Abstract
Instances of perioral and labial foreign body reactions to a variety of injectable dermal fillers were selected from the oral and maxillofacial pathology and dermatopathology archives at Pacific Pathology Laboratory of San Diego with the objective being to engender a compilation of histopathologic characteristics that allow the pathologist to identify the inciting materials. All cases of foreign body reactions located in the lips and perioral regions were reviewed by four pathologists, retaining those cases with a history of injection lip augmentation as well as those with histologic features previously documented to represent dermal filler substances. In selected cases, Alcian blue pH 2.5 with and without hyaluronidase pretreatment was performed. Immunohistochemical markers for macrophages (CD 68), adipocytes (S-100) and keratinocytes (AE1/AE2) were undertaken. All instances presented as single or multiple submucosal plaques, nodules or swellings. Natural polymers including collagen, hyaluronate, hydroxyapatite, poly-L-lactate and synthetic polymers including carboxymethyl cellulose, dimethylpolysiloxane, and polyethyl methacrylate induce histologically unique features that allow for their identification. Host histopathologic responses included nodule without foreign body reaction, nodule with chronic inflammation, granuloma with epithelioid histiocytic and multinucleated giant cell reaction. Dermal filler foreign body host reactions in conjunction with the morphology of the foreign materials themselves are unique and can be differentiated from one another microscopically.
Keywords: Dermal fillers, Lip augmentation, Foreign body reaction
Introduction
Minimally invasive procedures for lip augmentation have been performed extensively in recent years. Inert injectable biological and synthetic polymers can be precisely placed in the dermis in order to increase soft tissue bulk, produce esthetic contours and flatten wrinkles. Prior to release and marketing, these dermal fillers had been tested in animal models to ensure tissue compatibility [1, 2]. Subcutaneous injection with time lapse tissue sampling and subsequent histopathologic evaluation among human volunteer subjects has also been undertaken [3]. The natural/biologic injection fillers are ultimately resorbable and therefore temporary, requiring repeated treatments to maintain desired esthetic results. Many synthetic polymers are not easily phagocytized, remain in situ with minimal resorption for many years, and are therefore considered to be permanent [1, 4].
Whereas most patients tolerate these materials without complications, some will develop prominent, unplanned lesions with histologic evidence of inflammation and foreign body granuloma formation. Furthermore, these granulomas may migrate up to 3 cm away from the initial site of injection placement. Patients may not associate an augmentation procedure with development of a nodular mass months or years later [5, 6] Most reports in the literature focus on a series of cases limited to one substance; here we present a series of cases that evolved subsequent to lip recontouring by injection with a variety of substances that manifest specific histologic characteristics.
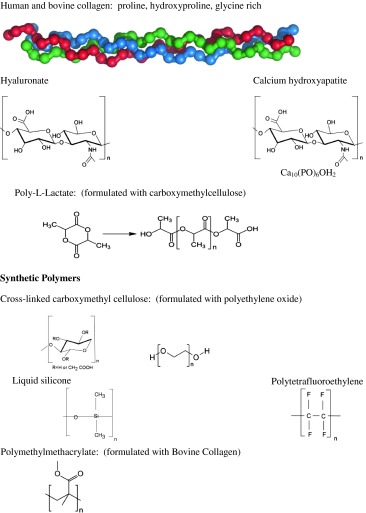
The more commonly employed augmentation materials that have been reported to evolve, albeit rarely, into foreign body granulomas are listed in Table 1. Natural polymers include human and bovine collagen, [6, 7] and the nonsulfated glycosaminoglycan, hyaluronic acid (HA) [8, 11, 12]. Many of these natural as well as the synthetic polymers serve as gelatinous matrices in which particulate or spherical filler substances, measuring 2–100 μm, are suspended. The synthetic fillers are usually not resorbed by phagocytic or lytic activity. These materials may also condense or coalesce into nodules with or without a classic foreign body response microscopically. In this communication, we have described the histopathologic features that will allow the pathologist to identify the more commonly encountered dermal filler reactions by virtue of their structural features and salient host response patterns.
