Abstract
Gene mutations that lead to decreased contraction of vascular smooth-muscle cells (SMCs) can cause inherited thoracic aortic aneurysms and dissections. Exome sequencing of distant relatives affected by thoracic aortic disease and subsequent Sanger sequencing of additional probands with familial thoracic aortic disease identified the same rare variant, PRKG1 c.530G>A (p.Arg177Gln), in four families. This mutation segregated with aortic disease in these families with a combined two-point LOD score of 7.88. The majority of affected individuals presented with acute aortic dissections (63%) at relatively young ages (mean 31 years, range 17–51 years). PRKG1 encodes type I cGMP-dependent protein kinase (PKG-1), which is activated upon binding of cGMP and controls SMC relaxation. Although the p.Arg177Gln alteration disrupts binding to the high-affinity cGMP binding site within the regulatory domain, the altered PKG-1 is constitutively active even in the absence of cGMP. The increased PKG-1 activity leads to decreased phosphorylation of the myosin regulatory light chain in fibroblasts and is predicted to cause decreased contraction of vascular SMCs. Thus, identification of a gain-of-function mutation in PRKG1 as a cause of thoracic aortic disease provides further evidence that proper SMC contractile function is critical for maintaining the integrity of the thoracic aorta throughout a lifetime.
Main Text
Thoracic aortic aneurysms, which develop in the first part of the aorta above the heart, can lead to life-threatening acute aortic dissections if not properly managed. The major risk factor for thoracic aortic diseases is uncontrolled hypertension, which is present in more than 70% of affected individuals.1 Thoracic aortic aneurysms and dissections are familial in 20% of cases and can be inherited as a single-gene disorder in an autosomal-dominant manner with variable expression and decreased penetrance that is more pronounced in women.2 Mutations causing inherited thoracic aortic aneurysms and dissections have been identified in ACTA2 (actin, alpha 2, smooth muscle, aorta [MIM 102620]), MYH11 (myosin, heavy chain 11, smooth muscle [MIM 160745]), MYLK (myosin light-chain kinase [MIM 600922]), TGFBR1 (transforming growth factor, beta receptor 1 [MIM 190181]), TGFBR2 (transforming growth factor, beta receptor II [70/80 kDa] [MIM 190182]), SMAD3 (SMAD family member 3 [MIM 603109]), and TGFB2 (transforming growth factor, beta 2 [MIM 190220]),3–10 revealing that disruption of proteins involved in either smooth-muscle cell (SMC) contraction or TGF-β signaling predispose to thoracic aortic disease. For example, mutations in the genes encoding α-actin and myosin heavy chain, the two major proteins in the SMC contractile complex, are predicted to disrupt the thin and thick filaments, respectively, and thus decrease the ability of the SMCs to contract in response to pulse pressures.4,5 SMC contraction is initiated by an influx of Ca2+, which binds to and activates the calmodulin-dependent myosin light-chain kinase, leading to phosphorylation of the regulatory light chain (RLC [MIM 160781]) and, consequently, activation of myosin ATPase to initiate movement of the thin filaments along thick filaments. MYLK loss-of-function mutations that are predicted to lead to decreased RLC phosphorylation in vascular SMCs have also been identified as a cause of inherited thoracic aortic disease.6
To identify additional genes associated with inherited predisposition to thoracic aortic aneurysms and dissections,11 we collected blood or saliva samples from affected individuals and family members after obtaining proper informed consent and approval at the University of Texas Health Science Center at Houston or the Centre de Référence pour les Syndromes de Marfan et Apparentés in France. Exome sequencing was pursued with DNA from two distantly related affected individuals (coefficient of relationship = 1/8) from a large family, TAA216, with autosomal-dominant inheritance of a predisposition to thoracic aortic disease as previously reported (dbGaP accession number of exome-sequencing data: phs000347.v1.p1).7 No mutation in known genes associated with familial thoracic aortic diseases