Abstract
Autosomal-dominant diffuse nonepidermolytic palmoplantar keratoderma is characterized by the adoption of a white, spongy appearance of affected areas upon exposure to water. After exome sequencing, missense mutations were identified in AQP5, encoding water-channel protein aquaporin-5 (AQP5). Protein-structure analysis indicates that these AQP5 variants have the potential to elicit an effect on normal channel regulation. Immunofluorescence data reveal the presence of AQP5 at the plasma membrane in the stratum granulosum of both normal and affected palmar epidermis, indicating that the altered AQP5 proteins are trafficked in the normal manner. We demonstrate here a role for AQP5 in the palmoplantar epidermis and propose that the altered AQP5 proteins retain the ability to form open channels in the cell membrane and conduct water.
Main Text
Inherited palmoplantar keratodermas (PPKs) are characterized by thickening of the stratum corneum (hyperkeratosis) on the palms and soles and have been grouped into diffuse, focal, and punctate forms according to the clinical appearance.1 PPKs can be further subdivided histologically into epidermolytic and nonepidermolytic forms depending on the presence of cytolysis in the upper spinous and granular layers.
An autosomal-dominantly inherited form of diffuse nonepidermolytic PPK (NEPPK [MIM 600231]) was mapped in Swedish and UK families to chromosomal region 12q11–q13.2–4 Diffuse NEPPK in these families usually manifests during childhood with a diffuse, even, hyperkeratosis with a yellowish tint over the whole of the palms and soles and sometimes extends onto the dorsal digits (Figure 1A). The phenotype varies, and milder cases are barely detectable clinically but have a typical white spongy appearance of affected areas upon exposure to water (Figure 1B). Fungal infection, probably related to an increase in sweating, is a frequent problem, and nails are curved and have ragged cuticles. This condition is distinct from aquagenic keratoderma, which is characterized by the development of translucent papules induced by water exposure and has been associated with cystic fibrosis.5
Figure 1.
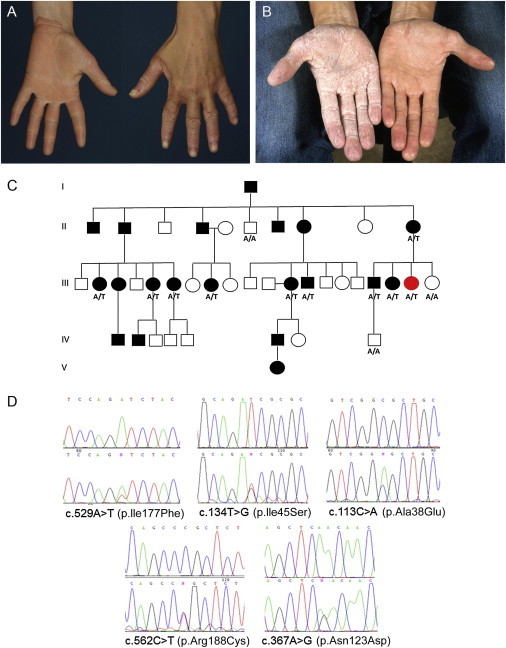
Mutations in AQP5 Underlie Autosomal-Dominant Diffuse NEPPK
(A) The clinical phenotype of a British individual affected by diffuse NEPPK (with the c.529A>T mutation in AQP5) shows some transgradiens onto the dorsal surface of the fingers and a livid, red edge.
(B) The clinical phenotype of a Swedish individual affected by diffuse NEPPK (with the c.113C>A mutation in AQP5). The hand on the right shows palmar skin with mild diffuse hyperkeratosis prior to water exposure. The hand on the left shows the white, spongy appearance of the palmar skin after 15 min of water exposure.
(C) The pedigree of the British family (affected by the c.529A>T mutation) shows how the mutant AQP5 allele segregates with disease in this family. Genotypes were confirmed in available individuals by Sanger sequencing: A/A represents two wild-type alleles, and A/T represents one mutant allele and one wild-type allele. The individual highlighted in red was used for exome sequencing.