Table 1.
Dermal fillers employed for lip augmentation natural polymers
Materials and Methods
Twelve cases of perioral foreign nodules attributable to injection dermal fillers were obtained from archived material accessioned over a 4 year period (9-1-2008 to 9-1-2011). Histopathologic features were recorded for both host response and foreign material morphology. All patients underwent nonsurgical, injection lip augmentation. The clinicians submitting these cases for pathologic assessment were either oral and maxillofacial surgeons or dermatologists. They were contacted and detailed information was obtained regarding the chemical nature of the injected materials. Special stains and markers applied to selected cases included Alcian blue pH 2.5, Alcian blue pretreated with streptococcal hyaluronidase, and standard immunoperoxidase staining for CD68, cytokeratins AE1,AE2 and S-100 protein expression.
Results
Clinical Presentation
Foreign body reactions to dermal fillers present as firm, often movable subdermal or submucosal focal, bosselated or multifocal nodules. They may be localized to the site of injection, or they may migrate, usually into the submucosa of the maxillary or mandibular sulcus, depending on whether the upper or lower lip was injected. The nodules often masquerade as an odontogenic infection (parulis), a mesenchymal neoplasm, a mucocele or a minor salivary tumor (Fig. 1). All twelve cases arose in females ranging in age from 30 to 77, mean = 55.5 years. All patients had noted a visible or palpable subcutaneous/submucosal pink or yellow nodule or raised plaque, yet were otherwise asymptomatic.
Fig. 1.
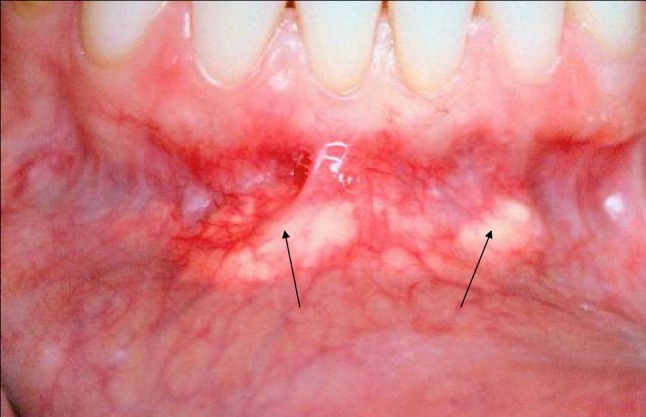
Diffuse nodular lesions in the labial vestibule represent hyaluronate dermal fill granulomas that migrated from the original injection sites in the vermillion/mucosal junction of the lower lip
It is noteworthy that during the early data gathering phase of this report, many discrepancies occurred with regard to testimony from patients and their surgeons. Frequently the wrong filler substances were reported to us by telephone contacts and the discrepancies were uncovered when our cases were compared with reported histologic findings in the literature. Furthermore, two patients denied ever receiving a filler material.
Microscopic Features
As seen in Table 2, reactions to six fillers were identified including: bovine collagen(n = 1), hyaluronic acid(n = 2), hydroxyapatite(n = 3), poly-L-lactate(n = 2), liquid silicone(n = 3) and hydroxyethyl-methacrylate(n = 1). The microscopic patterns for most of the dermal fillers used in practice today have been assessed in animal and human models. The histology in these studies mirrors that seen in case reports that clinically manifest as plaques or nodules. In those that become tumefactive, the host response is prominent although the structural features of the filler materials themselves are the same as those encountered in animal and human research models [1–3]. We have classified the host response in these patients as: Foreign material without inflammatory reaction (FM1), Foreign nodule with nonspecific inflammation (FN2), Foreign body granuloma with epithelioid histiocytic/multinucleated giant cell response (FBG 3).
Table 2.