was identified in this family. The phenotype in the family has been reported previously and is notable for aortic dissections in both men and women as young as 17 years of age.12 Gene variants identified by whole-exome sequencing were filtered, and only rare variants shared by the two affected relatives and not present in internal control exomes were pursued, as previously described.8 The variants meeting these criteria were assessed for segregation with aortic disease in TAA216, decreasing the number of candidate rare variants down to four missense variants, one nonsense variant, and one frameshift variant (Figure 1A and Table S1, available online). One of the genes, PRKG1, encodes type I cGMP-dependent protein kinase (PKG-1 [RefSeq accession number NM_001098512.2; MIM 176894]), which controls SMC relaxation, and was therefore an excellent candidate gene for thoracic aortic disease. Analysis of additional exome data from 55 unrelated probands identified the same variant, PRKG1 c.530G>A (p.Arg177Gln), in the probands of families TAA508 and TAA690, but no other rare variants were found in this gene. Sanger sequencing of PRKG1 in 307 affected probands identified the same variant in another family, TAA292. DNA sequencing of family members in these three additional families confirmed segregation of the PRKG1 variant with thoracic aortic disease (Figure 1A). Two-point linkage analysis of thoracic aortic disease with the PRKG1 variant resulting in p.Arg177Gln in these four families was performed with Superlink software and revealed a combined LOD score of 7.88.13 The Arg177 amino acid is highly conserved, and the p.Arg177Gln variant is absent from 8,600 European American chromosomes and 4,400 African American chromosomes in the National Heart, Lung, and Blood Institute (NHLBI) Exome Sequencing Project Exome Variant Server (Figure 1B). Six HapMap SNPs flanking the PRKG1 c.530G>A variant were assessed in these families, which showed that TAA508 and TAA690 share a common haplotype in this region, but this haplotype differs from the mutation-associated haplotype in TAA216 and TAA292 (Table S2). Sanger sequencing of PRKG1 also identified two variants, c.1997G>C (p.Gly666Ala) and c.1421A>T (p.Tyr474Phe), in families TAA165 and TAA561, respectively (Figure S1). Gly666 is conserved, and the p.Gly666Ala variant is predicted to be probably damaging by PolyPhen-2 analysis; however, TAA165 does not have enough affected family members to establish that this variant segregates with disease in the family. Tyr474 is conserved, but the p.Tyr474Phe variant is predicted to be benign and does not segregate with aortic disease in TAA561. Therefore, although other rare variants in PRKG1 have been identified in individuals with familial thoracic aortic diseases and are present in the general population (Figure 1C), only the PRKG1 mutation resulting in p.Arg177Gln has been shown to cause thoracic aortic disease.
Figure 1.

Identification of PRKG1 c.530G>A (p. Arg177Gln) as the Mutation Responsible for Thoracic Aortic Disease in Families TAA216, TAA292, TAA508, and TAA690
(A) Family pedigrees. The legend indicates the disease and mutation status of the family members. The age at diagnosis of aortic aneurysm or dissection (“dx”) and age at death (“d”) are shown in years. A diagonal line across a symbol indicates that an individual is deceased, an arrow indicates a proband, and a single asterisk indicates an individual whose DNA was used for exome sequencing. Mutation-positive children who are unaffected and two adults who have an unknown disease status are not included in the pedigrees.
(B) Ortholog conservation of PKG-1 variants identified in individuals with thoracic aortic disease and surrounding amino acids.
(C) Schematic representation of PRKG1. The boxes represent exons 1–18, and the UTRs and the open reading frame are designated. The PRKG1 rare variants identified in this study are on the top of the gene diagram, and the rare variants identified in European Americans in the NHLBI Exome Variant Server are indicated below. Bold type designates variants predicted to be possibly or probably damaging by PolyPhen-2 analysis, and regular type designates variants predicted to be benign.
