Abstract
Granular cell tumors (GCT) of the head and neck are not uncommon; however, involvement of the cervical esophagus is rare. Characterized by an infiltrative growth pattern, these benign tumors are historically difficult to surgically excise and are radioresistant. We present here a case of dysphagia caused by a GCT of the cervical esophagus. Work up with ultrasound-guided fine needle aspiration was suggestive of a GCT due to the presence of cohesive cells with granular cytoplasm that were S-100 and CD68 positive with immunostaining, and PAS positive with histochemistry. Resection required removal of a portion of the muscular wall of the esophagus sparing the overlying mucosa. The patient is currently asymptomatic and without recurrence after 10 month follow-up. Review of the literature revealed 19 reports of cervical esophageal GCTs. There is a female preponderance (75 %), with an average age of 41 years. Dysphagia and weight loss are the most common presenting symptoms. The average tumor size on presentation was 2.7 cm, with symptomatic tumors being significantly larger than asymptomatic lesions; the latter was present in 25 % of patients. Concurrent GCTs in the upper aerodigestive tract were identified in 35 % of cases. Approximately 30 % of tumors required segmental cervical esophageal resection. The purpose of this report is to describe the epidemiology and treatment of GCTs of the cervical esophagus. Lesions should be addressed early with complete surgical excision to prevent growth necessitating more morbid surgery. Due to the high rate of concurrent GCTs, upper endoscopy is advised in the workup of these patients.
Keywords: Granular cell tumor, Cervical esophagus, Management, Dysphagia
Introduction
Granular cell tumors (GCTs) are rare benign neoplasms of neural derivation, thought to arise specifically from Schwann cells. Most commonly found in subcutaneous tissue, other sites of origin include the oral cavity, breast, larynx, bronchus, and gastrointestinal tract, with approximately 45–65 % occurring in the head and neck [1]. GCTs of the cervical portion of the esophagus are particularly rare. In reviews of gastrointestinal tract GCTs, the incidence of cervical esophageal lesions has been found to be 1.3 % of all gastrointestinal GCTs, and only 4.2–5 % of all esophageal GCTs [2, 3]. Due to the rarity of this lesion and its benign pathology, prescribed treatment protocols have not been elucidated; however, some have recommended observation alone for lesions less than one centimeter [4]. In an effort to better characterize these uncommon lesions, we present a case of a cervical esophageal GCT and review the literature to determine trends in therapy in an effort to better establish treatment recommendations.
Methods and Materials
A case of granular cell tumor of the cervical esophagus treated in the Department of Otolaryngology—Head and Neck Surgery of Virginia Commonwealth University is described. The patient’s clinical presentation, workup including fine needle aspiration biopsy and imaging, and treatment were reviewed. To assess the current literature on cervical esophageal granular cell tumors, a MEDLINE search was performed through the United States National Library of Medicine’s “PubMed” online database. Using the search terms “esophagus” and “granular cell tumor” with results limited to the English language, over 83 papers were identified. Case reports, series, reviews and their citations were examined for further references. Eighteen references which included at least one patient with granular cell tumor involving the cervical esophagus were identified, comprising a total of nineteen unique patients. These publications were reviewed to extract information regarding patient demographics, presenting symptoms, tumor size, method of treatment, length of follow up, and presence of concurrent tumors. Statistical analysis of continuous variables was carried out using student’s t test.
Results
Case Report
A 55 year old female initially presented to the Emergency Department of Virginia Commonwealth University Health System following a motor vehicle accident. Evaluation included a CT of the cervical spine, which incidentally revealed a cervical esophageal mass. The Otolaryngology—Head and Neck Surgery service was consulted for evaluation of the lesion. The patient had a 35 pack-year history of smoking, but had quit 6 months prior to presentation. She reported a greater than 10 year history of progressive dysphagia, especially to pills, without concurrent odynophagia, weight loss, neck mass, otalgia, dysphonia, or hemoptysis. Head and neck examination including flexible fiberoptic laryngoscopy was unremarkable, showing normal vocal fold mobility with no mass nor mucosal lesions, and no pooling of secretions in the hypopharynx. Operative laryngoscopy showed no lesions, while rigid esophagoscopy revealed a submucosal mass immediately distal to the esophageal introitus. Ultrasound-guided fine needle aspiration was performed, revealing cohesive cells with granular cytoplasm that were S-100 protein and CD68 positive with immunostaining, and PAS positive with histochemistry. S-100 positivity suggested a neurally-derived tumor (Fig. 1a) and in combination with CD68 and PAS positivity was indicative of a granular cell tumor.
