment and classification of depressive symptoms through self-report questionnaires might have lead to overestimation of what is considered as depression thus obscuring its relationship with glycemic control. Another important issue, stressed recently, is that a significant part of what has been previously conceptualized as 'depression', might well reflect general emotional distress, or diabetes-related distress, rather than clinical depression, especially when self-report questionnaires are applied for depression assessment. Diabetes-related distress and depression though overlapping represent different constructs, with probably different impact on glycemic control and responsiveness to different treatment strategies47,48. Treating depression either as a continuous variable (scores in depression self-report questionnaires) or a categorical one (based either on standardized interviews' categorical diagnosis, or on established cut-offs in self-report questionnaires) could be another issue. Apart from depression, treating HBA1c also as a categorical variable might as well account for inconsistencies, since the cut-off value applied in order to discriminate between poor and good glycemic control varies across studies. Finally, antidepressant medication represents a factor of great significance that should be taken into account when evaluating the effects of depression on glycemic control, on the grounds that antidepressants have been associated with an increased risk for developing diabetes and a negative impact on glycemic control - depending on the type of the antidepressant, the dosage and the duration of the treatment49 - not necessarily through weight gain. Moreover, antidepressant effects on glycemic control have also been reported to depend upon the type of antidepressant, as will be discussed below. Thus, in order to investigate the association between depression and glycemic control, antidepressant medication is a factor that should be controlled for.
Regarding the investigation of the mechanisms implicated in the association between depression and glycemic control, adherence to self-care is regarded as a potential mediator34,50, though it cannot fully account for it. The latter implicates that depression might also have a direct negative effect on glycemic control, probably via psychoneuroimmunological and psychoneuroendocrinological pathways.
Diabetes-related symptoms
Diabetes-related symptoms are often amplified and more frequently reported in patients with comorbid depression. Ciechanowski et al33 found that depression - assessed with SCL-90R - as well as higher levels of diabetic complications were independently associated with the amount of diabetes-related symptoms reported (in Self-Completion Patient Outcome instrument) for both T1DM and T2DM.
Ludman et al51 in a study among 4168 patients with DM, found that the amount of diabetes symptoms reported - assessed with Self-Completion Patient Outcome instrument - was significantly higher (mean=4.40) in patients with major depression - defined as reporting ≥5 symptoms in PHQ-9 scale, including at least one core symptom of depression, such as depressed mood or anedonia - compared to patients without depression (mean=2.46; pp<0.001).
McKellar et al50, in a study among 307 patients with T2DM followed-up over 1 year, found that baseline depressive symptoms - assessed with CESD and the Mental Health Subscale of Mental Outcome Studies 36-short form (MOS-36-SF) - predicted the diabetes-related symptoms (categorized as hyperglycemic, hypoglycemic and microvascular) change over the follow-up period. However, when self-care adherence was entered in the structural equation model applied, the relationship between depressive and diabetes-related symptoms remained no longer significant, indicating that the negative impact of depressive symptoms on diabetes-related symptoms is indirect, probably mediated by the negative impact of depression on diabetes self-care.
Diabetic complications
A significant association between depression and diabetic complications has been identified. According to a meta-analysis by De Groot et al52, the effect sizes for each complication were as follows: 0.17 for retinopathy, 0.20 for macrovascular complications, 0.25 for nephropathy, 0.28 for neuropathy and 0.32 for sexual dysfunction. The overall effect size was small to moderate (r=0.25), comparable between the two types of diabetes.