(D) Sanger sequence traces of the five missense mutations identified in AQP5 in families affected by diffuse NEPPK.
Using the linkage data in combination with exome sequencing followed by Sanger sequencing, we have now identified missense mutations in AQP5, encoding the water-channel protein aquaporin-5 (AQP5), as the underlying cause of diffuse NEPPK. This study was approved by the research ethics committees of the National Health Service (08/H1102/73) and Umeå University, and informed consent was obtained from all participants. With the use of the SureSelect system for target enrichment (Agilent Technologies) and 2× 100 bp paired-end sequencing on the HiSeq system (Illumina) with a mean read depth of 46, exome sequencing of a single affected individual revealed missense mutation c.529A>T (p.Ile177Phe) in exon 3 of AQP5 (RefSeq accession number NM_001651.2) in a large British family affected by diffuse NEPPK (Figure 1C and Figure S1, available online). Using Sanger sequencing, we identified a further five missense mutations in AQP5 in families affected by diffuse NEPPK (see Figure 1D and Figure S2 for examples of other family pedigrees). Two families of British descent were found to be affected by the same mutation, c.134T>G (p.Ile45Ser) in exon 1, and a founder mutation, c.113C>A (p.Ala38Glu), also in exon 1, was identified in affected individuals sharing the same haplotype4 from seven Swedish families. An additional mutation, c.562C>T (p.Arg188Cys) in exon 3, was identified in one of the Swedish families in affected individuals displaying an alternative haplotype (Figure S2). A family in Scotland was found to harbor mutation c.367A>G (p.Asn123Asp) in exon 2. These variants were all found to segregate with the disease, and all are absent from dbSNP and 1000 Genomes.
Aquaporins are a family of cell-membrane proteins that allow the osmotic movement of water across the cell membrane independently of solute transport.6 Aquaporins can be subdivided into two groups: (1) aquaporins, which are only permeated by water, and (2) aquaglyceroporins, which also allow the passage of other small solutes, in particular, glycerol. AQP5 is a member of the former subgroup and has high water permeability. AQP5 is distributed in the apical plasma membrane and is known as an exocrine-type water-channel protein because of its involvement in the excretion of water from exocrine glands, such as the salivary gland, lacrimal gland, and sweat gland. However, AQP5 is also found in epithelial cells of the lung and the cornea, and so we investigated its localization in the palmar epidermis.
We performed immunohistochemistry to compare the localization of AQP5 between control and affected palmar-skin sections. Frozen skin sections were fixed with a methanol-acetone mixture (50:50), blocked for 30 min in 3% BSA, and stained by overnight incubation at 4°C with rabbit monoclonal AQP5 antibody (ab92320, Abcam) diluted 1:100 in 3% BSA. They were subsequently stained for 1 hr at room temperature with Alexa-Fluor-488 nm goat-anti-rabbit secondary antibody, diluted 1:800 in PBS. Images were taken with the Leica DFC350 epifluorescence microscope. AQP5 was strongly localized to the plasma membrane in the keratinocytes of the stratum granulosum (Figures 2A–2C and Figure S3; see Figure S4A for antibody specificity). The levels of AQP5 in the palmar epidermis were much lower than those seen in cells of the sweat glands (Figure S3B). Localization of AQP5 at the plasma membrane of the cells of the upper layers of the epidermis was retained in the diffuse NEPPK palmar sections (Figure 2B and Figure S3B).
Figure 2.