Fig. 1.
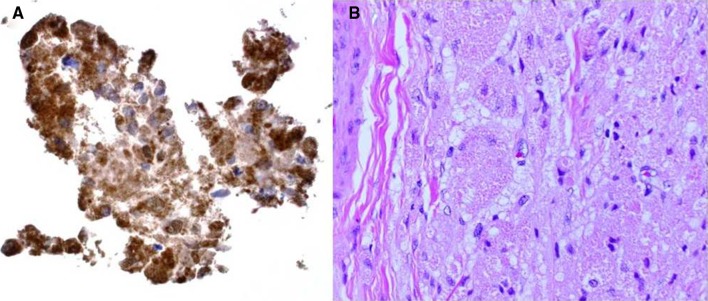
a Immunohistochemical staining for S-100 protein on cell block derived from needle rinse of fine needle aspiration (40x). b Section of granular cell tumor from resection specimen showing closely packed large cells with indistinct borders and granular cytoplasm and bland nuclei. (H and E stain, 20x)
Due to the patient’s underlying progressive dysphagia to solids, the decision was made to excise the mass. This was performed through a lower transcervical incision. The tumor was identified deep to the left recurrent laryngeal nerve, and was noted to be densely adherent to the submucosa and muscularis of the cervical esophagus. Grossly free tumor margins were obtained with resection of the muscular wall of the esophagus, while sparing the overlying mucosa. A superiorly-based left sternocleidomastoid muscle flap was used to reconstruct the cervical esophageal muscular wall defect in an effort to prevent diverticulum formation. Immediate post-operative examination showed intact vocal fold mobility bilaterally. Final pathologic review revealed a 4.1 cm × 2.6 cm × 1.8 cm mass in the submucoa composed entirely of large cells with indistinct cell borders, abundant granular cytoplasm and bland nuclei, consistent with a granular cell tumor (Fig. 1b). Six month follow up revealed resolution of dysphagia, both clinically and radiographically (Fig. 2), without evidence of tumor recurrence by CT imaging (Fig. 3).
Fig. 2.
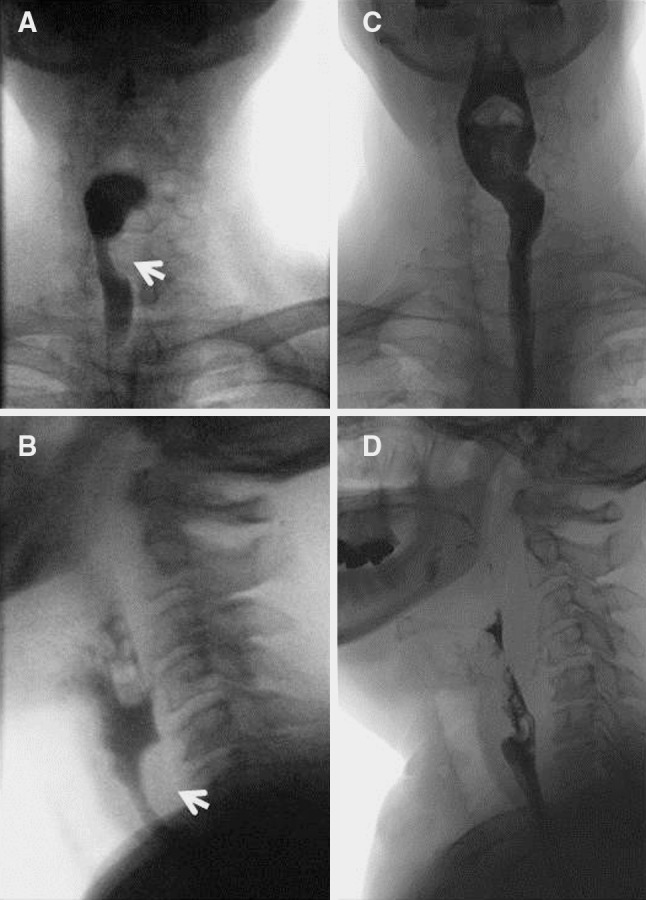
a, b Antero-posterior (a) and lateral (b) views from pre-operative esophagram showing extrinsic compression of barium column postero-laterally on the left side (arrows). c, d Antero-posterior (c) and lateral (d) views from post-operative esophagram, showing resolution of extrinsic compression
Fig. 3.