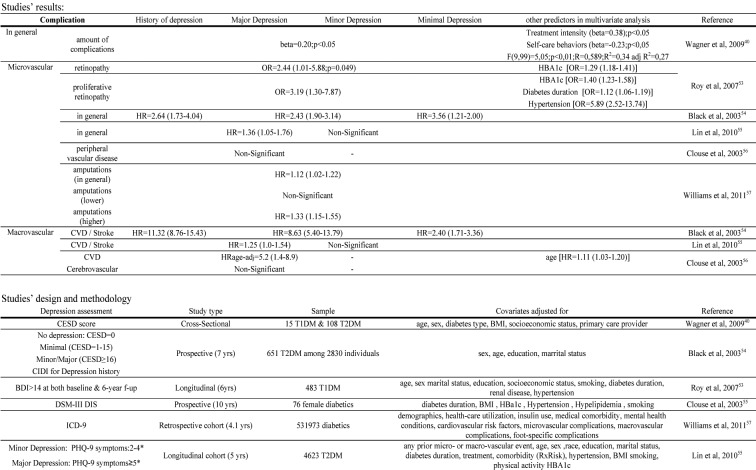
The majority of studies on the association between depression and diabetic complications have been cross-sectional, thus making causality difficult to infer. However, prospective studies have shown that depression is associated with a higher and more rapid incidence of diabetic complications (Table 2)40,53-57. The association between depression and diabetic complications seems to be bidirectional, since depression might result - probably with poor glycemic control as a mediator - in advanced course of complications on one hand, while, on the other hand, complications might also have a negative impact on patients' physical and mental health and quality of life, thus fostering the development of depression.
Table 2. Depression and diabetic complications risk.
Cognitive impairment
People with diabetes have been reported to be at 60% greater risk of developing dementia (OR=1.6; CI:1.4-1.8) according to a systematic review of prospective studies by Cukierman et al (2005)58.
Studies evaluating the impact of depression on cognitive impairment in patients with DM have produced mixed results.
Bruce et al59 assessed the longitudinal predictors of dementia in a study among 302 patients with DM. Dementia at follow-up was not significantly associated neither with depression at follow-up (cross-sectionally) nor with depression at baseline (longitudinally). Katon et al60, in a prospective cohort study of 3837 primary care patients with DM, evaluated the impact of depression on the risk for developing dementia over a 5-year follow-up period. They found a significantly increased incidence rate of dementia (21.5 per 1000 person-years) in patients with DM and major depression at baseline compared with patients with DM but no depression at baseline (incidence rate of 11.8 per 1000 person-years). Thus, comorbid major depression in DM was prospectively associated with an almost 3-fold higher risk of dementia (HR=2.69; CI:1.77-4.07).
Quality of life (QoL)
QoL has been recognized as a domain of major importance in patients with chronic diseases, including DM. Apart from the significance it entails in its own right, it is also regarded as a major outcome that should be taken into account when evaluating the goals and effectiveness of any therapeutic plan concerning DM management, since QoL has been also associated with several adverse health outcomes and increased mortality.
Depression has been associated with a significant impairment in QoL in patients with DM. However, since the majority of the studies conducted have been cross-sectional, no safe conclusion concerning causality can be easily drawn. Still, the relationship seems to be bi-directional.
Schram et al61.conducted a systematic literature review including 20 studies (18 cross-sectional and 2 longitudinal). They concluded that QoL (both physical and mental) was significantly impaired in diabetic patients with comorbid depression, demonstrating a mild to moderate impairment of QoL in studies that used generic or domain-specific QoL questionnaires and a moderate to severe impairment of QoL in studies that used disease-specific questionnaires. Despite the fact that potential confounders such as demographics or disease- and comorbidity-related factors were assessed in only half of the studies, controlling for confounders did not significantly affect the association between depression and QoL impairment.
Ali et al62 conducted a systematic literature review as well, including 14 cross-sectional studies, and concluded that depression had a significant negative impact on QoL of patients with DM, even in studies controlling for potential confounders such as diabetes duration, diabetic complications or medical comorbidity. This negative association was independent of the type of measures of QoL applied (generic or disease-specific). Notably, despite the negative association of depression with overall QoL, depression was not consistently associated with every specific domain of QoL across the studies reviewed.
