Abstract
Salivary gland tumors (SGT) are a heterogeneous group of lesions. There is conflicting data concerning the molecular events involving the tumour suppressor retinoblastoma protein (pRb) pathway in these tumors. Few studies examined the alterations in components of the Rb pathway by immunohistochemical (IHC) methods in benign and malignant SGTs. Furthermore, recent evidence implicates human papillomavirus (HPV) in mucoepidermoid carcinoma (MEC) carcinogenesis. The purpose of our study is to examine p16INK4A and cyclin D1 expression in a variety of benign and malignant salivary gland tumors, and to investigate p16INK4A expression as a surrogate marker for HPV infection in MEC. Our series includes 30 malignant tumors [14 MEC, 6 acinic cell carcinomas (ACC), 5 polymorphous low grade adenocarcinomas (PLGA), 5 (AdCC)] and 14 benign tumors (4 benign cysts, 5 Warthin tumors and 5 pleomorphic adenomas (PA). All cases were tested by IHC for p16INK4A and cyclin D1. Testing for HPV wide spectrum (HPV-WS) was performed by in situ hybridization in all MEC cases. Staining intensity was recorded semi quantitatively (on a scale from 0 to 4+). Fisher’s exact test and Pearson X2 test with a p < 0.05 were used. Cyclin D1 and p16INK4A are expressed similarly in malignant and benign tumors (p = 0.146 and p = 0.543, respectively). None of the MEC cases showed nuclear reactivity for HPV-WS. Statistical analysis showed positive correlation between cyclin D1 and p16INK4A expression. Our findings suggest that p16INK4A overexpression is likely secondary to cyclin D1 gene upregulation or amplification. Further molecular studies are warranted.
Keywords: Cyclin D1, p16INK4A, Rb pathway, Salivary glands
Background
Salivary gland tumors are relatively rare and comprise a group of morphologically and biologically heterogeneous lesions. Tumorigenesis frequently involves disruption to proteins in the pathway of the pRb. Currently, it is known that tumor cells typically sustain damage to genes that regulate G1-S progression [1]. The retinoblastoma gene product Rb protein serves as a checkpoint that restricts entry into the S phase by binding to transcription factor E2F and blocks the transcription of the S phase genes. Normally, this inhibition by Rb is relieved at the appropriate time by phosphorylation of the Rb protein, a process initially triggered by cyclin D–Cdk4/Cdk6 complexes [1, 2]. The activities of cyclin D–Cdk4/Cdk6 complexes are constrained by inhibitors such as p16INK4a [3, 4]. Thus, the loss of p16INK4a or Rb and overexpression of cyclin D have similar effects on G1 progression and represent a common pathway to tumorigenesis. Abnormalities in the Rb pathway can lead to uncontrolled cell proliferation and tumorigenesis in various types of human tumors. In oral squamous cell carcinomas (SCC), p16INK4A is frequently deleted, methylated, or mutated, and cyclin D1 gene amplified [5]. Currently, there is conflicting data concerning the molecular events involving the pRb pathway leading to tumorigenesis in salivary gland tumors. Published molecular studies suggest that promoter hypermethylation or homozygous deletion of p16INK4a gene in adenoid cystic carcinomas (ACC) and mucoepidermoid carcinomas (MEC) is the most common mechanism for p16INK4A gene silencing [6–8]. However, this alteration was identified in a minority of the studied cases. There are few studies investigating the alterations in native components of the Rb pathway in salivary gland tumors by immunohistochemical methods (including p16INK4A, CCND1, and other Rb pathway proteins), with results showing variable overexpression of both cyclin D1 and p16INK4A [9–12]. Furthermore, recent literature provides evidence of HPV implication in MEC carcinogenesis. Recently, Brandwein-Gensler et al. detected transcriptionally active HR HPV (16/18) using RT-PCR method for detection of E6/7 oncogenes in MEC [13]. To date, we are not aware of any published studies examining the performance of p16INK4A as a surrogate marker for HPV infection in MEC. In our study, we examined p16INK4A and cyclin D1 expression by immunohistochemical methods in a heterogeneous group of benign and malignant high and low grade salivary gland tumors in order to evaluate the implication of these molecular events in both subtypes of tumors. Additionally, we aimed to investigate the performance of p16INK4A as surrogate marker for HPV infection in the mucoepidermoid carcinoma subgroup.