Clinical features
| Age | Sex | Location | Filler |
|---|---|---|---|
| 49 | F | Nodule lower lip | Collagen |
| 45 | F | Granular yellow lesion | Hyaluronate |
| 51 | F | White nodules mandibular sulcus | Hyaluronate |
| 54 | F | Lower lip nodule | Hydroxyapatite |
| 77 | F | White mass mandibular sulcus | Hydroxyapatite, hyaluronate |
| 67 | F | Small mass buccal mucosa | Hydroxyapatite |
| 30 | F | Nodule lower lip | Poly-L-lactate |
| 62 | F | Mandibular sulcus | Poly-L-lactate |
| 38 | F | Nodule lower lip | Hydroxyethylmethacrylate |
| 69 | F | Lower and upper lip | Silicone |
| 54 | F | Multiple nodules lower lip | Silicone |
| 53 | F | Cheek | Silicone |
In this series, a single case of bovine collagen injection was encountered and was represented by a nodule of eosinophilic structureless material without any fibroblastic nuclei. Unlike human collagen, fibers were not evident and the material was found to be nonrefractile under crossed polar lenses. A thin fibrous capsule surrounded the nodule and was devoid of inflammation (Fig. 2). In this case the historical information obtained from the patient and clinician corresponded to the microscopic findings.
Fig. 2.
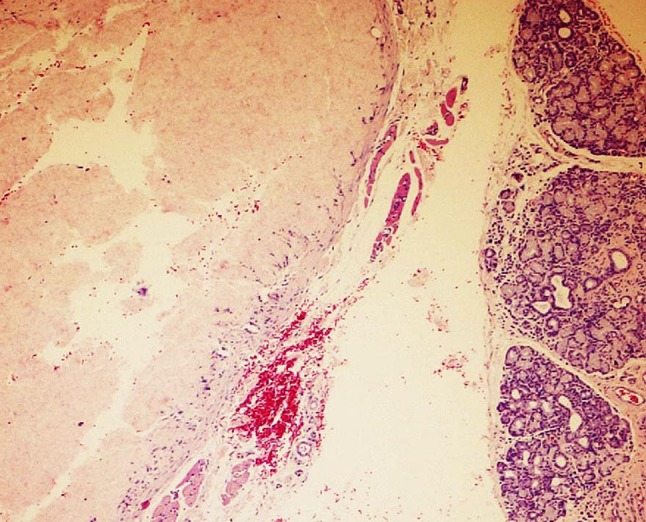
Collagen a diffuse eosinophilic lobule of bovine collagen with adjacent minor salivary lobules is acellular and structureless
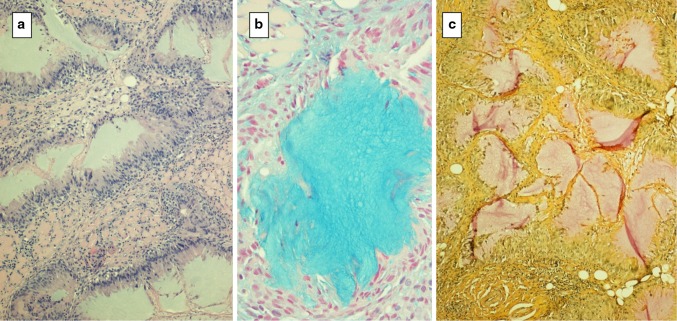
Two instances of hyaluronate filler reactions without particles or spheroids were seen. These lesions showed a FN 1 or FBG 3 response characterized by lakes of alcianophilic, hyaluronidase digestible basophilic amorphous material. One of these cases showed peripheral palisading by CD68 positive epithelioid histiocytes resembling the histopathologic features of a necrotizing granuloma with hyaluronate rather than necrosis in the center (Fig. 3). In surrounding areas small pools of hyaluronate could be seen and most of these smaller foci were not associated with any inflammatory reaction.
Fig. 3.