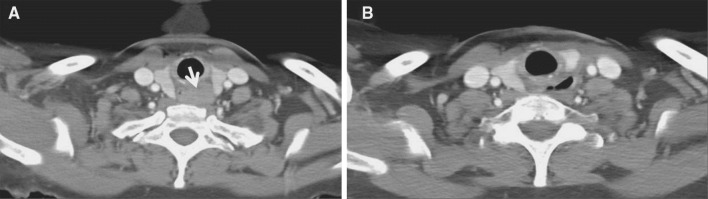
a Pre-operative CT scan revealing homogeneous soft tissue mass in cervical esophagus with compromise of esophageal lumen by GCT (arrow). b Post-operative CT scan revealing patent esophagus
Literature Review
Eighteen previous case reports and series of GCTs of the cervical esophagus, comprising a total of nineteen tumors, were identified in the English literature, which, along with the current study, comprises twenty unique subjects as summarized in Table 1. The average patient age at diagnosis was 41.2 years (±9.7 years, range 24–55 years). A female predominance was observed with a female-to-male ratio of 3:1. Minimally invasive diagnostic techniques were employed in eight patients (40 %), four by endoscopic biopsy and four by image-guided needle aspirations. Success rates of 50 and 75 % were achieved by endoscopic and radiographic needle biopsy interventions, respectively. Diagnosis was achieved by open biopsy or surgical excision in the remainder of patients (75 %). The average size of GCTs on intervention was available in seventeen cases and measured 2.6 cm (±1.6 cm, range 0.4–5.0 cm). The most common presenting symptoms were: dysphagia (65 %), weight loss (40 %), hoarseness (15 %), pain (10 %), and globus (5 %). One-quarter of patients were reported as asymptomatic on diagnosis. Comparing tumors either causing or not causing symptoms on presentation, a significant size difference was discovered with symptomatic masses over three times larger than asymptomatic masses (3.4 ± 1.2 cm versus 1.1 ± 1.1 cm; p < 0.05). Thirty-five percent of patients presented with concurrent granular cell tumors at other sites in the aerodigestive tract. Histologically, infiltration of esophageal musculature or muscular changes including thickening and degeneration were noted in 20 % of cases. No instances of mitotic figures or other concerning nuclear features were reported; however, in the seven cases where nuclear position was discussed, 43 % were described as eccentrically located and 57 % centrally situated. Mucosal ulceration or disruption was not present in any case reviewed, with all tumors either located submucosally or in the muscular layer of the esophagus. Length of follow up was available in sixteen reports, with the average length being 54.9 weeks (±72.7 weeks, range 1–260 weeks). No documented cases of tumor recurrence were discovered.
Table 1.