Materials and Methods
Forty-four archival cases of benign and malignant salivary gland tumors were retrieved from pathology files at Beth Israel Medical Center in New York. Clinical information on the patients was collected from the pathology database (Powerpath®) including: age, gender, and primary tumor location. Hematoxylin-eosin stained slides from representative formalin-fixed, paraffin–embedded tissue blocks were used to define diagnostic areas exhibiting unequivocal tumor morphology. The diagnostic criteria included dual epithelial (abluminal) and myoepithelial/basal (luminal) differentiation for AdCC. An admixture of epidermoid, mucous and intermediate cells and presence of cytoplasmic (zymogen–type) secretory granules helped us identify MEC and ACC cases respectively. Cytologic uniformity, morphologic diversity and infiltrative growth pattern were all used to identify PLGA cases. Epithelial differentiation (ductal structures with associated non-ductal elements) and mesenchymal differentiation (myxoid, hyaline, chondroid) served as diagnostic clues in all PA cases. The presence of a bilayered epithelium with a dual population of columnar oncocytic and smaller basaloid cells with a lymphoid stroma in cystic and papillary configuration characterized Warthin’s tumors. Flattened or cuboidal epithelium surrounded by collagenized connective was present in all four benign salivary duct cysts.
Immunohistochemistry
Immunohistochemical staining for p16INK4A and cyclin D1 was performed on all selected cases. Five micrometer sections were obtained from formalin-fixed, paraffin-embedded archival tissue following the avidin–biotin–immunoperoxidase technique (LSAB; Dako, Glostrup, Denmark). On board epitope retrieval was accomplished according to the manufacturers’ instructions. The immunostaining for p16INK4A and cyclin D1 was accomplished with a fully automated, random access, open system immunostainer (Bond III™—Leica Microsystems) using a polymer approach. The immunohistochemistry with primary antibodies directed against p16INK4A (E6H4, 1:2, CINtec®—Roche laboratories AG, Germany) and cyclin D1 (Monoclonal SP4, 1:10, Thermo Scientific—Labvision®—Fremont, CA), was performed. The chromogen diaminobenzidine tetrachloride was used to visualize the antibody–antigen complex. The tissue was counterstained with hematoxylin. Appropriate positive and negative control slides were prepared. The negative controls consisted of slides processed without addition of the primary antigen. Evaluation of the immunostaining was performed separately by two authors and then reviewed with a head and neck pathologist (BMW) to reach a consensus. Staining was scored using the following scoring system: 0 = negative, focal (1+) = (1–25 % of tumoral cells), moderate (2+) = (25–50 % of tumoral cells), diffuse (3+) = (50–75 % of tumoral cells), and very diffuse (4+) = (>75 % of tumoral cells). For p16INK4A, staining intensity was recorded using a three tiered system (weak, moderate, strong). Only cases showing moderate and/or strong immunoreactivity were considered positive. The percentage of reactive cells was also recorded for each case.
In Situ Hybridization (ISH) Procedure for Wide Spectrum HPV
Testing for HPV wide spectrum (HPV-WS) was performed by ISH technique in all fourteen MEC cases. A cocktail of HPV Biotinylated probes covering both high-risk (HR) and low-risk (LR) HPV genotypes (HPV 6, 11, 16, 18,31,33,35, 45, 51, and 52) (Dako, Glostrup, Denmark) was used. Briefly, 5 μ sections were rehydrated and treated with HCl and pepsin. After incubation in the pre hybridization buffer for a short period, tissue DNA was denatured and hybridized at 48 °C for 30 min with the denatured biotinylated labeled probe cocktail. Then, the slides were washed, blocked and incubated in appropriate dilution of primary and secondary streptavidin-HRP solution for 1 h at room temperature after addition of the amplification reagent (biotinyl tyramide). The signal was detected by adding the chromogen diaminobenzidine tetrachloride in order to visualize the antibody–antigen complex. The tissue was counterstained with hematoxylin. Appropriate positive (tonsilar tissue) and negative control slides were prepared.
Statistical Analysis
Fisher exact test was used to compare the immunoreactivity scores for p16INK4A and cyclin D1 within different benign and malignant tumor types. The correlation between p16INK4A and cyclin D1 expression with tumor type (benign vs. malignant) was assessed using the Pearson correlation test (X2). A p value < 0.05 was considered significant. SPSS software package 9.0 was used for data analysis.
Results
Our series included 25 males (57 %) and 19 females (43 %). Patients’ age ranged from 15 to 78 years with a mean age of 58 years. The clinical and histopathological features of benign and malignant tumors are summarized in (Table 1).
Table 1.