Hyaluronate a lakes of hyaluronate surrounded by palisaded epithelioid histiocytes, balcian blue staining of injected material, c mucinous lakes show pale mucicarmine staining
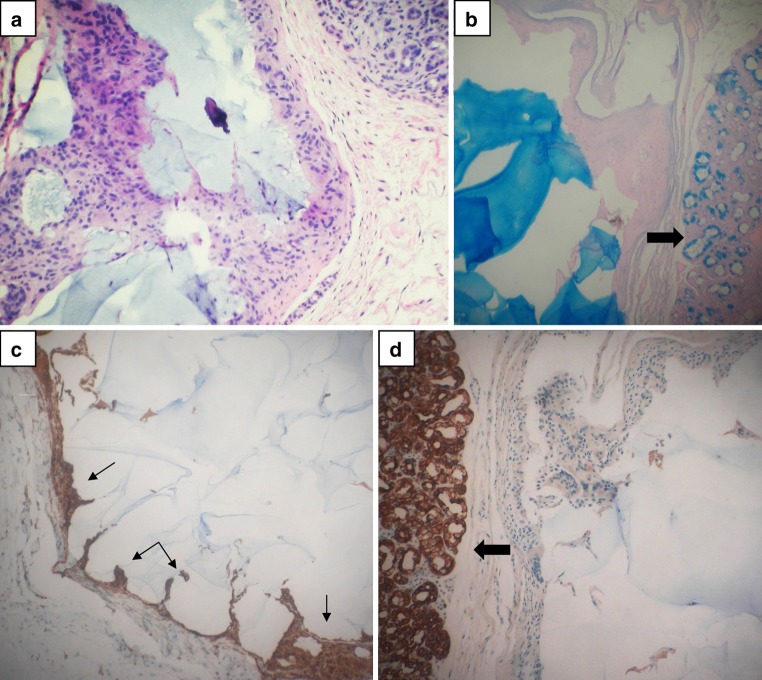
One of the cases showed multiple cystic areas with HA lakes lined by epithelioid cells showing a papillary cystic pattern that could be misinterpreted as a papillary cystadenoma or low grade mucoepidermoid carcinoma (Fig. 4). The lining epithelioid histiocytes and numerous admixed giant cells were found to be CD68 positive, cytokeratin negative confirming their histiocytic nature.
Fig. 4.
Hyaluronate cystic lakes: a cystic areas lined by epithelioid cells and b filled with basophilic homogeneous hyaluronate, alcianblue pH 2.5, c lining epithelioid cells are positive for CD68 and d negative for cytokeratins AE1, AE2. (large arrows: salivary lobules; small arrows: histiocytes)
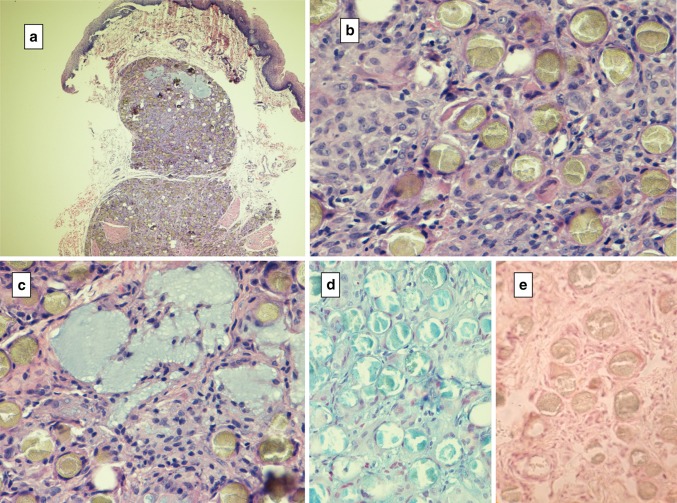
Hydroxyapatite granulomas are quite distinctive, inducing a FBG type 3 reaction in the region of the filler’s microspherules, the latter of which are round, grey-green, granular and measuring 25–40 μl. These microspheres are densely surrounded by compacted epithelioid histiocytes with interposed multinucleated giant cells. Small HA lakes are scattered throughout (Fig. 5).
Fig. 5.