Mortality
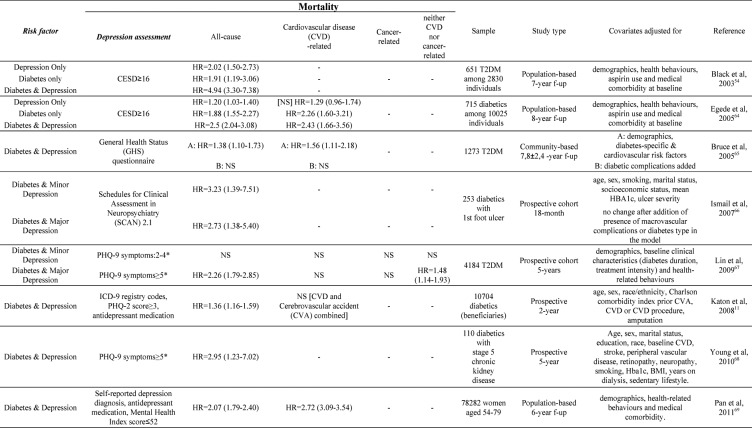
Comorbid depression in patients with DM establishes a potentially life-threatening combination63. Prospective studies have shown that depression is associated – besides the increased risk for diabetic complications – with increased risk for cardiovascular disease (CVD) and all-cause mortality, even after controlling for potential mediators, such as health-related behaviors. Concerning mortality risk, a 2-3fold higher risk has mainly been reported, with hazard ratios ranging from 1.36 to 4.94 (Table 3)11,54,64-68.
Table 3. Depression and mortality risk.
Treatment of depression in diabetes
Depression in diabetes is still underdiagnosed and undertreated, despite its high prevalence and its association with adverse health outcomes and QoL impairment.
Concerning the interventions strategies for treating depression in patients with DM, they fall into three broad catecories: diabetes self-management education, psychotherapy and pharmacotherapy. These strategies are, of course, not mutually exclusive.
Van der Feltz-Cornellis et al70 conducted a meta-analysis of 14 RCTs (6 out of 7 studies of pharmacotherapy, 5 studies of psychotherapy, 3 studies of psychotherapy combined with a diabetes self-management intervention and 3 studies of collaborative care intervention) evaluating the efficacy of interventions applied for the treatment of depression in DM. A moderate (-0.512) overall effect size was identified. It was large (-0.581) for psychotherapeutic interventions combined with educational interventions concerning diabetes self-care and moderate (-0.467) for pharmacological interventions. Collaborative care, with the option of initiating with pharmacotherapy or psychotherapy, yielded a small to moderate effect size (-0.292). With regard to glycemic control, the psychotherapeutic interventions often accompanied by self-care educational interventions yielded a moderate to large effect size. On the contrary, pharmacotherapy, except for sertraline (other pharmacological agents evaluated in RCTs included in this meta-analysis were nortriptyline, fluoxetine and paroxetine) and collaborative care had no significant influence upon glycemic control. Van der Feltz-Cornelis et al concluded that psychotherapy combined with self-care educational interventions emerges as the first-line treatment for depression in DM, based on its large effect size on both depression and glycemic control.
Summarizing in redard with antidepressant pharmacotherapy in DM: it is effective for treating depressive symptoms, but its effect on glycemic control might depend on the type of antidepressant. However, the small number of double-blind randomized-controlled clinical trials as well as the small sample sizes and short duration of the trials performed so far, does not allow definite conclusions to be drawn. Another methodolical issue possibly interfering with the inability to provide a significant association between depression and glycemic control might lie into the levels of glycemic control within the studies' samples. Larger sample studies with larger variance of glycemic control allowing to investigate the effect of antidepressant treatment among subgroups of patients with poor, moderate or good glycemic control are needed.