Clinical and histopathological features of benign and malignant salivary gland tumors
| Malignant tumors (n = 30) | Benign Tumors (n = 14) | ||||||
|---|---|---|---|---|---|---|---|
| MEC (n = 14) | AdCC (n = 5) | PLGAa (n = 5) | ACCb (n = 6) | Benign cyst (n = 4) | PA (n = 5) | WT (n = 5) | |
| Site | |||||||
| Buccal | 3/14 (22 %) | 0 | 2/5 (33 %) | 0 | 2/4 (50 %) | 0 | 1/5 (20 %) |
| Parotid | 7/14 (50 %) | 1/5 (20 %) | 0 | 6/6 (100 %) | 2/4 (50 %) | 4/5 (80 %) | 4/5 (80 %) |
| Palatal | 4/14 (28 %) | 0 | 3/5 (66 %) | 0 | 0 | 1/5 (20 %) | 0 |
| Maxillary sinus | 0 | 2/5 (40 %) | 0 | 0 | 0 | 0 | 0 |
| Nasopharynx | 0 | 2/5 (40 %) | 0 | 0 | 0 | 0 | 0 |
| Grade | |||||||
| Low | 7/14 (50 %) | 0 | 5/5 (100 %) | 5/5 (100 %) | N/A | N/A | N/A |
| Intermediate | 5/14 (36 %) | 5/5 (100 %) | N/A | N/A | N/A | N/A | N/A |
| High | 2/14 (14 %) | 0 | N/A | N/A | N/A | N/A | N/A |
MEC mucoepidermoid carcinoma, AdCC adenoid cystic carcinoma, PLGA polymorphous low-grade adenocarcinoma, ACC acinic cell carcinoma, PA pleomorphic adenoma, WT warthin tumor
aACC and PLGA are histologically considered as low grade tumors
bOne case was identified within the external auditory canal and represented a local extension from a primary parotid gland origin
p16INK4A
Cases were considered positive when cytoplasmic and/or nuclear reactivity were present. All benign tumors showed positive cytoplasmic and nuclear immunoreactivity for p16INK4A (Table 2). Pleomorphic adenomas (PA) expressed p16INK4A in both epithelial and myoepithelial cells (Fig. 1). Twenty-eight malignant tumors (93 %) expressed p16INK4A. One low grade MEC case and another ACC case did not exhibit positive immunoreactivity for p16INK4A. Positive immunoreactivity was noted in both luminal and abluminal cells in AdCC. A diffuse (3+) immunoreactivity was present in all AdCCs cases except one case that showed a moderate (2+) immunoreactivity. PLGA showed a reproducible haphazard staining pattern in all positive cases (Fig. 1). Four low grade MECs (4/7) showed a focal (1+) immunoractivity while the rest of MECs (10/14) showed variable moderate (2+) to very diffuse (4+) expression (Table 2). Interestingly, both mucocytes and epidermoid cells showed immunoreactivity in low and high grade MECs (Fig. 2). No immunoreactivity was detected in the adjacent non neoplastic salivary gland parenchyma in all studied cases.
Table 2.
Cyclin D1 and p16INK4A expression in benign and malignant salivary gland tumors
| Malignant tumors (n = 30) | Benign tumors (n = 14) | |||||||
|---|---|---|---|---|---|---|---|---|
