Abstract
We describe the clinical and radiographic characteristics of fungus balls in the bilateral paranasal sinuses. The medical records of 8 of 245 patients with fungus balls of the bilateral paranasal sinuses between 2000 and 2010 were retrospectively reviewed. The incidence of bilateral paranasal sinus fungus balls was 3.3%. Fungus balls were located in the maxillary sinuses bilaterally in 4 cases (50%), followed by the maxillary sinus and contralateral sphenoid sinus in 3 cases (37.5%), and the sphenoid sinuses bilaterally in 1 case (12.5%). There were no predisposing anatomic variations for the occurrence of bilateral paranasal fungus balls. Although the presenting symptoms and signs were non-specific, CT findings were helpful in the diagnosis of bilateral fungus balls. Endonasal removal by an endoscopic approach was performed in all patients. No peri-operative complications or recurrences were noted. Fungus balls in the bilateral paranasal sinuses are most frequently found in the maxillary sinuses bilaterally. Because symptoms of bilateral paranasal fungus balls and findings on nasal endoscopic examination are frequently non-specific, a high index of suspicion is needed and imaging studies, such as CT, are essential to establish the correct pre-operative diagnosis.
Keywords: Fungus disease, Sinusitis, Bilateral, Paranasal sinuses, Endoscopic surgical procedure
Introduction
The fungus ball of the paranasal sinuses is a non-invasive form of fungal sinusitis occurring in immunocompetent hosts [1]. Fungus balls (FBs) are characterized by a mass of inspissated fungal debris and mucus progressively growing into the sinus cavity without invasion of the underlying mucosa [2]. FBs are usually found in only one sinus; FBs are most frequently found in the maxillary sinus [3]. Multiple sinus involvement by FBs has been reported to occur in 6.4% of cases in the largest review of this disease entity [3]. Although FBs can be involved in multiple sinuses, these sinuses are mostly contiguous. Bilateral paranasal sinus involvement is rare [3–5], which has not been described in detail.
In this study, we report the largest case series with respect to clinical and radiographic characteristics and treatment outcomes of FBs of the bilateral paranasal sinuses.
Patients and Methods
After obtaining Institutional Review Board (IRB) approval, we identified 245 patients with paranasal sinus FBs who were treated at Chonnam National University Hostpital and Hwasun Hospital between January 2000 and October 2010. Among the 245 patients, 8 had FBs of the bilateral paranasal sinuses and the medical records were reviewed retrospectively. The data collected included medical and surgical histories, presenting symptoms, physical examination findings, radiographic findings, complications, and surgical outcome.
Surgery was performed via an endoscopic approach. When the FBs were localized in the maxillary sinus, a uncinectomy and maxillary antrostomy were performed, and all of the fungal debris was removed. A sphenoidotomy was performed via the transnasal or transethmoidal approach. The fungal materials were removed and sent for pathologic examination, and fungus cultures were performed if necessary. The nasal cavities were packed with Merocel (Medtronic, Jacksonville, FL, USA), which was removed on post-operative day 1.
Results
Eight patients (4 males and 4 females) were included in the study and 16 isolated sinuses were affected. The age of the patients ranged between 42 and 69 years, with a mean of 59.3 years. The underlying diseases included diabetes, hypertension, and lung cancer in 3 patients. The incidence of bilateral paranasal sinus FBs was 3.3%.
Patients presented with a chief complaint of purulent rhinorrhea (75%), nasal obstruction (38%), or headaches (25%). Endoscopic findings of the middle or superior meatus revealed polyps, discharge, or swelling in 9 sides; abnormal findings were not shown in 7 sides. All patients underwent pre-operative computed tomography (CT) of the paranasal sinuses (Fig. 1). Bilateral maxillary sinuses were the most common pattern of involvement (4 of 8 patients [50%]), followed by the maxillary sinus and contralateral sphenoid sinus (3 of 8 patients [37.5%]), and bilateral sphenoid sinuses (1 of 8 cases [12.5%]). The CT findings of maxillary FBs included microcalcifications (8 of 11 involved sinuses [73%]), diffuse thinning of the medial wall (1 of 11 involved sinuses [9%]), and sinus wall sclerosis (7 of 11 involved sinuses [64%]). The CT findings of sphenoid FBs included sinus wall sclerosis (5 of 5 involved sinuses [100%]) focal erosions (3 of 5 involved sinuses [60%]), and calcifications (3 of 5 involved sinuses [60%]). The anatomic variations associated with FBs were examined. Septal deviation and concha bullosa were noted in each 3 of 8 patients, but these variations were not related to the development of maxillary FBs. Despite no evidence of endodontic treatment, all five dentulous patients had FBs, which may suggest low probability of the causal relationship between endodontic treatment and FB.