Conclusion
Depression is a matter of great concern in patients with DM. It is not only highly prevalent, but also highly persistent and recurrent leading to a significant negative impact on both clinical outcomes and QoL. Besides, impaired QoL further deteriorates clinical outcomes and has been prospectively associated with increased mortality in DM71. Nevertheless, depression stills remain rather underdiagnosed and undertreated. Katon et al72, in a retrospective population-based study among 4385 patients with DM, identified an inadequate rate of correct depression recognition (51%) over a 12-month period prior to the study. Furthermore, only 31% of the patients correctly diagnosed with depression received adequate dosage of antidepressants, while only 6.7% of them received an adequate amount (defined as ≥ 4) of psychotherapy sessions over the 12-month period. Frequency of primary care visits (≥7), alongside with female gender, poor self-rated physical health, panic attacks and dystymia were factors independently associated with increased likelihood for correct depression recognition. A further sensitization of health care professionals, especially in primary care, is imperative, in order to enhance timely detection and treatment of depression in DM. American Diabetes Association recommends that patients with DM, particularly those with poor disease control, should be screened for psychosocial and psychological disturbances or disorders, such as depression73. Concerning the management of depression in DM, psychotherapy combined with psychoeducational interventions or collaborative care (psychotherapy or pharmacological treatment combined with psychoeducation and psychosocial interventions) seem to be cost-effective74 and yield beneficial results, both on mental health outcomes as well as diabetes management and glycemic control70. Pharmacotherapy alone is a significant therapeutic option, particularly in contexts where more integrated strategies are not easily applicable, though its effectiveness in treating depression alongside with improving glycemic control seems not to be equivalent. Furthermore, given the disparities among different antidepressants concerning both their effect on glycemic control and their potential side-effects, further research with longer and larger clinical trials and with larger variance of glycemic control among the samples is needed, in order to provide sufficient data on the optimal antidepressant treatment in patients with DM. Still, even in contexts were a highly organized collaborative care can not be applied, the enhancement of patient-doctor relationship providing the patient with the opportunity to verbalize concerns and emotions related to living with diabetes, could be therapeutic.
Conflict of Interest
None
References
- 1.Kruse J, Schmitz N, Thefeld W. On the association between diabetes and mental disorders in a community sample. Results from the German National Health Interview and Examination Survey. Diabetes Care. 2003;26:1841–1846. doi: 10.2337/diacare.26.6.1841. [DOI] [PubMed] [Google Scholar]
- 2.Das-Munshi J, Stewart R, Ismail K, Bebbington PE, Jenkins R, Prince MJ. Diabetes, Common Mental Disorders, and disability: Findings form the UK National Psychiatric Morbidity Survey. Psychosom Med. 2007;69:543–550. doi: 10.1097/PSY.0b013e3180cc3062. [DOI] [PubMed] [Google Scholar]
- 3.Pouwer F, Beekman ATF, Nijpels G, Dekker JM, Snoek FJ, Kostense PJ, et al. Rates and risks for co-morbid depression in patients with type 2 diabetes mellitus: results from a community-based study. Diabetologia. 2003;46:892–898. doi: 10.1007/s00125-003-1124-6. [DOI] [PubMed] [Google Scholar]
- 4.Egede LE, Zheng D. Independent factors associated with Major Depressive Disorder in a national sample of individuals with diabetes. Diabetes Care. 2003;26:104–111. doi: 10.2337/diacare.26.1.104. [DOI] [PubMed] [Google Scholar]