| MEC | AdCC | PLGA | ACC | Benign Cyst | PA | Warthin’s | ||
| p16INK4A score | Neg | 1/14 (7 %) | 0 | 0 | 1/6 (17 %) | 0 | 0 | 0 |
| 1+ | 5/14 (36 %) | 0 | 0 | 1/6 (17 %) | 1/4 (25 %) | 1/5 (20 %) | 2/5 (40 %) | |
| 2+ | 5/14 (36 %) | 1/5 (20 %) | 3/5 (60 %) | 2/6 (33 %) | 1/4 (25 %) | 1/5 (20 %) | 1/5 (20 %) | |
| 3+ | 2/14 (14 %) | 4/5 (80 %) | 2/5 (40 %) | 2/6 (33 %) | 2/4 (50 %) | 3/5 (60 %) | 2/5 (40 %) | |
| 4+ | 1/14 (7 %) | 0 | 0 | 0 | 0 | 0 | 0 | |
| CCDN1 score | Neg | 0 | 0 | 1/5 (20 %) | 0 | 1/4 (25 %) | 0 | 0 |
| 1+ | 7/14 (50 %) | 0 | 2/5 (40 %) | 2/6 (33 %) | 1/4 (25 %) | 2/5 (40 %) | 2/5 (40 %) | |
| 2+ | 4/14 (29 %) | 0 | 1/5 (20 %) | 2/6 (33 %) | 1/4 (25 %) | 3/5 (60 %) | 3/5 (60 %) | |
| 3+ | 3/14 (21 %) | 5/5 (100 %) | 1/5 (20 %) | 1/6 (17 %) | 1/4 (25 %) | 0 | 0 | |
| 4+ | 0 | 0 | 0 | 1/6 (17 %) | 0 | 0 | 0 | |
MEC mucoepidermoid carcinoma, AdCC adenoid cystic carcinoma, PLGA polymorphous low-grade adenocarcinoma, ACC acinic cell carcinoma; PA pleomorphic adenoma, WT warthin tumor
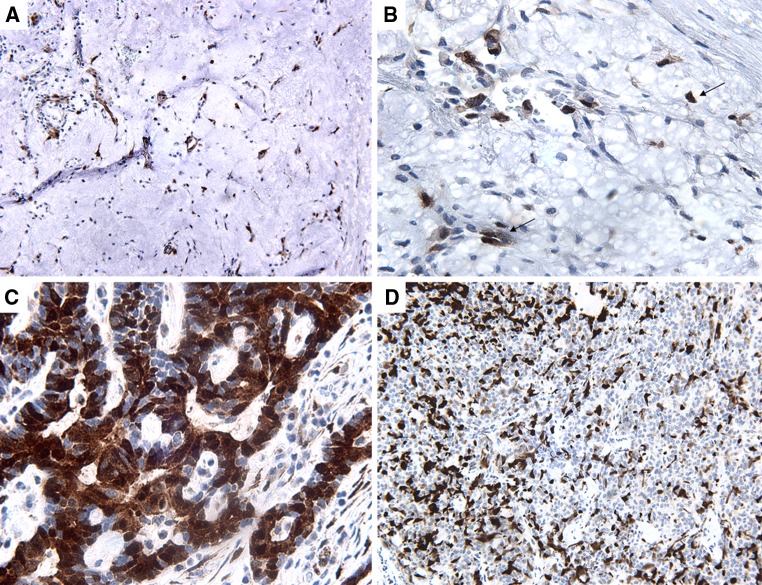
Fig. 1.
p16INK4A immunostain. a PA showing diffuse (3+) nuclear and cytoplasmic staining in epithelial and myoepithelial tumor cells (20×). b high power shows positive immunoreactivity in myoepithelial cells (arrows) (60×). c AdCC showing diffuse (3+) nuclear and cytoplasmic staining in luminal and abluminal tumor cells (60×). d PLGA showing moderate (2+) haphazard dendritic like staining pattern (20×)
Fig. 2.
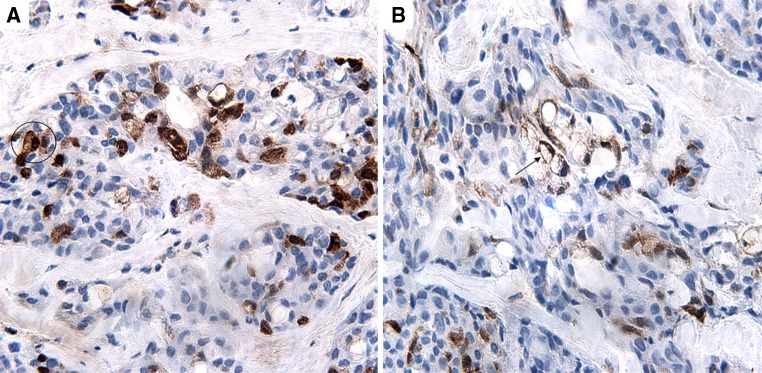
p16INK4A immunostain. a MEC showing focal (1+) nuclear and cytoplasmic reactivity in epidermoid cells (circle) (60×). b Focal (1+) nuclear and cytoplasmic reactivity in mucocytes (arrow) in the same case (60×)
Cyclin D1
Cases were considered positive when nuclear reactivity was present. Thirteen (13/14) benign tumors expressed cyclin D1 (93 %). One case was completely negative for cyclin D1 (benign cyst) (Table 2). PAs expressed cyclin D1 in both epithelial and myoepithelial cells in a similar pattern to that seen with p16INK4A (Fig. 3). Within the malignant tumor group, one case was completely negative. Twenty-nine cases (29/30) expressed cyclin D1. Among low grade MEC tumors, 5 showed a (1+) score, while the remaining two cases showed (2+) and (3+) score, respectively. Immunoreactivity was present in both mucocytes and epidermoid cells (Fig. 3). All AdCCs showed a (3+) diffuse pattern of staining. Both luminal and abluminal cells expressed cyclin D1 constantly (Fig. 3). No immunoreactivity was present in the adjacent non neoplastic salivary gland parenchyma in all studied cases.