Fig. 1.
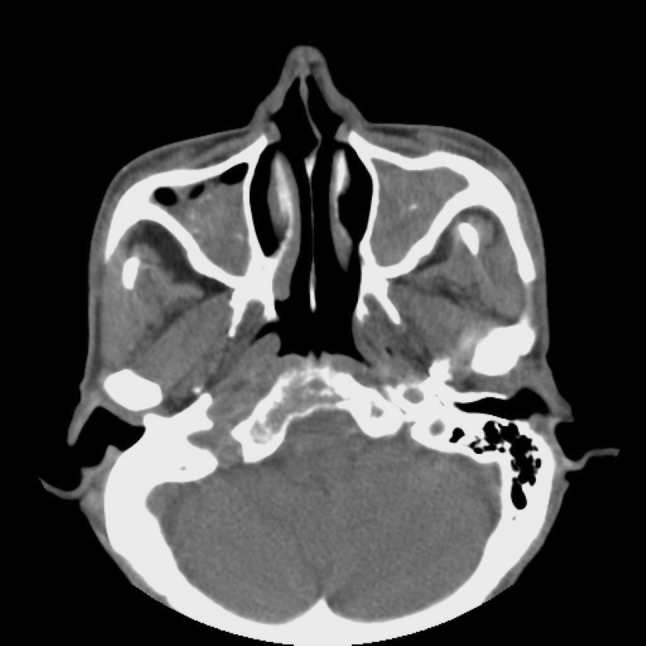
A CT scan shows a typical appearance of fungus ball in both maxillary sinuses (microcalcifications and sinus wall sclerosis)
All patients underwent removal of FBs through an endoscopic approach. A middle meatal antrostomy and removal of the hyphal mass was performed in maxillary FBs and a transnasal approach was performed in all patients with sphenoid FBs. Histopathologic examination of the specimens demonstrated Aspergillus species in all cases. Fungal cultures were performed in two cases, but there was no fungal growth. No peri-operative complications were noted, and there were no recurrences during the follow-up, which ranged from 8 to 61 months (mean, 30 months). The involved sinuses and clinical summary are shown in Table 1.
Table 1.
Summary of fungus balls of the bilateral paranasal sinsuses
| Case | Age/gender | Underlying diseases | Involved site (R/L) | Septal deviation | Concha bullosa | Endodontic treatment | Follow-up (months) |
|---|---|---|---|---|---|---|---|
| 1 | 69/M | DM, HTN | M/S | Left side | None | Edentulous | NED (53) |
| 2 | 63/F | DM, HTN | S/S | None | None | None | NED (61) |
| 3 | 50/M | None | S/M | None | Both | None | NED (38) |
| 4 | 64/F | None | S/M | None | None | Edentulous | NED (37) |
| 5 | 62/M | None | M/M | Left side | None | None | NED (12) |
| 6 | 57/F | None | M/M | None | None | None | NED (10) |
| 7 | 42/F | None | M/M | Left side | Both | None | NED (24) |
| 8 | 67/M | Lung ca | M/M | None | None | None | NED (8) |
FB fungus ball, DM diabetes, HTN hypertension, M maxillary sinus, S sphenoid sinus, NED no evidence of disease
Discussion
Paranasal sinus fungal infection is an uncommon disease; however, the incidence may be increasing for a number of reasons, including better detection through endoscopic and radiologic evaluation and longer life expectancies [6]. FBs are an extramucosal fungal proliferation usually occurring in immunocompetent individuals as a maxillary sinus involvement caused by Aspergillus species [4]. Because FBs are usually unilateral paranasal sinus lesions, bilateral involvement is rare. In the largest series involving FBs of the paranasal sinuses, 2 of 109 patients (1.8%) had bilateral maxillary sinus involvement [3]. Nicholai et al. [2] and Ferreiro et al. [5] reported 2 of 160 patients (1.25%) and 1 of 29 patients (3.4%) with bilateral paranasal sinuses involvement, respectively. In our study, 8 of 245 patients (3.3%) had bilateral paranasal sinuses involvement.