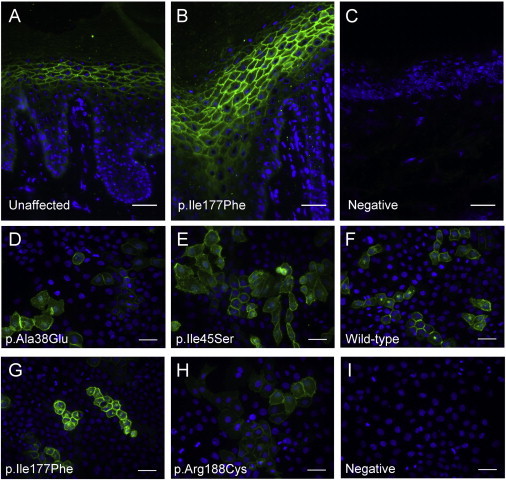
AQP5 Variants Appear to Demonstrate Normal Trafficking to the Plasma Membrane
(A–C) Immunofluorescent staining of AQP5 with an antibody raised against the C terminus of human AQP5 (green) in frozen sections from an unaffected, control palm (A) and a British diffuse NEPPK (with the p.Ile177Phe substitution) palm (B). The primary antibody was omitted in the negative control (C). Nuclei were counterstained with DAPI (blue). AQP5 appears to be present throughout the palmar epidermis and shows strong localization to the plasma membrane of the keratinocytes of the stratum granulosum. A similar AQP5 distribution is seen in the diffuse NEPPK palm compared to the unaffected control.
(D–I) Monolayers of Neb1 HpV-immortalized keratinocyte cells transfected with constructs expressing wild-type or variant AQP5 and then fixed in 4% paraformaldehyde and stained with rabbit monoclonal AQP5 antibody (ab92320, green). The levels of cell-membrane localization in all four AQP5 variants tested—p.Ala38Glu (D), p.Ile45Ser (E), p.Ile177Phe (G), and p.Arg188Cys (H)—are similar to that seen for wild-type AQP5 (F). Primary antibody was omitted in the negative control (I). Nuclei are counterstained with DAPI (blue).
Scale bars represent 40 μm.
To investigate four of the AQP5 variants in comparison to wild-type AQP5, we performed site-directed mutagenesis on an untagged human AQP5 cDNA clone (Origene) with the QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies). Wild-type and mutant AQP5 constructs were overexpressed in Neb1 cells, an HpV-immortalized keratinocyte cell line, by transfection with FuGENE 6 (Promega) at a FuGENE-6-to-DNA ratio of 3 to 1. Cells were fixed with 4% paraformaldehyde 72 hr after transfection, and immunofluorescent staining was performed with rabbit monoclonal AQP5 antibody (ab92320) as detailed above. Compared to wild-type AQP5, all four AQP5 variants studied (p.Ala38Glu, p.Ile45Ser, p.Ile177Phe, and p.Arg188Cys) showed similar levels of localization in the plasma membrane (Figures 2D–2I). Thus, these AQP5 variants seem to retain the ability to traffic to the cell membrane.
More than a dozen aquaporin structures have been solved at medium-to-high resolution by X-ray or electron crystallography,7,8 and the conformation of these proteins is fairly well understood. Aquaporins share a common protein fold with six transmembrane alpha helices (TMHs) and their amino and carboxy termini in the cytosol (Figure S5A). Two short half helices abut in the center of the membrane to form a virtual seventh TMH that is critical to the selectivity of the channel. Each half helix begins in the center of the membrane with an asparagine, proline, and alanine (the NPA motif). The dipole moments created by the half helices within the membrane electrostatically repulse protons and ensure the selectivity of the channel for water without dissipation of the proton gradient.9 Human AQP5 has been crystallized as a tetramer (Figure S5B), which is considered the relevant physiological complex of aquaporins.10 The water molecules move through the channel formed by each of the four aquaporin monomers rather than through the central pore of the aquaporin tetramer, which is blocked by a lipid molecule. The aromatic and arginine (ar/R) constriction point where each channel narrows to an average 2.04 Å diameter is defined by Arg188 in half helix “E,” Phe49 in TMH2, and His173 in TMH5 and is located one turn of their respective helices extracellular to the NPA motif of half helix “E” (Figure 3A and Figures S5A and S5C). The ar/R constriction point is an energy barrier to conduction and a determinant of specificity for water.
Figure 3.