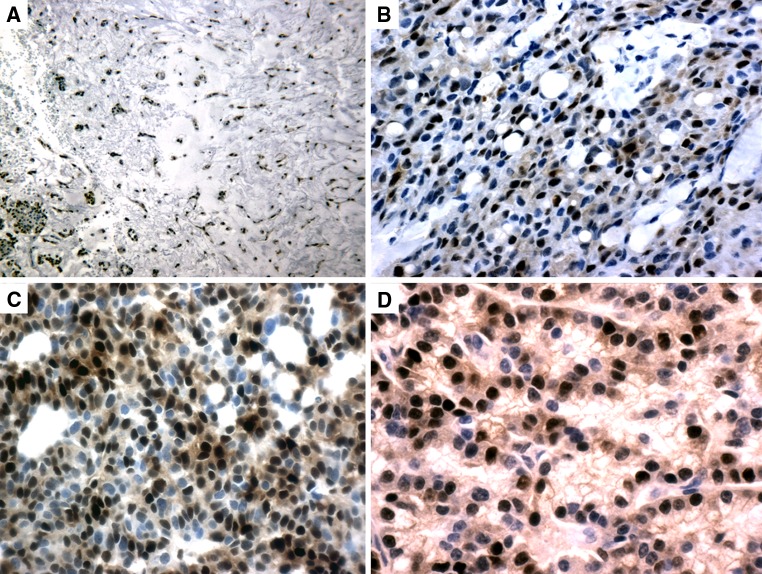
Fig. 3.
cyclin D1 immunostain. a PA showing diffuse (3+) staining in epithelial and myoepithelial tumor cells (20×). b MEC showing epidermoid cells and mucocytes expressing diffuse (3+) immunoreactivity (20×). c AdCC showing diffuse (3+) nuclear staining in luminal and abluminal tumor cells (20×). d Cyclin D1 immunostain in ACC moderate (2+) nuclear staining (20×)
ISH-WSHPV
None of the MEC cases showed positive nuclear reactivity for HPV-WS.
Discussion
Salivary gland tumors (SGTs) are rare. With a worldwide incidence less than 1 per 100,000 population, they account for less than 0.5 % of all malignancies and less than 10 % of head and neck tumors [14]. According to the 2005 World Health Organization (WHO) classification, there are 24 different histologic subtypes [15, 16]. Deregulation of the pRb pathway is of fundamental importance in the development and progression of a number of human tumors, including squamous cell carcinomas of the head and neck [17, 18]. Cyclin D1 and p16INK4A modulate the phosphorylation of pRb and under normal circumstances control G1 to S transition and cellular proliferation. There are few studies investigating immunohistochemical expression of cyclin D1 and p16 proteins in salivary gland tumors.
In a series of 42 cases including 29 PA and 11 carcinoma ex-pleomorphic adenomas (CXPA), Patel et al. [10] showed frequent cyclin D1 and p16INK4A expression in the neoplastic components of both subgroups (epithelial and myoepithelial components) concomitantly. The authors reported a higher expression of p16INK4A in the benign epithelial component than in the malignant component in CXPA and hypothesized a “protective” effect for p16INK4A against progression from PA to CXPA. In line with these findings, our series showed expression for both p16INK4A and cyclin D1 in both epithelial and myoepithelial components of PA but not in the normal salivary gland parenchyma. Also, positive immunoreactivity with the aforementioned markers was noted in benign cysts and Warthin tumors (Fig. 4). No CXPA were included in our study. Hence, such a hypothesis could not be further investigated.
Fig. 4.
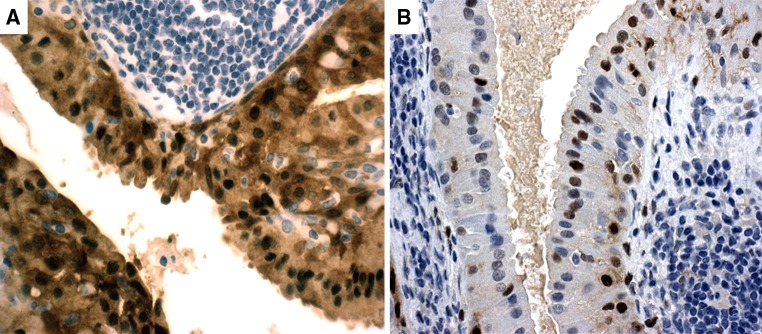
p16INK4A immunostain. a Warthin tumor showing moderate (2+) nuclear reactivity in basal and epithelial layers (20×). b moderate (2+) nuclear cyclin D1 immunoreactivity in the same case (60×)
By using methylation-specific PCR, Maryua et al. [19] demonstrated hypermethylation of the p16INK4A promoter gene in 4 cases of primary AdCC (21 %), all of which showed negative or low expression of the p16INK4A protein. Furthermore, the study showed a trend for a decreased expression of p16INK4A with higher grade of malignancy. The authors concluded that p16INK4A expression decreases in ACC cases of higher histological grade and that hypermethylation of its promoter gene may be involved in its process in some but not all cases. Adenoid cystic carcinoma (AdCC) is one of the most frequent malignant SGT showing a dual epithelial and myoepithelial/basal differentiation. It is characterized by slow growth, multiple late recurrences and distant metastases. It presents with different histologic patterns which confer the grade for the tumor: low grade (tubular pattern), intermediate grade (cribriform pattern) and high grade (solid pattern), the latter defined as present in at least 30 % of a given tumor often accompanied by increased mitotic activity and necrosis [20]. In our study, all AdCC cases were intermediate grade (cribriform) and showed diffuse (3+) cyclin D1 expression. Similarly, 4/5 (80 %) of the cases showed diffuse (3+) p16INK4A expression, and one case showed moderate (2+) p16INK4A expression. For both markers, immunoreactivity was noted in tubular, cribriform and solid areas (present in less than 30 % of the tumor) in all cases. There was a trend toward increased expression of both p16INK4A and cyclin D1 in solid tumor foci compared to tubular and cribriform areas. A reproducible luminal and abluminal pattern of staining for both p16INK4A and cyclin D1 was noted in all examined cases (Figs. 1, 3). Though limited by the sample size, our results demonstrate overexpression of both p16INK4A and cyclin D1 at the protein level, and suggest that molecular deregulations other than p16INK4A gene silencing (by promoter hypermethylation or by homozygous deletion) may be involved in tumorigenesis in AdCC.