- 5.Katon W, Russo J, Lin EH, Heckbert SR, Karter AJ, Williams LH, et al. Diabetes and poor diseases control: is comorbid depression associated with poor medication adherence or lack of treatment intensification. Psychosom Med. 2009;23:588–594. doi: 10.1097/PSY.0b013e3181bd8f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engum A, Mykletun A, Midthjell K, Holen A, Dahl AA. Depression and Diabetes. A large population-based study of sociodemographic, lifestyle and clinical factors associated with depression in type 1 and type 2 diabetes. Diabetes Care. 2005;28:1904–1909. doi: 10.2337/diacare.28.8.1904. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence JM, Standiford DA, Loots B, Klingensmith GJ, Williams DE, Ruggiero A, et al. Prevalence and correlates of depressed mood among youth with diabetes: The SEARCH for Diabetes in Youth Study. Pediatrics. 2006;117:1348–1358. doi: 10.1542/peds.2005-1398. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Ford ES, Strine TW. Prevalence of depression among U.S. adults with diabetes. Findings from the 2006 Behavioral Risk Factor Surveillance System. Diabetes Care. 2008;31:105–107. doi: 10.2337/dc07-1154. [DOI] [PubMed] [Google Scholar]
- 9.Lin EH, Von Korff MV. Mental disorders among persons with diabetes-Results from the World Mental Health Surveys. J Psychosom Res. 2008;65:571–580. doi: 10.1016/j.jpsychores.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer L, Skaff MM, Mullan JT, Areant P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med. 2008;25:1096–1101. doi: 10.1111/j.1464-5491.2008.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katon W. Depression and diabetes: Unhealthy bedfellows. Depress Anxiety. 2010;27:323–326. doi: 10.1002/da.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egede LE, Ellis C. The effects of depression on metabolic control and quality of life in indigent patients with type 2 diabetes. Diabetes Technol The. 2010;12:257–262. doi: 10.1089/dia.2009.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pouwer F, Geelhoed-Duijvestijn LM, Tack CJ, Bazelmans E, Beekman AJ, Heine RJ, et al. Prevalence of comorbid depression is high in out-patients with type 1 or type 2 diabetes mellitus. Results from three out-patient clinics in the Netherlands. Diabet Med. 2010;27:217–224. doi: 10.1111/j.1464-5491.2009.02903.x. [DOI] [PubMed] [Google Scholar]
- 14.Dirmaier J, Watzke B, Koch U, Schulz H, Lehnert H, Pieper L, et al. Diabetes in primary care: prospective associations between depression, nonadherence and glycemic control. Psychother Psychosom. 2010;79:172–178. doi: 10.1159/000296135. [DOI] [PubMed] [Google Scholar]
- 15.Trento M, Raballo M, Trevisan M, Sicuro J, Passera P, Cirio L, et al. A cross-sectional survey of depression, anxiety, and cognitive function in patients with type 2 diabetes. Acta Diabetol. 2012;49:199–203. doi: 10.1007/s00592-011-0275-z. [DOI] [PubMed] [Google Scholar]
- 16.Gavard JA, Lustman PJ, Clouse RE, et al. Prevalence of depression in adults with diabetes. An epidemiological evaluation. Diabetes Care. 1993;16:1167–1178. doi: 10.2337/diacare.16.8.1167. [DOI] [PubMed] [Google Scholar]
- 17.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ, et al. The prevalence of comorbid depression in adults with diabetes. A meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 18.Grey M, Whittemore R, Tamborlane W. Depression in Type 1 diabetes in children. Natural history and correlates. J Psychosom Res. 2002;53:907–911. doi: 10.1016/s0022-3999(02)00312-4. [DOI] [PubMed] [Google Scholar]
- 19.Barnard KD, Skinner TC, Peveler R. The prevalence of co-morbid depression in adults with Type 1 diabetes: systematic literature review. Diabet Med. 2006;23:445–448. doi: 10.1111/j.1464-5491.2006.01814.x. [DOI] [PubMed] [Google Scholar]
- 20.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23:1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 21.Mezuk B, Eaton W, Albrecht S, Golden SH. Depression and type 2 diabetes over life span. Diabetes Care. 2008;31:2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nouwen A, Winkley K, Twisk J, Lloyd CE, Peyrot M, Ismail K, et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. 2010;53:2480–2486. doi: 10.1007/s00125-010-1874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lustman PJ, Griffith LS, Freedland KE, Clouse RE. The course of major depression in diabetes. Gen Hosp Psychiatry. 1997;19:138–143. doi: 10.1016/s0163-8343(96)00170-3. [DOI] [PubMed] [Google Scholar]