Hydroxyapatite granuloma a low power depicting granulomas in the submucosa, b spheres with a granular, crinkled appearance and concentric stromal cells, c lakes of basophilic hyaluronic acid (alcian blue pH 2.5), d spheroids are alcianophilic, e pretreatment hyaluronidase digests hyaluronic acid in spheroids and surrounding connective tissues
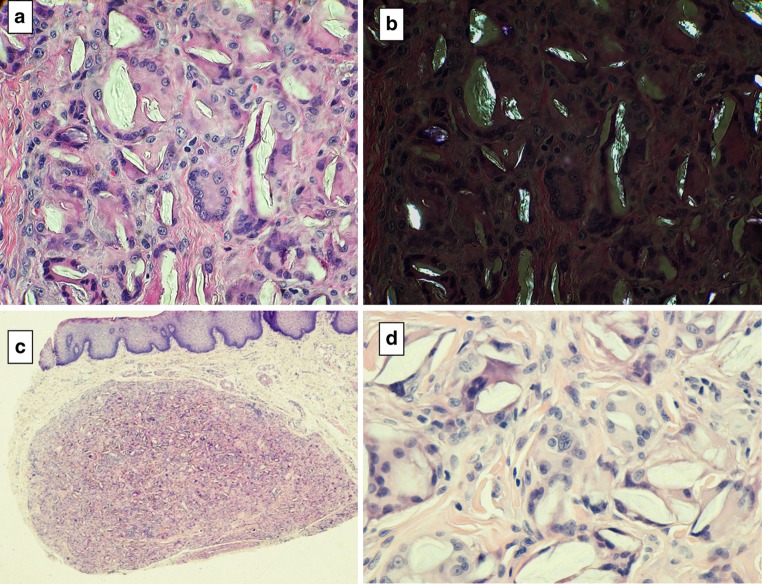
Poly-L-lactate is formulated with carboxymethylcellulose and may induce a classic foreign body granuloma (FBG 3) characterized by epithelioid cells and giant cells that surround “surfboard” vacuoles that contain a clear translucent poly-L-lactate substance that is refractile under polarized light (Fig. 6a, b). In contrast granuloma formation to hydroxyethylmethacrylate shows nonrefractile material within clear clefts (Fig. 6c, d). Otherwise, the granuloma shows the same microscopic features as Lactate granulomas (Table 3).
Fig. 6.
“Surf-board” vacuoles with foreign body reaction: poly-L-lactate a clefts with surrounding histioctes and giant cells b poly-L-lactate crystals are birefringent under polarized light. Hydroxyethylmethacrylate granuloma c well circumscribed granuloma, d higher magnification depicting stretched multinucleated giant cells; the cleft contents are nonrefractile
Table 3.
Morphologic patterns in dermal filler foreign body granuloma
| Material | Trade name | Morphology | Host responsea |
|---|---|---|---|
| Hydroxyapatite | Radiance, Radiesse | 25–50 um grey/green spherules | FBG 3 |
| Hyaluronate | Restylane Juvederm | Basophilic lakes alcianophilic, hyaluronidase digestible | FBG 3 |
| Liquid Silicone | Silicone medical grade | Adipocytoid vacuoles, variable size, 5–50 μm, vacant | FBG 3 |
| Collagen Bovine | Zyplast Zyderm | Multilobular eosinophilic acellular coagulum | FN 1 |
| Poly-L-lactate | New-Fill Sculptra | Angulate, elliptical particles of Equal size, refractilea | FBG 2 |
| Polymethyl-methacrylate | Artecoll Artefill | Microspheres 40 μm, vacant | FBG 3 |
| Hydroxyethyl-methacrylate | Dermalive | Translucent, broken glass-like nonrefractileb 20–120 μm Vacuoles |
FBG 3 |
| Polyacrylamide | Aquamid | Thin fibrous capsule | FN 2 |
| Polyvinylhydroxide | Evolution | Microspheres 25–40 μm | FN 2 |
aFN 1 foreign nodule, FN 2 foreign nodule chronic inflammation, FBG 3 foreign body granuloma with epithelioid histiocytes, multinucleated giant cells
bRefractile = birefringent under crossed polars
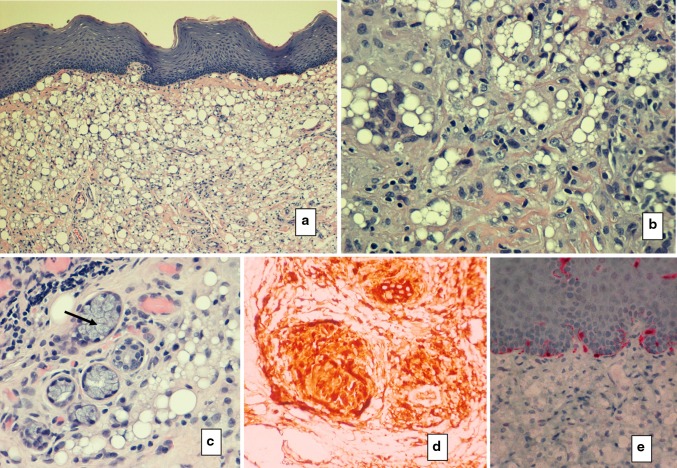
Lastly are the silicone reactions. Silicone is a permanent filler that may cause a foreign body reaction with epithelioid cells and giant cells. The silicone material is microlobular with clear bubbly spaces that may be mistaken for lipoblasts. In some silicon granulomas, the vacuoles are about the same size whereas in others they are polymorphic. An S100 stain disclosed negative results eliminating adipocytes and a consideration of liposarcoma (Fig. 7).
Fig. 7.