In a study of 38 cases of MEC, Guo et al. [8] demonstrated that homozygous deletion and hypermethylation are the most frequent molecular events leading to p16INK4A gene silencing and loss of its tumor suppressor function in these tumors. Furthermore, the authors showed higher incidence of hypermethylation in high grade MEC than in low/intermediate grade MEC. Nevertheless, no immunohistochemical stains were used in order to correlate these molecular findings with protein expression. In contrast, Nishiminie et al. [7] reported a low frequency (8.3 %) of p16INK4A gene promoter hypermethylation in a heterogeneous group of 36 salivary gland carcinomas including ACC, AdCC, MEC, CXPA, squamous cell carcinoma, adenocarcinomas NOS and basal cell adenocarcinoma. Interestingly, none of the seven MEC cases included in that series showed p16INK4A gene promoter hypermethylation. The same study showed significant correlation between promoter hypermethylation (p16INK4A and other Rb genes, i.e. p14ARF) and cyclin D1 nuclear expression. In our series, p16INK4A and cyclin D1 expression was present in 13/14 (93 %) and 100 % of MEC cases, respectively. At least two cell populations (epidermoid cells and mucocytes) were positive for p16INK4A and cyclin D1. Further analysis within the MEC subgroup did not reveal any significant association between p16INK4A and cyclin D1 expression and tumor grade (p = 0.689, p = 0.610, respectively). This suggests that molecular derangements affecting the pRb pathway leading to tumorigenesis in MEC are not associated with tumor aggressiveness. Although p16INK4A is established as surrogate marker to detect HPV associated SCC (40 % of oropharyngeal and 25 % of all HNSCC), our study showed that it lacks similar performance in detecting HPV infections in salivary gland MEC. In our series, ISH for WS-HPV was negative in all MEC cases. Positive controls consisting of tonsilar tissue were prepared on separate slides. Similar incubation times, water baths, retrieval methods and reagents were used while examining the MEC cases to ascertain that the technique is performed correctly. Thus, it is highly unlikely that the lack of reactivity in all of MEC cases is due to a technical error; rather we believe it is a true result. This finding along with the significant (93 %) positive expression of p16INK4A in MEC cases suggests that this protein has a poor performance as a surrogate maker for HPV detection in these tumors.
Acinic cell carcinoma is an uncommon low grade malignant salivary gland tumor that is associated with local tissue infiltration and recurrences. Men are more frequently affected than women with a predilection for the parotid gland [20]. In a review of 18 cases of ACC, Liu et al. [11] reported frequent overexpression of p16INK4A and cyclin D1 proteins in neoplastic epithelium. Using two different types of antibodies targeting different phosphorylation sites on pRB proteins (serine 795 and 780), the authors suggested that serine 795 but not serine 780 is the preferred phosphorylation site induced by cyclin D1. These results raised the possibility that tumorigenesis in ACC is related to phosphorylation of pRb proteins secondary to overexpression of cyclin D1 rather than silencing of the p16INK4A gene by promoter hypermethylation [11]. In agreement with these findings, our series showed all except one case of ACC to be reactive for p16INK4A. All other cases showed positive immunoreactivitty that ranged from focal to very diffuse with no reactivity in the non neoplastic parenchyma (Table 2).