The pathogenesis of FBs is unknown. A possible contributing factor of FBs may be ostio-meatal complex closure with development of an anaerobic environment that is a favorable condition for fungus growth [3]. However, Tsai et al. [7] reported that maxillary FBs are not associated with ostio-meatal complex obstruction. Another theory is that endodontic treatment with root-filling materials containing zinc oxide-eugenol has been reported to promote the growth of Aspergillus fumigates [8]. However, this theory does not explain the occurrence of FBs in the ethmoid, sphenoid, or frontal sinuses, or in the maxillary sinus in the absence of a history of dental treatment. In review of our cases, anatomic variations, such as septal deviation, concha bullosa, and endodontic treatment were not related to the development of FBs.
Symptoms of FBs are non-specific and may be similar to chronic rhinosinusitis. Klosseck et al. [3] reported that nasal obstruction was the most common symptom and Robey et al. [6] reported that the top three symptoms of FBs were headaches, nasal obstruction, and mucus discharge. In agreement with previous reports [3, 6], purulent rhinorrhea, nasal obstruction, and headaches were the presenting symptoms in this study. On endoscopic examination, identification of gritty or cheesy and fragmented clay-like material was a reliable finding in the diagnosis of FBs, but there were no cases in which fungal material was noted in this study. Instead, non-specific findings, such as polyps and discharge, were noted in 9 of 16 sides with lesions. In our study, endoscopic examination revealed normal findings in 7 of 16 sides with lesions, which makes the diagnosis of bilateral paranasal sinus FBs difficult. Therefore, the diagnosis of bilateral fungal sinus lesions mainly depends on imaging studies, such as CT.
On CT images of FBs, the partial or irregularly calcified lesions, heterogeneous opacities in the paranasal sinus, or partial bone erosions were found frequently [9]. In our study, CT scans showed microcalcifications within the sinus cavity in 11 of 16 involved sinuses (69%), which is similar to the results of a previous report [2]. The calcification patterns of maxillary FBs on CT are different from the calcification patterns of chronic sinusitis with respect to location [10]. The location of the calcifications was central in 95% of maxillary FBs and peripheral near the sinus wall in 81% of cases of non-fungal sinusitis. Punctuate micro-calcifications occurred were most frequently in maxillary FBs, and in contrast, smooth marginated calcifications occurred more commonly in maxillary non-fungal sinusitis [10]. Sinus wall sclerosis was one of the common findings and the presence of both calcifications and sinus wall sclerosis was highly suggestive of bilateral maxillary FBs. The CT findings of sphenoid FBs was somewhat different from of the CT findings of maxillary FBs; specifically, sinus wall sclerosis was the most common finding on CT of maxillary FBs. In contrast to maxillary FBs, focal bony erosions appeared to be more common in sphenoid FBs, which may be attributable to expansion of FBs within the relatively small sphenoid sinus cavity.
Histopathologic examination, by assessing the presence of fungal hyphae in the specimens and excluding mucosal invasion, is essential for the diagnosis of FBs [2]. FBs are usually caused by Aspergillus species [4]. In our study, histopathologic examination of FBs with bilateral paranasal sinuses demonstrated Aspergillus species in all cases. Fungal culture is useful to identify fungal species, but failure of the fungus to grow on fungal culture is common. The treatment of choice for FBs of the paranasal sinuses is complete removal of FBs from the involved sinus to re-establish proper ventilation and drainage [11]. The endoscopic approach has been used as the primary treatment for FBs of the paranasal sinuses and has the advantage for easy access to the affected sinus, perfect visualization into the sinus, and low morbidity [1]. Our patients underwent endoscopic treatment and had excellent treatment outcomes without recurrence.