Reported cases of cervical esophageal granular cell tumors
| Author | Age (years) | Sex | Presenting symptoms | Tumor size | Method of diagnosis | Method of excision | Length of follow-up | Concurrent tumor |
|---|---|---|---|---|---|---|---|---|
| Bayerdorffer [9](2 cases) | 47 35 |
M F |
Asymptomatic Asymptomatic |
0.5 cm 0.4 cm |
Excision | Diathermy snare Endoscopic forceps |
1 year 2 years |
None None |
| Boncoeur-Martel [10] | 44 | M | Dysphagia, globus, weight loss, hoarseness | 4 cm | Excision | Transcervical excision, thyroidectomy | 3 weeks | None |
| Cohle [11] | 52 | M | Asymptomatic/autopsy | 1.2 cm | Autopsy | N/A | N/A | Mid and distal esophagus |
| Collett [12] | 48 | F | Dysphagia | 3.5 cm | Excision | Excision | 22 months | None |
| Crawford [7] | 31 | F | Dysphagia | 4 cm | Excision | Excision (cervical esophageal resection with primary anastomosis) | N/A | Skin |
| Tongue | ||||||||
| Retroperitoneum | ||||||||
| DeGouveia [13] | 19 | F | Dysphagia, hypersalivation, weight loss | N/A | Excision | Excision | 28 days | None |
| Farrell [6] | 24 | F | Dysphagia, weight loss | 4 cm | Excision | Excision | 7 days | None |
| Gloor [14] | 48 | M | Weight loss, epigastric pain, hematemesis | 0.8 cm | Endoscopic biopsy | Biopsy | 4 months | Distal esophagus |
| Goldblum [4] | 46 | F | Asymptomatic | 0.5 cm | Endoscopic biopsy | Biopsy alone | 23 months | Gastric, cutaneous |
| Halum [15] | 34 | F | Dysphonia, dysphagia | N/A | Excision | Transcervical excision | N/A | None |
| John [16] | 30 | F | Dysphagia, weight loss | 4 cm | CT-guided biopsy | Esophagogastrectomy | 1 month | Gastric |
| Keshishian [17] | 48 | F | Indigestion | N/A | Excision | Excision | 3 weeks | Posterior pharyngeal wall |
| Lack [5] | 50 | F | Asymptomatic | 3 cm | Open biopsy | Segmental esophageal resection | N/A | None |
| Marin [18] | 51 | F | Dysphagia, weight loss, hoarseness | <5 cm | CT-guided biopsy | Cervical esophageal resection, radial forearm free flap | 1 year | None |
| Rella [19] | 43 | F | Dysphagia | N/A | Excision | Total laryngectomy, cervical esophagectomy | 6 weeks | None |
| Reyes [20] | 38 | M | Dysphagia, retrosternal pain | 2.3 cm | Excision | Excision | 5 years | Mid esophagus |
| Vuyk [21] | 40 | F | Dysphagia, weight loss | 4 cm | Excision | Esohpagectomy, pectoralis major flap | 3 years | None |
| Wypkema [22] | 40 | F | Dysphagia, weight loss, regurgitation | 2 cm | Excision | Esophageal bypass | 14 days (patient died) | None |
| Huang (current report) | 55 | F | Dysphagia | 4.1 cm | US-guided biopsy | Excision | 6 months | None |
N/A not available
Discussion
In a review of 410,000 surgical specimens from all sites at one institution over 32 years, Lack et al. [5] found the incidence of granular cell tumors to be 0.03 %. While esophageal granular cell tumors are a rare entity, accounting for only 1.7 % of all granular cell tumors [5] and approximately 1.2 % of all esophageal masses [6], GCTs of the cervical portion of the esophagus are even more uncommon. The case presented herein brings the total reported number of cervical esophageal GCTs to twenty cases, with this report being the first literature review on the subject.
GCTs are characterized histologically by large cells with abundant granular cytoplasm, bland nuclei, and indistinct nucleoli. Granules within the cell cytoplasm may stain positive with Periodic Acid-Schiff. S-100 protein positivity is typical with immunostaining [1]. Although benign, these GCTs may be incorrectly diagnosed as squamous lesions since a high percentage also present with pseudoepitheliomatous hyperplasia on superficial biopsy. Not uncommonly, GCTs may also require difficult surgical dissection to achieve removal due to their infiltrative growth pattern [7, 8]. This difficulty in dissection may raise suspicion of a squamous cell carcinoma and distinguishes it surgically from more common benign tumors of the esophagus, including leiomyomas. Other benign lesions to be considered in this region include adult rhabdomyomas, xanthomas, or other tumors with granular cytoplasm. As benign and malignant esophageal tumors require extremely different surgical approaches, pre-operative biopsy is warranted. Endoscopic biopsy, with only a 50 % success rate in this review, may be technically difficult due to its submucosal nature, placement lower down in the upper digestive tract, and need for deeper tissue in order to obtain an adequate specimen. Ultrasound- or CT-guided needle biopsies offer the advantage of requiring minimal sedation and precise needle localization which possibly contributes to the higher diagnostic success rate observed in this study. Due to the proximity of the cervical esophagus to the larynx, thyroid, and great vessels of the neck, cases should be evaluated on an individual basis by a skilled cytopathologist.