Individuals with the p.Arg177Gln alteration presented with either thoracic aortic dissection or mild to severe aortic root dilatation (Table S3). Thoracic aortic disease was fully penetrant in family members over 18 years of age. Of the 31 mutation-positive individuals and affected relatives, including five who must have carried the mutation on the basis of their position in the pedigree and six who were at a 50% risk of inheriting the mutation, the majority (63%) presented with acute aortic dissections (Stanford type A, n = 11; type B, n = 6; unspecified, n = 2) and 37% had aortic root enlargement; one died suddenly of an unknown cause. Acute aortic dissections occurred as young as 17 years of age and were equally penetrant in men and women. There was no gender difference in the age of onset of dissections (women: mean age 31 years, range 17–45 years; men: mean age 31 years, range 18–51 years). Women with the p.Arg177Gln alteration presented with dissections at a significantly younger age than they did with aortic root dilatation (31 years for dissections, 43 years for aortic dilatation, p = 0.009, n = 19), whereas men did not (31 years for dissection, 39 years for aortic dilatation, p = 0.18, n = 11). Aortic measurements were available for two individuals who had type A dissections: TAA216 IV:3 had a 5.7 cm aortic root prior to dissection, and TAA690 III:3 had a 3.7 cm aortic root and 4.3 cm ascending aorta at the time of dissection. There were family members with mild to severe aortic root dilatation, and the largest aortic diameter was noted to be 5.7 cm. The medical records were reviewed for additional risk factors of aortic dissection, specifically pregnancy and hypertension. Of the 19 individuals who had dissections, five had a diagnosis of hypertension and five had evidence of end-organ damage associated with hypertension, specifically mild to moderate left ventricular hypertrophy or chronic small-vessel cerebrovascular disease at the time of presentation with aortic dissection; ten individuals had insufficient information in their medical records. Two women, TAA508 III:1 and TAA690 III:1, had dissections in the postpartum period and in the third trimester of pregnancy, respectively.
Records were reviewed for additional vascular disease, and some individuals were noted to have enlargement of the descending thoracic aorta, abdominal aorta, and other arteries. In family TAA508, individual II:3 had a giant coronary artery aneurysm treated with covered stent and individual II:1 had a spontaneous coronary artery dissection and ectatic coronary arteries. Three individuals (TAA216 III:3 and III:9 and TAA292 III:1) were noted to have tortuosity of the thoracic aorta. None of the individuals with the mutation and examined by a clinical geneticist had features of Marfan syndrome (MIM 154700) or any other genetic syndrome associated with thoracic aortic disease.
Although two PKG-1 splice variants (PKG-1α and PKG-1β) exist in mammalian tissues, PKG-1α is the major isoform present in vascular SMCs.14 PKG-1α is a homodimer with the N-terminal regulatory (R) and C-terminal catalytic (C) domains. The R domain consists of a leucine zipper motif that is involved in kinase dimerization and cellular localization, an autoinhibitory sequence that binds the active site of the C domain in the absence of cGMP, and tandem cGMP binding domains (CNB-A and CNB-B) that bind cGMP and activate the kinase. Upon binding of cGMP to CNB-A and CNB-B, the R domain undergoes major conformational change—releasing and activating the C domain.15 Arg177 is located in CNB-A, which binds cGMP with nanomolar affinity. PKG-1α and PKG-1β have the same CNB-A domain, and the PKG-1β (amino acids 92–227)-cGMP complex is the only structure available for CNB-A with bound cGMP.16 In the crystal structure of the PKG-1β (92–227)-cGMP complex, Arg192, which corresponds to Arg177 in PKG-1α, interacts directly with the cyclic phosphate moiety and is essential for cGMP binding (Figure 2A).16 A p.Arg177Gln alteration would substitute the guanidinium side chain with a carboxamide, which would disable its interaction with the cyclic phosphate. To confirm decreased cGMP binding, we purified the altered and wild-type CNB-A of PKG-1α (residues 79–212) and used a fluorescence polarization assay to measure the binding affinity to 8-Fluo-cGMP (Figure 2B). The half maximal effective concentration (EC50) for the altered CNB-A was more than 32,000-fold lower than the wild-type, indicating that p.Arg177Gln results in a CNB-A that does not bind or respond to cGMP.
Figure 2.