Silicone granuloma a early phase with vacuoles dispersed through out the submucosa, bsmall racemose bubbles resembling lipoblasts, c silicone vacuoles surrounding salivary mucous gland acini (arrow on salivary acinar cluster), d stromal cells are CD68 positive and e S-100 negative; positively labeled melanocytes and dendritic cells are seen in the overlying epithelium
Discussion
A variety of other cosmetic dermal fillers have been reported to induce foreign body reactions in skin yet were not encountered in our archived files. Table 4 compares and contrasts foreign body features as well as host response reactions. Natural fillers such as collagen and hyaluronic acid, as evidenced in animal studies, are resorbed by macrophages and/or giant cells with progressive elimination of the material that will result in clinically observable shrinkage over time [1]. Why some patients react with an exuberant increase in size and nodular granuloma formation is unknown yet probably involves numerous variables such as continued trauma, rubbing or irritation, iatrogenic factors, infection, immunogenic mechanisms from protein contaminants or genetic and molecular variations in host response. Particle surface roughness as well as surface chemistry probably initiate heterogeneous host responses to these materials [1, 3, 4].
Table 4.
Histologic differential diagnosis of dermal filler foreign body reactions
| Eosinophilic coagulum |
| Cross linked collagens, human and bovine |
| Basophilic lakes |
| All Hyaluronic acid polymers |
| Spheroid filler particles |
| Hyaluronate polymers with hydroxyapatite |
| Broken-glass like particles |
| Poly-L-lactate, refractile |
| Hydroxyethylmethacrylate, nonrefractile |
Recognition and identification of augmentation materials microscopically is important because granuloma formation to foreign materials may simulate an inflammatory reaction to a specific microbial infection. The host response to the fillers reported here have been categorized into three microscopic reactions including: (1). a nodular retention of filler without an inflammatory cell infiltrate, (2). a nodule with nonspecific chronic inflammation, (3). an epithelioid histiocytic/giant cell granulomatous reaction. The foreign material is unique for each augmentation filler, dependent upon its morphology and chemical makeup.
Cross linked collagen is an amorphous acellular material being eosinophilic, appearing as proteinacious pools [7, 8]. A host response is nonexistent, showing only a thin fibrous capsule unless a true allergic reaction has occurred. Fibrin and amyloid deposition should be considered in the differential diagnosis. We only identified one case of bovine collagen, a substance that differs morphologically from human collagen; in the former no structure is evident whereas in human collagen the fiber morphology is retained. The picture seen with hyaluronic acid fillers is typically represented by small lakes of basophilic material [9–11]. One of the cases in this series showed features that could be confused with a salivary neoplasm (papillary cystadenoma, low grade mucoepidermoid carcinoma).