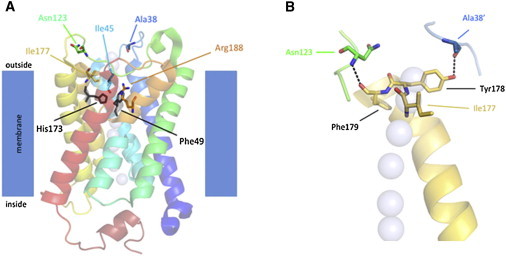
The Five AQP5 Variants Most Likely Affect the Water Conductance of the AQP5 Channel
(A) A side view of the AQP5 monomer shows the half helices formed by loops B and E. The cartoon is transparent so that the side chains of the residues substituted in diffuse NEPPK can be visualized. Residues Ile45, Ile177, and Arg188 line the water channel one helical turn extracellular to the ar/R constriction point, which is defined in AQP5 by Arg188, Phe49, and His173. Residues Ala38 and Asn123 are located on the extracellular surface of AQP5.
(B) A close-up view of the tripeptide consisting of Ile177, Tyr178, and Phe179 of the extracellular end of helix 5 (gold) shows the intramolecular hydrogen bond formed with Asn123 in extracellular loop C (green) and the intermolecular hydrogen bond formed with Ala38′ in extracellular loop A′ (blue) of the adjacent monomer. The intrachannel water molecules (which are in the foreground) are shown as light-blue transparent spheres. Structural models were generated in MacPyMOL (Delano Scientific). Despite the fact that the AQP5 variants display a wide distribution of the primary sequence of AQP5, they are clustered in the tertiary structure of the protein and either line the extracellular end of the water channel or are situated in extracellular loops important for stabilizing the tertiary structure of AQP5.
The five AQP5 variants described here are distributed widely in the primary sequence of the aquaporin channel (Figure S5A) but clustered in the tertiary structure of the aquaporin monomer (Figure 3A and Figure S5C). Three of the residues substituted in diffuse NEPPK—Ile45, Ile177, and Arg188—line the extracellular end of the water channel, and Ala38 and Asn123 are located on the extracellular surface of AQP5. With protein modeling, it is tempting to speculate that the amino acid residues (Ile45, Ile177, and Arg188) that are substituted in diffuse NEPPK and that line the extracellular end of the water channel are likely to have a direct impact on water conductance. Arg188 is a key component of the ar/R constriction point in the water channel and is the most highly conserved of all the AQP5 amino acid variants identified in diffuse NEPPK, and the simplest interpretation of substitution by the smaller cysteine is the widening of the channel at its narrowest point. Ile45 and Ile177 are one turn (of helices 2 and 5, respectively) extracellular to the ar/R constriction point, and we speculate that replacing Ile45 with the smaller serine might also widen the diameter of the constriction point and that replacing Ile177 with the bulkier phenylalanine might have an effect on the diameter of the channel via steric hindrance.
For the substituted residues (Ala38 and Asn123) located on the extracellular surface of AQP5, the explanation is more complicated, but the extracellular loops in which both are situated appear important to stabilize the tertiary (and possibly quaternary) structure of AQP5. The peptide backbone of Ala38′ (from the adjacent monomer) and the intramolecular interaction of the backbone of Asn123 form hydrogen bonds with residues in helix 6 (Tyr178 and Phe179, respectively; Figure 3B). The positions of both Ala38 and Asn123 in the tertiary structure of AQP5 would be expected to be altered if these residues are replaced with charged side chains, as is the case with the p.Ala38Glu and p.Asn123Asp substitutions, and so the position of helix 5 is likely to change in these altered proteins. Because helix 5 also contains His173 of the ar/R constriction point and Ile177, which is changed to Phe in another diffuse NEPPK substitution, this would imply that Ala38 and Asn123 are also critical to water conductance despite their being located outside the channel itself.
Recent molecular-dynamics simulations have revealed a gating mechanism for AQP5 and highlighted the importance of the constriction point for the regulation of water flow through AQP5 in its open state.11 Therefore, the diffuse NEPPK variants could have a direct influence on AQP5 gating and/or water flow through the channel, but further simulations and functional data are required for testing the modeling. Given this protein-structure modeling, the immunhistochemical data, and the diffuse NEPPK phenotype, we propose that the variant AQP5 monomers retain the ability to form open channels and conduct water and are thus likely to represent gain-of-function alleles.