Most studies investigating pRb pathway focused on AdCC and MEC given that these represent the most frequently encountered salivary gland tumors. In a series that included four cases of PLGA, Etges et al. [9] reported overexpression of cyclin D1 and p16INK4A protein in a heterogeneous group of malignant salivary gland tumors. All four PLGA described in that study showed no reactivity for cyclin D1 but variable reactivity with p16INK4A ranging from 9 to 20 % of the total cell population. In our study, p16INK4A stained both luminal and non luminal cells in all five cases of PLGA. One case did not reveal any immunoreactivity for cyclin D1, while the rest (4/5) of the cases showed similar luminal and non luminal nuclear pattern of staining that ranged from focal to diffuse. In addition, we identified a reproducible pattern of immunoreactivity in PLGAs characterized by a haphazard distribution in the lobular/solid areas with p16INK4A. This finding is in concordance with the previously described pattern and may be helpful in differentiating PLGA’s from other neoplasms in small biopsy specimens [21].
To the best of our knowledge, our series is the first to examine p16INK4A and cyclin D1 expression in benign conditions such as Warthin tumors and salivary duct cysts. Interestingly, the cells lining the cavities in duct cysts were easily confused with histiocytes by light microscopy. CD68 and S100 immunostains were applied to all of these cases and failed to reveal immunoreactivity within the cyst lining. This finding helped establish the epithelial nature of the cells expressing p16INK4A and cyclin D1 in this subgroup. On the other hand, p16INK4A and cyclin D1 labeled both basal and epithelial layer cells in Warthin tumors in a focal (1+) to moderate (2+) fashion (Table 2).
In conclusion, our study showed overexpression of both p16INK4A and cyclin D1 proteins in a heterogeneous group of malignant and benign salivary gland tumors. The lack of statistical significance in p16INK4A and CCND1 expression (p = 0.543 and p = 0.146 respectively) between the two tumor groups suggests that these deregulations are unrelated to their biological behavior (Tables 3, 4). Nevertheless, such a conclusion should be interpreted cautiously given the relatively small sample size of cases in our study. The significant correlation between p16INK4A and cyclin D1 expression among benign and malignant salivary gland tumors (p = 0.01) suggests that p16INK4A overexpression occurs as a secondary event due to cyclin D1 overexpression (Table 5). This may represent an adaptative positive feedback mechanism (similar to that seen in HPV infection) in a deranged pRB pathway that is involved in salivary gland tumorigenesis. Further molecular studies targeting the cyclin D1 gene locus are warranted in order to provide accurate insight about these molecular pathogenic events, which would guide targeted gene therapy in cases where surgery may not assure curative results. Larger multicenter studies are needed to achieve such goals.
Table 3.
Comparison of p16INK4A expression in benign and malignant tumors
| p16INK4A score | Benign tumorsa | Malignant tumorsa | Total |
|---|---|---|---|
| 0 | 0 | 2 | 2 |
| 1 | 4 | 6 | 10 |
| 2 | 3 | 11 | 14 |
| 3 | 7 | 10 | 17 |
| 4 | 0 | 1 | 1 |
| Total | 14 | 30 | 44 |
| p value** | 0.543 |
aFisher exact test was used for each staining category, ** Pearson Chi squared test X2(4) statistical test was used to compare categories altogether in benign and malignant tumors
Table 4.
Comparison of cyclin D1 expression in benign and malignant tumors
| Cyclin D1 score | Benign tumorsa | Malignant tumorsa | Total |
|---|---|---|---|
| 0 | 1 | 1 | 2 |
| 1 | 4 | 11 | 15 |
| 2 | 7 | 7 | 14 |
| 3 | 2 | 11 | 13 |
| Total | 14 | 30 | 44 |
| p value** | 0.146 |
No tumor showed very strong (4+) staining for cyclin D1
aFisher exact test was used for each staining category, ** Pearson Chi squared test X2 (3) statistical test was used to compare categories altogether in benign and malignant tumors
Table 5.
Correlation of p16INK4A and cyclin D1 expression in benign and malignant salivary gland tumors
| p16INK4A | ||||||
|---|---|---|---|---|---|---|
| Cyclin D1 | 0 | 1 | 2 | 3 | 4 | Total |
| 0 | 0 | 0 | 2 | 0 | 0 | 2 |
| 1 | 1 | 9 | 3 | 2 | 0 | 15 |
| 2 | 0 | 1 | 5 | 7 | 0 | 13 |
| 3 | 1 | 0 | 4 | 8 | 1 | 14 |
| Total | 2 | 10 | 14 | 17 | 1 | 44 |
| p value* | 0.01 | |||||
Pearson Correlation X2 (12) test was used [r = 26.33]
Conflict of interest
None.