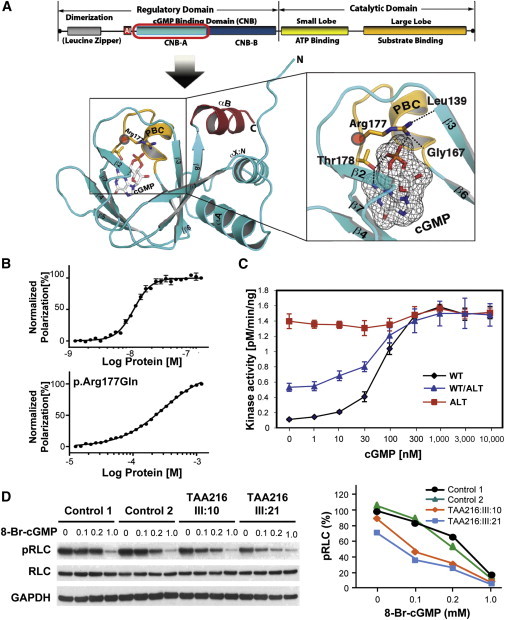
Effect of PKG-1α p.Arg177Gln on cGMP Binding and Kinase Activity
(A) Structural model of the CNB-A domain of PKG-1α. The crystal structure of the PKG-1β (92–227):cGMP complex (Protein Data Bank ID 3OD0) was used for modeling the CNB-A domain of PKG-1α because PKG-1α and PKG-1β have the same protein sequence at this region and this is the only structure available for CNB-A with bound cGMP. The phosphate-binding cassette (PBC) is colored in yellow, the αB helix is in red, and the rest is in cyan. cGMP is colored by atom type with white and black carbons. The secondary-structure elements and both termini are labeled. In the right panel, a zoomed-in view shows the region around Arg177 (corresponding to Arg192 in PKG-1β). Arg177 not only binds the cyclic phosphate of cGMP but also stabilizes the PBC by interacting with Leu139 (corresponding to Leu154 in PKG-1β) at strand β5 and Gly167 (corresponding to Gly181 in PKG-1β) at the base of PBC.
(B) Direct fluorescence-polarization assay using 8-Fluo-cGMP. PKG-1α amino acids 79–212 and p.Arg177Gln PKG-1α were cloned into pQTEV, and both protein fragments were purified from E. coli Δcya TP2000, which lacks adenylyl cyclase activity.17 The proteins were purified as described previously.16 The direct fluorescence-polarization assay was performed according to the procedure described by Moll.18
(C) Kinase activities of wild-type (“WT”) and altered (“ALT”) proteins, as well as of a 1:1 mixture of wild-type and altered peptides, were assayed with 35 ng protein in reactions containing 8 μg kemptide. Flag-tagged wild-type and altered PKG-1α proteins were purified from transfected HEK293T cells with the use of anti-Flag M2 agarose (Sigma-Aldrich). Reactions were performed for 5 min at 30°C in 40 mM HEPES (pH 7.0), 8 μg kemptide (Sigma-Aldrich), 10 mM MgCl2, 60 μM ATP, 0.6 μCi 32P-γ-ATP, and variable amounts of cGMP. Reactions were stopped by spotting on P81 phosphocellulose paper, and activity was measured by liquid scintillation counting. The results show that p.Arg177Gln led to constitutive activation of kinase when assayed alone and increased activity of the kinase when purified with the wild-type protein. Assays were performed in triplicate.
(D) Fibroblasts explanted from individuals TAA216 III:10 and III:21 and age-matched control fibroblasts were exposed to variable doses of 8-Br-cGMP. The methods of fibroblast cell culture and 8-Br-cGMP treatment followed the procedures previously described.19,20 RLC phosphorylation was lower in the mutant fibroblasts when exposed to 8-Br-cGMP than in control fibroblasts (left panel). On the x axis, the percentage of pRLC is based on the pRLC level in each sample over the pRLC level averaged from the two control fibroblast lines in the absence of 8-Br-cGMP (right panel). The concentration unit of 8-Br-cGMP is mM, and assays were performed in triplicate.