The most common presenting symptoms of cervical esophageal GCTs include dysphagia and weight loss, with symptomatic tumors being significantly larger than asymptomatic lesions, as shown by analysis of reported cases above. Recommended treatment for GCTs has generally included complete excision of tumors, especially those greater than 1 cm, to prevent recurrence and eliminate the possibility of malignant transformation, the latter estimated to occur in 1–2 % of cases. Though up to 25 % of lesions are asymptomatic, excision may cause significant morbidity, often requiring sacrifice of contiguous esophageal musculature due to the infiltrative growth pattern. Resection of larger symptomatic lesions is especially problematic due to the close proximity of the cervical esophagus to vital structures such as the recurrent laryngeal nerve, larynx, and pharyngeal constrictor muscles. In fact, 40 % of symptomatic lesions identified in our review required segmental esophageal resections, with 20 % also requiring local pedicled or free flap reconstruction. While 20 % (1 patient) of asymptomatic patients also required segmental esophageal resection, the smaller average size of these tumors (1.12 cm) would be expected to less likely require sacrifice of important contiguous structures to achieve complete excision. Although the growth rate of these tumors is not known due to its rarity, it should be recommended that these tumors be addressed as early as possible after diagnosis to prevent subsequent growth and potential additional morbidity related to more extensive esophageal resection and reconstruction. This would hold particularly true in the case of a smaller, asymptomatic lesion discovered incidentally, at which point treatment would be less likely to entail segmental esophageal resection and its potential morbidity. Further, as 35 % (7/20) of cases reviewed had concurrent GCTs, all of which were present in the upper aerodigestive tract and stomach, it should be advocated that initial evaluation upon diagnosis of cervical esophageal GCTs include a complete survey of the upper aerodigestive tract, either by endoscopy or imaging. This high percentage of concurrent tumors seems unique to GCTs of the cervical esophagus, as the rate of concurrent GCTs with lesions at other primary sites is estimated to be only 2–15 % [1].
Conclusion
Cervical esophageal granular cell tumors, although benign, are difficult to manage lesions due to infiltrative growth and often large size on presentation. Even asymptomatic tumors should be excised early to prevent the risk of further growth, and with it risk to nearby structures and the potential for malignant transformation. In addition, due to a high rate of concurrent lesions seen with GCTs of the cervical esophagus, endoscopy or imaging of the upper gastrointestinal tract is recommended to exclude second primary lesions.
Conflict of interest
The authors declare they have no financial disclosures or conflicts of interest to report.
References
- 1.Barnes L. Surgical Pathology of the Head and Neck: Third Edition. Informa Healthcare, 2009, p. 698–701.