References
- 1.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 2.Quelle DE, Ashmun RA, Shurtleff SA, et al. Overexpression of mouse D-type Cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7(8):1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 3.Lukas J, Parry D, Aagaard L, et al. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumor suppressor p16. Nature. 1995;375:503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- 4.Kamb A, Gruis NA, Weaver-Feldhaus J, et al. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994;264:436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- 5.Nakahara Y, Shintani S, Mihara M, et al. High frequency of homozygous deletion and methylation of p16INK4A gene in oral squamous cell. Cancer Lett. 2001;163:221–228. doi: 10.1016/S0304-3835(00)00699-6. [DOI] [PubMed] [Google Scholar]
- 6.Kishi M, Nakamura M, Nishimine M, et al. Genetic and epigenetic alteration profiles for multiple genes in salivary gland carcinomas. Oral Oncol. 2005;41(2):161–169. doi: 10.1016/j.oraloncology.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Nishimine M, Nakamura M, Kishi M, et al. Alterations of p14ARF and p16INK4a genes in salivary gland carcinomas. Oncol Rep. 2003;10(3):555–560. [PubMed] [Google Scholar]
- 8.Guo XL, Sun SZ. Wang WX et al Alterations of p16INK4a tumour suppressor gene in mucoepidermoid carcinoma of the salivary glands. Int J Oral Maxillofac Surg. 2007;36(4):350–353. doi: 10.1016/j.ijom.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Etges A, Nunes FD, Ribeiro KC, et al. Immunohistochemical expression of retinoblastoma pathway proteins in normal salivary glands and in salivary gland tumours. Oral Oncol. 2004;40:326–331. doi: 10.1016/j.oraloncology.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Patel RS, Rose B, Bawdon H, et al. Cyclin D1 and p16 expression in pleomorphic adenoma and carcinoma ex pleomorphic adenoma of the parotid gland. Histopathology. 2007;51(5):691–696. doi: 10.1111/j.1365-2559.2007.02853.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu T, Zhu E, Wang L, et al. Abnormal expression of Rb pathway-related proteins in salivary gland acinic cell carcinoma. Hum Pathol. 2005;36(9):962–970. doi: 10.1016/j.humpath.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Shintani S, Mihara M, Nakahara Y, et al. Infrequent alternations of RB pathway (Rb-p16INK4A-cyclin D1) in adenoid cystic carcinoma of salivary glands. Anticancer Res. 2000;20(3B):2169–2175. [PubMed] [Google Scholar]
- 13.Isayeva T, Bai S, Said-Al-Naief N et al. Transcriptionally active high-risk human papillomavirus in salivary mucoepidermoid carcinoma: a novel finding. [Abstract]. Mod Pathol 2011; 24:278A.
- 14.Ferrario F, Roselli R, Spriano G. Epidemiology and risk factors. In: Spriano G, editor. Tumors of the major salivary glands. Official report of the 95th National congress. Turin, Italy: Italian Society of Othroinolaryngology and Cervico-facial surgery; 2008. p. 55–82.
- 15.Barnes L, Everson JW, Reichart P, editors. Pathology and genetics of head and neck tumours. In: Kleihues P, Sobin LH, series editors. World Health Organization classification of tumours. Lyon: IARC Press; 2005.
- 16.Skalova A. Rare tumours of salivary glands. Pathol Case Rev. 2004;9:264–269. doi: 10.1097/01.pcr.0000143779.06074.aa. [DOI] [Google Scholar]
- 17.Barbieri F, Cagnoli M, Ragni N, et al. Increased cyclin D1 expression is associated with features of malignancy and disease recurrence in ovarian tumors. Clin Cancer Res. 1999;5:1837–1842. [PubMed] [Google Scholar]
- 18.Lammie GA, Fantl V, Smith R, et al. D11S287, a putative oncogene on chromosome 11q13, is amplified and expressed in squamous cell and mammary carcinomas and linked to BCL-1. Oncogene. 1991;6:439–444. [PubMed] [Google Scholar]
- 19.Maruya S, Kurotaki H, Shimoyama N, et al. Expression of p16 protein and hypermethylation status of its promoter gene in adenoid cystic carcinoma of the head and neck. ORL J Otorhinolaryngol Relat Spec. 2003;65(1):26–32. doi: 10.1159/000068658. [DOI] [PubMed] [Google Scholar]
- 20.Wenig BM, Heffes CS, editors. Atlas of head and neck pathology. 2. Philadelphia: W.B. Saunders; 2008. [Google Scholar]
- 21.Perez-Ordonez B, Linkov I, Huvos AG. Polymorphous low-grade adenocarcinoma of minor salivary glands: a study of 17 cases with emphasis on cell differentiation. Histopathology. 1998;32(6):521–529. doi: 10.1046/j.1365-2559.1998.t01-2-00410.x. [DOI] [PubMed] [Google Scholar]