In addition to playing a role in transcellular water transport, aquaporin proteins have alternative functions.12–14 AQP5 can regulate paracellular permeability in airway epithelial cells via a mechanism involving microtubule stabilization.15 When levels of AQP5 were reduced, lower levels of microtubule assembly and decreased paracellular permeability were seen. In contrast, higher levels of AQP5 led to increased microtubule assembly and stability.15 We decided to investigate the levels of tubulin acetylation in our diffuse NEPPK biopsy samples given that an increase in tubulin acetylation is associated with increased microtubule stabilization.16 Using immunohistochemistry as detailed above, we looked at total α-tubulin (ab7291, Abcam) and acetylated α-tubulin (ab24610, Abcam) in both a diffuse NEPPK palm and an unaffected, control palm (Figure 4). Although there might have been a small increase in the amount of total α-tubulin present in the diffuse NEPPK biopsy samples (Figures 4B and 4C) compared to the control (Figure 4A), the fact that there was a more obvious increase in the levels of acetylated α-tubulin in the diffuse NEPPK sections (Figures 4F and 4G) compared to the control palm (Figure 4E) suggests increased levels of microtubule stabilization in the diffuse NEPPK palmar epidermis. AQP5 is regulated in lung epithelial cells in response to shear stress,17,18 and a role involving the cytoskeleton for AQP5 could be very relevant in the palmoplantar skin that sustains high levels of stress, but additional functional assays are required for investigating this further.
Figure 4.
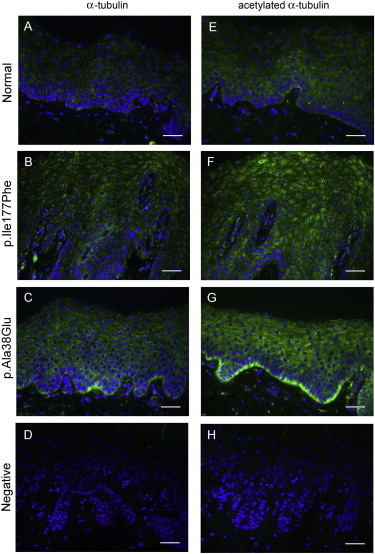
Microtubules in Diffuse NEPPK Palm Skin Show Evidence of Increased Stabilization
Immunofluorescent staining of α-tubulin (green) (A–D) and acetylated α-tubulin (green) (E–H) in paraffin-wax-embedded sections from an unaffected, control palm (A and E), a British diffuse NEPPK (with the p.Ile177Phe substitution) palm (B and F), and a Swedish diffuse NEPPK (with the p.Ala38Glu substitution) palm (C and G). Primary antibody was omitted in negative controls (D and H). Nuclei were counterstained with DAPI (blue). There appears to be a slight increase in the level of α-tubulin and a more pronounced increase in the level of acetylated α-tubulin in the diffuse NEPPK palmar biopsy samples compared to the control palm, indicating an increase in microtubule stabilization in the diffuse NEPPK palmar skin. Scale bars represent 40 μm.
We describe here inherited aquaporin alterations in a skin disease and propose a gain-of-function effect for the identified AQP5 variants, which are still able to traffic to the plasma membrane (Figure 3). This is in contrast to missense variants identified in other aquaporin family members, such as AQP2, in which variants underlying the autosomal-dominant form of nephrogenic diabetes insipidus (MIM 125800) not only exhibit a trafficking defect but also exert a dominant-negative effect by interfering with the trafficking of the wild-type AQP2.19,20 The exact mechanisms by which defective epidermal-water-barrier function associated with mutant AQP5 leads to palmoplantar hyperkeratosis are still unclear. Mutations in other junctional- and channel-protein-coding genes, including those involved with the desmosome21,22 and gap junction, also result in hyperkeratosis.23,24 However, unlike other skin-barrier disorders, diffuse NEPPK involves increased keratinocyte water uptake rather than transepidermal water loss.