Conclusion
FBs of the bilateral paranasal sinuses are a rare presenting form of paranasal FBs. Because of non-specific symptoms and frequent negative endoscopic findings, a high index of suspicion is required in the diagnosis of bilateral paranasal FBs. To establish the correct pre-operative diagnosis, imaging studies, such as a CT scan, are essential.
Key Messages
The incidence of bilateral paranasal sinus fungus balls was 3.3%.
Fungus balls in the bilateral paranasal sinuses are most frequently found in the maxillary sinuses bilaterally.
Because symptoms of bilateral paranasal fungus balls and findings on nasal endoscopic examination are frequently non-specific, a high index of suspicion is needed and imaging studies, such as CT, are essential to establish the correct pre-operative diagnosis.
Contributor Information
Dong Hoon Lee, Email: leen3l@hanmail.net.
Sang Chul Lim, Phone: +82-62-220-6776, FAX: +82-62-226-6369, Email: limsc@chonnam.ac.kr.
References
- 1.Dufour X, Kauffmann-Lacroix C, Ferrie JC, Goujon JM, Rodier MH, Karkas A, et al. Paranasal sinus fungus ball and surgery: a review of 175 cases. Rhinology. 2005;43:34–39. [PubMed] [Google Scholar]
- 2.Nicolai P, Lombardi D, Tomenzoli D, Villaret AB, Piccioni M, Mensi M, et al. Fungus ball of the paranasal sinuses: experience in 160 patients treated with endoscopic surgery. Laryngoscope. 2009;119:2275–2279. doi: 10.1002/lary.20578. [DOI] [PubMed] [Google Scholar]
- 3.Klossek JM, Serrano E, Péloquin L, Percodani J, Fontanel JP, Pessey JJ, et al. Functional endoscopic sinus surgery and 109 mycetomas of paranasal sinuses. Laryngoscope. 1997;107:112–117. doi: 10.1097/00005537-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 4.deShazo RD, O’Brien M, Chapin K, Soto-Aguilar M, Swain R, Lyons M, et al. Criteria for the diagnosis of sinus mycetoma. J Allergy Clin Immunol. 1997;99:475–485. doi: 10.1016/S0091-6749(97)70073-3. [DOI] [PubMed] [Google Scholar]
- 5.Ferreiro JA, Carlson BA, Cody DT., 3rd Paranasal sinus fungus balls. Head Neck. 1997;19:481–486. doi: 10.1002/(SICI)1097-0347(199709)19:6<481::AID-HED4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Robey AB, O’Brien EK, Richardson BE, Baker JJ, Poage DP, Leopold DA. The changing face of paranasal sinus fungus balls. Ann Otol Rhinol Laryngol. 2009;118:500–505. doi: 10.1177/000348940911800708. [DOI] [PubMed] [Google Scholar]
- 7.Tsai TL, Guo YC, Ho CY, Lin CZ. The role of ostiomeatal complex obstruction in maxillary fungus ball. Otolaryngol Head Neck Surg. 2006;134:494–498. doi: 10.1016/j.otohns.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Legent F, Billet J, Beauvillain C, Bonnet J, Miegeville M. The role of dental canal fillings in the development of aspergillus sinusitis. A report of 85 cases. Arch Otorhinolaryngol. 1989;246:318–320. doi: 10.1007/BF00463584. [DOI] [PubMed] [Google Scholar]
- 9.Roithmann R, Shankar L, Hawke M, Chapnik J, Kassel E, Noyek A. Diagnostic imaging of fungal sinusitis: eleven new cases and literature review. Rhinology. 1995;33:104–110. [PubMed] [Google Scholar]
- 10.Yoon JH, Na DG, Byun HS, Koh YH, Chung SK, Dong HJ. Calcification in chronic maxillary sinusitis: comparison of CT findings with histopathologic results. AJNR Am J Neuroradiol. 1999;20:571–574. [PMC free article] [PubMed] [Google Scholar]
- 11.Dufour X, Kauffmann-Lacroix C, Ferrie JC, Goujon JM, Rodier MH, Klossek JM. Paranasal sinus fungus ball: epidemiology, clinical features and diagnosis. A retrospective analysis of 173 cases from a single medical center in France, 1989–2002. Med Mycol. 2006;44:61–67. doi: 10.1080/13693780500235728. [DOI] [PubMed] [Google Scholar]