Hydroxyapatite granulomas are distinctive in that the foreign body reaction is directed to the characteristic spheroids present in these preparations. Hyaluronate lakes are also present and the glycosaminoglycan is digestible with hyaluronidase as evidenced by lack of staining with alcian blue [12].
Many reports of silicone granuloma have been reported. The characteristic morphology is that of a lipoblastic lesion which has been mistaken for a low grade, well differentiated liposarcoma. Clear vacuoles of varying size are encountered yielding a bubbly appearance with interposed histiocytes and multinucleated cells. Over time, fibrosis evolves with minimal vacuolar features [13–17]. The nuclei are round, small and monomorphic and immunohistochemical markers depict histiocytic differentiation. When lipsosarcoma is a consideration, S-100 protein negativity may be helpful.
Lactate polymers are also unique showing a classic foreign body granuloma (FBG 3) with sheets of histiocytes and multinucleated giant cells surrounding “surf board” shaped large vacuoles that contain “broken glass-like particles” that are refractile to polarized light [17, 18]. Conversely, hydroxyethylmethacrylate induces a foreign body reaction, also with vacuoles containing “broken glass” like particles yet these particles fail to exhibit birefringence. The most common substance seen in this series was hydroxyapatite with it’s distinctive spheroids [12]. Less commonly used fillers have also been reported to induce foreign body reactions [19–22].
In reactions to natural polymers, the implanted material is ultimately resorbed and is considered to be a temporary procedure for most patients, thereby requiring periodic readministration. In patients presenting with nodules at or in proximity to injection sites, clinical observation, simple excision or enucleation is the treatment of choice taking care to ensure a cosmetic/esthetic outcome [1, 5].
Conclusions
Dermal filler injectables display distinctive histomorphology. Similarly, the host response generated to the foreign material among sensitive patients is, for the most part, unique for each material. All of these resorbable molecules rely on resident macrophages and emigrating monocytes with epithelioid features; many of these reactions are accompanied by multinucleated giant cells. There are unique variations in the morphology of the foreign material itself as well as the patterns of host reactivity that have been compiled herein, thereby allowing for substance recognition.
Excision or enucleation is the treatment of choice. Smaller nodules with a history of prior injection augmentation can be subjected to excisional biopsy, whereas larger or lobulated masses with no prior history of augmentation can be subjected to incisional biopsy to rule out a neoplastic process.
References
- 1.Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2003;27(5):354–367. doi: 10.1007/s00266-003-3022-1. [DOI] [PubMed] [Google Scholar]
- 2.Alster TS, West TB. Human-derived and new synthetic injectable materials for soft-tissue augmentation: current status and role in cosmetic surgery. Plast Reconstr Surg. 2000;105:2515. doi: 10.1097/00006534-200006000-00034. [DOI] [PubMed] [Google Scholar]
- 3.Smith KC. Reversible versus nonreversible fillers in facial aesthetics: concerns and considerations. Dermatol Online J. 2008;14:3. [PubMed] [Google Scholar]
- 4.Johl SS, Burgett RA. Dermal filler agents: a practical review. Curr Opin Ophthalmol. 2006;17:471–479. doi: 10.1097/01.icu.0000243021.20499.4b. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch RJ, Stier M. Complications of soft tissue augmentation. J Drugs Dermatol. 2008;7:841–845. [PubMed] [Google Scholar]
- 6.Salles AG, Lotierzo PH, Gemperli R, Besteiro JM, Ishida LC, Gimenez RP, Menezes J, Ferreira MC. Complications after polymethylmethacrylate injections: report of 32 cases. Plast Reconstr Surg. 2008;121:1811–1820. doi: 10.1097/PRS.0b013e31816b1385. [DOI] [PubMed] [Google Scholar]