Acknowledgments
D.P.K. is supported by Barts and the London Charity. V.P. is supported by a grant from the UK Medical Research Council (G1001158) and by the National Institute for Health Research Moorfields Biomedical Research Council. F.J.D.S. is supported by a project grant from the Pachyonychia Congenita Project. A.L. is supported by the Welander-Finsen Foundation. We thank the Barts and the London Genome Centre and the Blizard Advanced Light Microscopy core facilities.
Contributor Information
Diana C. Blaydon, Email: d.blaydon@qmul.ac.uk.
David P. Kelsell, Email: d.p.kelsell@qmul.ac.uk.
Supplemental Data
Web Resources
The URLs for data presented herein are as follows:
1000 Genomes, http://www.1000genomes.org/
Integrative Genomics Viewer, http://www.broadinstitute.org/igv/
Online Mendelian Inheritance in Man (OMIM), http://www.omim.org
UCSC Genome Browser, http://genome.ucsc.edu/
References
- 1.Itin P.H., Fistarol S.K. Palmoplantar keratodermas. Clin. Dermatol. 2005;23:15–22. doi: 10.1016/j.clindermatol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Kelsell D.P., Stevens H.P., Purkis P.E., Talas U., Rustin M.H., Leigh I.M. Fine genetic mapping of diffuse non-epidermolytic palmoplantar keratoderma to chromosome 12q11-q13: exclusion of the mapped type II keratins. Exp. Dermatol. 1999;8:388–391. doi: 10.1111/j.1600-0625.1999.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 3.Kelsell D.P., Stevens H.P., Ratnavel R., Bryant S.P., Bishop D.T., Leigh I.M., Spurr N.K. Genetic linkage studies in non-epidermolytic palmoplantar keratoderma: evidence for heterogeneity. Hum. Mol. Genet. 1995;4:1021–1025. doi: 10.1093/hmg/4.6.1021. [DOI] [PubMed] [Google Scholar]
- 4.Lind L., Lundström A., Hofer P.A., Holmgren G. The gene for diffuse palmoplantar keratoderma of the type found in northern Sweden is localized to chromosome 12q11-q13. Hum. Mol. Genet. 1994;3:1789–1793. doi: 10.1093/hmg/3.10.1789. [DOI] [PubMed] [Google Scholar]
- 5.Rongioletti F., Tomasini C., Crovato F., Marchesi L. Aquagenic (pseudo) keratoderma: a clinical series with new pathological insights. Br. J. Dermatol. 2012;167:575–582. doi: 10.1111/j.1365-2133.2012.11003.x. [DOI] [PubMed] [Google Scholar]
- 6.King L.S., Kozono D., Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 7.Engel A., Fujiyoshi Y., Gonen T., Walz T. Junction-forming aquaporins. Curr. Opin. Struct. Biol. 2008;18:229–235. doi: 10.1016/j.sbi.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Törnroth-Horsefield S., Hedfalk K., Fischer G., Lindkvist-Petersson K., Neutze R. Structural insights into eukaryotic aquaporin regulation. FEBS Lett. 2010;584:2580–2588. doi: 10.1016/j.febslet.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 9.Jung J.S., Preston G.M., Smith B.L., Guggino W.B., Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J. Biol. Chem. 1994;269:14648–14654. [PubMed] [Google Scholar]
- 10.Horsefield R., Nordén K., Fellert M., Backmark A., Törnroth-Horsefield S., Terwisscha van Scheltinga A.C., Kvassman J., Kjellbom P., Johanson U., Neutze R. High-resolution x-ray structure of human aquaporin 5. Proc. Natl. Acad. Sci. USA. 2008;105:13327–13332. doi: 10.1073/pnas.0801466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janosi L., Ceccarelli M. The gating mechanism of the human aquaporin 5 revealed by molecular dynamics simulations. PLoS ONE. 2013;8:e59897. doi: 10.1371/journal.pone.0059897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin M.H., Verkman A.S. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest. Ophthalmol. Vis. Sci. 2006;47:4365–4372. doi: 10.1167/iovs.06-0335. [DOI] [PubMed] [Google Scholar]