- 24.Peyrot M, Rubin RR, Lauritzen T, Skovlund SE, Snoek FJ, Matthews DR, et al. Resistance to insulin therapy among patients and providers. Diabetes Care. 2005;28:2673–2679. doi: 10.2337/diacare.28.11.2673. [DOI] [PubMed] [Google Scholar]
- 25.Katon WJ, Simon G, Russo J, Von Korff M, Lin EH, Ludman E, et al. Quality of depression care in a population-based sample of patients with diabetes and major depression. Med Care. 2004;42:1222–1229. doi: 10.1097/00005650-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Katon W, Fan MU, Unützer J, Taylor J, Pincus H, Schoenbaum M. Depression and diabetes: a potentially lethal combination. J Gen Intern Med. 2008;23:1571–1578. doi: 10.1007/s11606-008-0731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tellez-Zenteno JF, Cardiel MH. Risk factors associated with depression in patients with type 2 diabetes mellitus. Arch Med Res. 2002;33:53–60. doi: 10.1016/s0188-4409(01)00349-6. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, et al. Depression and diabetes treatment non-adherence: a meta-analysis. Diabetes Care. 2008;31:2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez JS, Safren SA, Delahanty LM, Cagliero E, Wexler DJ, Meigst JB, et al. Symptoms of depression prospectively predict poorer self-care in patients with type 2 diabetes. Diabet Med. 2008;25:1102–1107. doi: 10.1111/j.1464-5491.2008.02535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katon W, Russo J, Lin EH, Heckbert SR, Karter AJ, Ciechanowski P, et al. Depression and diabetes: factors associated with major depression at five-year follow-up. Psychosomatics. 2009;50:570–579. doi: 10.1176/appi.psy.50.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lustman PJ, Anderson RJ, Freedland KE, De Groot M, Carney RM, Clouse RE. Depression and poor glycemic control. A meta-analytic review of the literature. Diabetes Care. 2000;23:934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 32.Van Tilburg MAL, McCaskill CC, Lane JD, Edwards CL, Bethel A, Feinglos MN, et al. Depressed mood is a factor in glycemic control in type 1 diabetes. Psychosom Med. 2001;63:551–555. doi: 10.1097/00006842-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Ciechanowski PS, Katon WJ, Russo JE, Hirsch IB. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. Gen Hosp Psychiatry. 2003;25:246–252. doi: 10.1016/s0163-8343(03)00055-0. [DOI] [PubMed] [Google Scholar]
- 34.Lustman PJ, Clouse RE. Depression in diabetes: the chicken or the egg? Psychosom Med. 2007;69:297–299. doi: 10.1097/PSY.0b013e318060cc2d. [DOI] [PubMed] [Google Scholar]
- 35.Helgeson VS, Siminerio L, Escobar O, Backer D. Predictors of metabolic control among adolescents with diabetes:a 4-year longitudinal study. J Pediatr Psychol. 2009;34:254–270. doi: 10.1093/jpepsy/jsn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hood KK, Rausch JR, Dolan LM. Depressive symptoms predict change in glycemic control in adolescents with type 1 diabetes:rates, magnitude and moderators of change. Pediatric Diabetes. 2011;12:718–723. doi: 10.1111/j.1399-5448.2011.00771.x. [DOI] [PubMed] [Google Scholar]
- 37.Herzer M, Hood KK. Anxiety symptoms in adolescents with type 1 diabetes; Association with blood glucose monitoring and glycemic control. J Pediatr Psychol. 2010;35:415–425. doi: 10.1093/jpepsy/jsp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Surwit RS, van Tilburg MAL, Parekh PI, Lane JD, Feinglos MN. Treatment regimen determines the relationship between depression and glycemic control. Diabetes Res Clin Pract. 2005;69:78–80. doi: 10.1016/j.diabres.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Papelbaum M, Moreira RO, Countinho W, Kupfer R, Zagury L, Freitas S, et al. Depression, glycemic control and type 2 diabetes. Diabetol Metab Syndr. 2011;3:26–29. doi: 10.1186/1758-5996-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner JA, Abbott GL, Heapy A, Yong L. Depressive symptoms and diabetes control in African Americans. J Immigr Minor Health. 2009;11:66–70. doi: 10.1007/s10903-008-9147-1. [DOI] [PubMed] [Google Scholar]