- 7.Cockerham K, Hsu VJ. Collagen-based dermal fillers: past, present, future. Facial Plast Surg. 2009;25:106–113. doi: 10.1055/s-0029-1220650. [DOI] [PubMed] [Google Scholar]
- 8.Anderson TD, Sataloff RT. Complications of collagen injection of the vocal fold: report of several unusual cases and review of the literature. J Voice. 2004;18:392–397. doi: 10.1016/j.jvoice.2002.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Aceñero MJ, Zamora E, Borbujo J. Granulomatous foreign body reaction against hyaluronic acid: report of a case after lip augmentation. Dermatol Surg. 2003;29:1225–1226. doi: 10.1111/j.1524-4725.2003.29392.x. [DOI] [PubMed] [Google Scholar]
- 10.Lombardi T, Samson J, Plantier F, Husson C, Küffer R. Orofacial granulomas after injection of cosmetic fillers. Histopathologic and clinical study of 11 cases. J Oral Pathol Med. 2004;33(2):115–120. doi: 10.1111/j.1600-0714.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- 11.Edwards PC, Fantasia JE, Iovino R. Foreign body reaction to hyaluronic acid (Restylane): an adverse outcome of lip augmentation. J Oral Maxillofac Surg. 2006;64:1296–1299. doi: 10.1016/j.joms.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Daley T, Damm DD, Haden JA. Kolodychak MT:oral lesions associated with injected hydroxyapatite cosmetic filler. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:107–111. doi: 10.1016/j.oooo.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Bigatà X, Ribera M, Bielsa I, Ferrándiz C. Adverse granulomatous reaction after cosmetic dermal silicone injection. Dermatol Surg. 2001;27(2):198–200. doi: 10.1046/j.1524-4725.2001.00020.x. [DOI] [PubMed] [Google Scholar]
- 14.Ficarra G, Mosqueda-Taylor A, Carlos R. Silicone granuloma of the facial tissues: a report of seven cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(1):65–73. doi: 10.1067/moe.2002.124459. [DOI] [PubMed] [Google Scholar]
- 15.Maly A, Regev E, Meir K, Maly B. Tissue reaction to liquid silicone simulating low-grade liposarcoma following lip augmentation. J Oral Pathol Med. 2004;33:314. doi: 10.1111/j.0904-2512.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 16.Fulton JE, Jr, Porumb S, Caruso JC, Shitabata PK. Lip augmentation with liquid silicone. Dermatol Surg. 2005;31:1577–1585. doi: 10.2310/6350.2005.31244. [DOI] [PubMed] [Google Scholar]
- 17.Jham BC, Nikitakis NG, Scheper MA, Papadimitriou JC, Levy BA, Rivera H. Granulomatous foreign-body reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280–285. doi: 10.1016/j.joms.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 18.Dionyssopoulos A, Nikolis A, Patsatsi A, Sotiriadis D. Granulomas of the lips: a rare complication after injection of polylactic acid for aesthetic augmentation. J Plast Reconstr Aesthet Surg. 2007;60:1079–1080. doi: 10.1016/j.bjps.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Akrish S, Dayan D, Taicher S, Adam I, Nagler RM. Foreign body granulomas after injection of bio-alcamid for lip augmentation. Am J Otolaryngol. 2009;30:356–359. doi: 10.1016/j.amjoto.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Hubmer MG, Hoffmann C, Popper H, Scharnagl E. Expanded polytetrafluoroethylene threads for lip augmentation induce foreign body granulomatous reaction. Plast Reconstr Surg. 1999;103(4):1277–1279. doi: 10.1097/00006534-199904040-00029. [DOI] [PubMed] [Google Scholar]
- 21.Mercer SE, Kleinerman R, Goldenberg G, Emanuel PO. Histopathologic identification of dermal filler agents. J Drugs Dermatol. 2010;9:1072–1078. [PubMed] [Google Scholar]
- 22.Requena L, Requena C, Christensen L, Zimmermann US, Kutzner H, Cerroni L. Adverse reactions to injectable soft tissue fillers. J Am Acad Dermatol. 2011;64:1–34. doi: 10.1016/j.jaad.2010.02.064. [DOI] [PubMed] [Google Scholar]