- 13.Liu J., Xu J., Gu S., Nicholson B.J., Jiang J.X. Aquaporin 0 enhances gap junction coupling via its cell adhesion function and interaction with connexin 50. J. Cell Sci. 2011;124:198–206. doi: 10.1242/jcs.072652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loitto V.M., Forslund T., Sundqvist T., Magnusson K.E., Gustafsson M. Neutrophil leukocyte motility requires directed water influx. J. Leukoc. Biol. 2002;71:212–222. [PubMed] [Google Scholar]
- 15.Sidhaye V.K., Chau E., Srivastava V., Sirimalle S., Balabhadrapatruni C., Aggarwal N.R., D’Alessio F.R., Robinson D.N., King L.S. A novel role for aquaporin-5 in enhancing microtubule organization and stability. PLoS ONE. 2012;7:e38717. doi: 10.1371/journal.pone.0038717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takemura R., Okabe S., Umeyama T., Kanai Y., Cowan N.J., Hirokawa N. Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J. Cell Sci. 1992;103:953–964. doi: 10.1242/jcs.103.4.953. [DOI] [PubMed] [Google Scholar]
- 17.Hansel N.N., Sidhaye V., Rafaels N.M., Gao L., Gao P., Williams R., Connett J.E., Beaty T.H., Mathias R.A., Wise R.A. Aquaporin 5 polymorphisms and rate of lung function decline in chronic obstructive pulmonary disease. PLoS ONE. 2010;5:e14226. doi: 10.1371/journal.pone.0014226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sidhaye V.K., Schweitzer K.S., Caterina M.J., Shimoda L., King L.S. Shear stress regulates aquaporin-5 and airway epithelial barrier function. Proc. Natl. Acad. Sci. USA. 2008;105:3345–3350. doi: 10.1073/pnas.0712287105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuwahara M., Iwai K., Ooeda T., Igarashi T., Ogawa E., Katsushima Y., Shinbo I., Uchida S., Terada Y., Arthus M.F. Three families with autosomal dominant nephrogenic diabetes insipidus caused by aquaporin-2 mutations in the C-terminus. Am. J. Hum. Genet. 2001;69:738–748. doi: 10.1086/323643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulders S.M., Bichet D.G., Rijss J.P., Kamsteeg E.J., Arthus M.F., Lonergan M., Fujiwara M., Morgan K., Leijendekker R., van der Sluijs P. An aquaporin-2 water channel mutant which causes autosomal dominant nephrogenic diabetes insipidus is retained in the Golgi complex. J. Clin. Invest. 1998;102:57–66. doi: 10.1172/JCI2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKoy G., Protonotarios N., Crosby A., Tsatsopoulou A., Anastasakis A., Coonar A., Norman M., Baboonian C., Jeffery S., McKenna W.J. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355:2119–2124. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- 22.Norgett E.E., Hatsell S.J., Carvajal-Huerta L., Cabezas J.C., Common J., Purkis P.E., Whittock N., Leigh I.M., Stevens H.P., Kelsell D.P. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum. Mol. Genet. 2000;9:2761–2766. doi: 10.1093/hmg/9.18.2761. [DOI] [PubMed] [Google Scholar]
- 23.Heathcote K., Syrris P., Carter N.D., Patton M.A. A connexin 26 mutation causes a syndrome of sensorineural hearing loss and palmoplantar hyperkeratosis (MIM 148350) J. Med. Genet. 2000;37:50–51. doi: 10.1136/jmg.37.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamartine J., Munhoz Essenfelder G., Kibar Z., Lanneluc I., Callouet E., Laoudj D., Lemaître G., Hand C., Hayflick S.J., Zonana J. Mutations in GJB6 cause hidrotic ectodermal dysplasia. Nat. Genet. 2000;26:142–144. doi: 10.1038/79851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