- 41.Richardson LK, Egede LE, Mueller M, Echols CL, Gebregziabher M. Longitudinal effects of depression on glycemic control in veterans with type 2 diabetes. Gen Hosp Psychiatry. 2008;30:509–514. doi: 10.1016/j.genhosppsych.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Paschalides C, Wearden AJ, Dunkerley R, Bundy C, Davies R, Dickens CM. The associations of anxiety, depression and personal illness representations with glycaemic control and health-related quality of life in patients with type 2 diabetes mellitus. J Psychosom Res. 2004;57:557–564. doi: 10.1016/j.jpsychores.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Egede LE, Ellis C, Grubaugh AL. The effect of depression on self-care behaviors and quality of care in a national sample of asults with diabetes. Gen Hosp Psychiatry. 2009;31:422–427. doi: 10.1016/j.genhosppsych.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Fischer L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23–28. doi: 10.2337/dc09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aikens JE, Perkins DW, Lipton B, Piette JD. Longitudinal analysis of depressive symptoms and glycemic control in type 2 diabetes. Diabetes Care. 2009;32:1177–1181. doi: 10.2337/dc09-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heckbert S, Rutter R, Oliver M, Williams LH, Ciechanowski P, Lin EHB, et al. Depression in relation to long-term control of glycemia, blood pressure, and lipids in diabetes patients. J Gen Intern Med. 2010;24:524–529. doi: 10.1007/s11606-010-1272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer L, Skaff MM, Mullan JT, Arean P, Mohr D, Masharani U, et al. Clinical depression versus distress among patients with type 2 diabetes. Not just a question of semantics. Diabetes Care. 2007;30:542–548. doi: 10.2337/dc06-1614. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez JS, Fischer L, Polonsky W. Depression in diabetes: Have we been missing something important? Diabetes Care. 2011;34:236–239. doi: 10.2337/dc10-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersohn F, Schade R, Suissa S, Garbe E. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry. 2009;166:591–598. doi: 10.1176/appi.ajp.2008.08071065. [DOI] [PubMed] [Google Scholar]
- 50.McKellar JD, Humphreys K, Plette JD. Depression increases diabetes symptoms by complicating patients' self-care adherence. The Diabetes Educator. 2004;30:485–492. doi: 10.1177/014572170403000320. [DOI] [PubMed] [Google Scholar]
- 51.Ludman EJ, Katon W, Russo J, Von Korff M, Simon G, Ciechanowski P, et al. Depression and diabetes symptom burden. Gen Hosp Psychiatry. 2004;26:430–436. doi: 10.1016/j.genhosppsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 52.De Groot M, Anderson R, Freedland K, Clouse R, Lustman P. Association of Depression and Diabetes Complications: A meta-analysis. Psychosom Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 53.Roy MS, Roy A, Affouf M. Depression is a risk factor for poor glycemic control and retinopathy in African-Americans with type 1 diabetes. Psychosom Med. 2007;69:537–542. doi: 10.1097/PSY.0b013e3180df84e2. [DOI] [PubMed] [Google Scholar]
- 54.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26:2822–2828. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 55.Lin EH, Rutter CM, Katon W, Heckbert SR, Ciechanowski P, Oliver MM, et al. Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care. 2010;33:264–269. doi: 10.2337/dc09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clouse RE, Lustman PJ, Freedland KE, Griffith LS, McGill JB, Carney RM. DDepression and coronary heart disease in women with diabetes. Psychosom Med. 2003;65:376–383. doi: 10.1097/01.psy.0000041624.96580.1f. [DOI] [PubMed] [Google Scholar]