- 2.Johnston MJ, Helwig EB. Granular cell tumors of the gastrointestinal tract and perianal region. Dig Dis Sci. 1981;26:807–816. doi: 10.1007/BF01309613. [DOI] [PubMed] [Google Scholar]
- 3.Voskuil JH, Dijk MM, Wagenaar SSC, Van Vliet ACM, Timmer R, Van Hees PAM. Occurrence of esophageal granular cell tumors in the Netherlands between 1988 and 1994. Dig Dis Sci. 2001;46:1610–1614. doi: 10.1023/A:1010676715046. [DOI] [PubMed] [Google Scholar]
- 4.Goldblum JR, Rice TW, Zuccaro G, Richter JE. Granular cell tumors of the esophagus: a clinical and pathologic study of 13 cases. Ann Thor Surg. 1996;62:860–865. doi: 10.1016/S0003-4975(96)00443-2. [DOI] [PubMed] [Google Scholar]
- 5.Lack EE, Worsham GF, Callihan MD, Crawford BE, Klappenback S, Rowden G, Chun B. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301–316. doi: 10.1002/jso.2930130405. [DOI] [PubMed] [Google Scholar]
- 6.Farrell KH, Devine KD, Harrison EG, Olsen AM. Granular cell myoblastoma of the esophagus: incidence and surgical treatment. Ann Otol. 1973;82:784–789. doi: 10.1177/000348947308200606. [DOI] [PubMed] [Google Scholar]
- 7.Crawford ES, DeBakey ME. Granular cell myoblastoma: two unusual cases. Cancer. 1953;6:786–789. doi: 10.1002/1097-0142(195307)6:4<786::AID-CNCR2820060420>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 8.David O, Jakate S. Multifocal granular cell tumor of the esophagus and proximal stomach with infiltrative growth. Arch Pathol Lab Med. 1999;123:967–973. doi: 10.5858/1999-123-0967-MGCTOT. [DOI] [PubMed] [Google Scholar]
- 9.Bayerdorffer E, Ottenjann R. Granular cell tumor in upper GI-tract endoscopy: five cases of esophageal location. Endoscopy. 1986;18:97–100. doi: 10.1055/s-2007-1018341. [DOI] [PubMed] [Google Scholar]
- 10.Boncoeur-Martel MP, Loevner LA, Yousem DM, Elder DE, Weinstein GS. Granular cell myoblastoma of the cervical esophagus: MR findings. AJNR. 1996;17:1794–1797. [PMC free article] [PubMed] [Google Scholar]
- 11.Cohle SD, McKechnie JC, Truong L, Jurco S. Granular cell tumor of the esophagus. Am J Gastroenterol. 1981;75:431–435. [PubMed] [Google Scholar]
- 12.Collett HS. Granular cell myoblastoma of the esophagus. Rocky Mountain Medical Journal. 1974;71:145–147. [PubMed] [Google Scholar]
- 13.DeGouveia OF, Pereira AA, Netto B, Vilhena AM, Dutra G, Bryk D. Granular cell myoblastoma of the esophagus (Abrikossoff’s tumor) Gastroenterology. 1960;38:805–809. [PubMed] [Google Scholar]
- 14.Gloor F, Clemencon G. Granular cell tumors (“myoblastomas”) of the esophagus. Endoscopy. 1975;7:239–242. doi: 10.1055/s-0028-1098581. [DOI] [Google Scholar]
- 15.Halum SL, Yates C. Granular cell tumor of the esophagus presenting as a duplication cyst. ENT Journal. 2008;86:135. [PubMed] [Google Scholar]
- 16.John BK, Dang NC, Hussain SA, et al. Multifocal granular cell tumor presenting as an esophageal stricture. J Gastrointest Canc. 2008;39:107–113. doi: 10.1007/s12029-009-9056-0. [DOI] [PubMed] [Google Scholar]
- 17.Keshishian JM, Alford TC. Granular cell myoblastoma of the esophagus: a report of a case. The American Surgeon. 1964;30:263–266. [PubMed] [Google Scholar]
- 18.Marin VP, Yu P, Weber RS. Isolated cervical esophageal reconstruction for rare esophageal tumors. Head Neck. 2006;28:856–860. doi: 10.1002/hed.20442. [DOI] [PubMed] [Google Scholar]
- 19.Rella AJ, Conte AJ, Farrell JT. Granular cell myoblastoma of the esophagus. Archives of Otolaryngology. 1963;78:105–108. doi: 10.1001/archotol.1963.00750020729018. [DOI] [PubMed] [Google Scholar]
- 20.Reyes CV, Kathuria S, Molnar Z. Granular cell tumor of the esophagus: a case report. J Clinc Gastroenterol. 1980;2:365–368. doi: 10.1097/00004836-198012000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Vuyk HD, Snow GB, Tiware RM, Van Velzen D, Veldhuizen RW. Granular cell tumor of the proximal esophagus: a rare disease. Cancer. 1985;55:445–449. doi: 10.1002/1097-0142(19850115)55:2<445::AID-CNCR2820550226>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 22.Wypkema W, Schmaman A, Berson D. Granular cell myoblastoma of the oesophagus: a report of 2 cases. SA Medical Journal. 1967;41:911–912. [PubMed] [Google Scholar]