- 57.Williams LH, Miller DR, Fincke G, Lafrance J, Etzioni R, Maynard C, et al. Depression and incident lower limb amputations in veterans with diabetes. J Diabetes Complications. 2011;25:175–182. doi: 10.1016/j.jdiacomp.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes-systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 59.Bruce DG, Davis WA, Casey GP, Starkstein SE, Clarnett RM, Foster JK, et al. Predictors of cognitive impairment and dementia in older people with diabetes. Diabetologia. 2008;51:241–248. doi: 10.1007/s00125-007-0894-7. [DOI] [PubMed] [Google Scholar]
- 60.Katon WJ. The Comorbidity of Diabetes Mellitus and Depression. Am J Med. 2008;121:S8–S15. doi: 10.1016/j.amjmed.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schram MT, Ban CA, Pouwer F. Depression and Quality of Life in Patients with Diabetes: A Systematic Review from the European Depression in Diabetes (EDID) Research Consortium. Curr Diabetes Rev. 2009;5:112–119. doi: 10.2174/157339909788166828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ali S, Stone M, Skinner TC, Robertson N, Davies M, Khunti K, et al. The association between depression and health-related quality of life in people with type 2 diabetes: a systematic literature review. Diabetes Metab Res Rev. 2010;26:75–89. doi: 10.1002/dmrr.1065. [DOI] [PubMed] [Google Scholar]
- 63.Katon W, Von Korff M, Chiechanowski P, Russo J, Lin E, Simon G, et al. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914–920. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 64.Egede LE, Nietert PJ, Zheng D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care. 2005;28:1339–1345. doi: 10.2337/diacare.28.6.1339. [DOI] [PubMed] [Google Scholar]
- 65.Bruce DG, Davis WA, Starkstein SE, Davis TME. A prospective study of depression and mortality in patients with type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2005;48:2532–2539. doi: 10.1007/s00125-005-0024-3. [DOI] [PubMed] [Google Scholar]
- 66.Ismail K, Winkley K, Stahl D, Chalder T, Edmonds M. A cohort study of people with diabetes and their first foot ulcer. The role of depression on mortality. Diabetes Care. 2007;30:1473–1479. doi: 10.2337/dc06-2313. [DOI] [PubMed] [Google Scholar]
- 67.Lin EH, Heckbert SR, Rutter CM, Katon WJ, Ciechanowski P, Ludman EJ, et al. Depression and increased mortality in diabetes: unexpected causes of death. Ann Fam Med. 2009;7:414–421. doi: 10.1370/afm.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Young B, Von Korff M, Heckbert S, Ludman EJ, Rutter C, Lin EHB, et al. Association of depression and mortality in stage 5 diabetic chronic kidney disease. Gen Hosp Psychiatry. 2010;32:119–124. doi: 10.1016/j.genhosppsych.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pan A, Lucas M, Sun Q, van Dam RM, Franco OH, Willett WC, et al. Increased mortality risk in women with depression and diabetes mellitus. Arch Gen Psychiatry. 2011;68:42–50. doi: 10.1001/archgenpsychiatry.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van der Feltz-Cornelis CM, Nuyen J, Stoop C, Chan J, Jacobson AM, Katon W, et al. Effect of interventions for major depressive disorder and significant depressive symptoms in patients with diabetes mellitus: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2010;32:380–395. doi: 10.1016/j.genhosppsych.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 71.Kleefstra N, Landman GWD, Houweling ST, Ubink-Veltmaat LJ, Logtenberg SJJ, Meyboom-De Jong B, et al. Prediction of mortality in type 2 diabetes from health-related quality of life (ZODIAC-4) Diabetes Care. 2008;31:932–933. doi: 10.2337/dc07-2072. [DOI] [PubMed] [Google Scholar]
- 72.Katon WJ, Lin EHB, Williams LH, Ciechanowski P, Heckbert SR, Ludman E, et al. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: a prospective cohort study. J Gen Intern Med. 2010;25:423–429. doi: 10.1007/s11606-009-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.American Diabetes Association Standards of medical care in diabetes (Position Statement) Diabetes Care. 2004;27:S15–S35. doi: 10.2337/diacare.27.2007.s15. [DOI] [PubMed] [Google Scholar]
- 74.Simon GE, Katon WJ, Lin EH, Rutter C, Manning WG, Von Korff M, et al. Cost-effectiveness of systematic depression treatment among people with diabetes mellitus. Arch Gen Psychiatry. 2007;64:65–72. doi: 10.1001/archpsyc.64.1.65. [DOI] [PubMed] [Google Scholar]