To test the effect of the p.Arg177Gln alteration on full-length PKG-1α, we purified altered and wild-type PKG-1α in human embryonic kidney 293T (HEK293T) cells and measured phosphotransferase activity. Interestingly, the measurements indicated that p.Arg177Gln PKG-1α was highly active even without cGMP, whereas wild-type PKG-1α required cGMP for activation (Figure 2C). When equal amounts of the altered and wild-type PKG-1α constructs were cotransfected into HEK293T cells to mimic the heterozygous mutation identified in affected individuals, kinase activity was higher both at baseline and with cGMP exposure than that of wild-type PKG-1α (Figure 2C). The activity in the mixed samples was lower than expected if the wild-type and altered peptides were acting independently of each other but higher than expected if the wild-type was completely inhibiting the altered protein in heterodimeric complexes (Table 1). The kinase assays of p.Gly666Ala PKG-1α showed activity levels similar to those of the wild-type, providing further evidence that this variant does not cause thoracic aortic disease (Figure S2). Taken together, these results suggest that the p.Arg177Gln alteration structurally perturbs PKG-1α and thus abolishes the ability of the R domain to inhibit kinase activity in the absence of cGMP and results in a kinase whose activity is no longer modulated by cGMP.
Table 1.
Kinase-Activity Assays of Wild-Type and Altered PKG-1α
| cGMP (nM) | WT | Alt | WT/Alt Mixeda | WT/Altb | WT/Altc |
|---|---|---|---|---|---|
| 0 | 0.11 | 1.4 | 0.53 | 0.76 | 0.43 |
| 1 | 0.15 | 1.36 | 0.55 | 0.76 | 0.45 |
| 10 | 0.21 | 1.35 | 0.68 | 0.78 | 0.5 |
| 30 | 0.41 | 1.31 | 0.8 | 0.86 | 0.64 |
| 100 | 1.04 | 1.35 | 1.21 | 1.2 | 1.12 |
| 300 | 1.47 | 1.48 | 1.4 | 1.48 | 1.47 |
| 1,000 | 1.59 | 1.56 | 1.5 | 1.58 | 1.58 |
| 3,000 | 1.49 | 1.49 | 1.5 | 1.49 | 1.49 |
| 10,000 | 1.51 | 1.51 | 1.48 | 1.51 | 1.51 |
PKG-1α activity observed in cells that were transfected with equal amounts of altered and wild-type constructs to mimic the heterozygous PRKG1 mutations identified in thoracic aortic disease.
Hypothetical kinase activity of the mixed sample was calculated under the assumption that the wild-type and altered proteins act independently (0.5 [WT] + 0.5 [Alt]).
Hypothetical kinase activity of the mixed sample was calculated under the assumption that the wild-type completely inhibits the altered protein in heterodimers (0.5 [WT] + 0.25 [WT + Alt]).
PKG-1α is activated in SMCs when nitric oxide stimulates soluble guanylyl cyclase to increase cellular cGMP levels. The activated PKG-1α regulates many cellular systems that lead to relaxation of SMCs, including phosphorylation of the regulatory myosin-binding subunit (MYPT) of the phosphatase that dephosphorylates RLC. MYPT1 is specifically phosphorylated by PKG-1α at Ser695, which leads to enhanced phosphatase activity and increased dephosphorylation of the RLC. Thus, the increased kinase activity associated with the PKG-1α p.Arg177Gln alteration should lead to decreased levels of phosphorylated RLC (pRLC). Fibroblasts explanted from two individuals with the PKG-1α Arg177Gln alteration and matched control fibroblasts were exposed to variable doses of 8-Fluo-cGMP. The levels of pRLC were slightly lower in the mutant cells than in the control cells, and the reduced pRLC levels in the mutant fibroblasts were more pronounced with exposure to low doses of 8-Fluo-cGMP (Figure 2D).
Given that MYLK and PRKG1 mutations are predicted to decrease aortic SMC contraction as a result of decreased phosphorylation and increased dephosphorylation of the RLC, respectively, we expected similar pathological features in the aortas of individuals with mutations in these genes. Aortic tissues from two individuals (TAA216 III:10 and TAA292 II:4) with the PRKG1 mutation demonstrated the typical pathology associated with thoracic aortic disease: increased proteoglycan accumulation, decreased SMCs, and elastic fiber loss and fragmentation (Figure 3). These aortas showed a substantial increase in the small arteries in the medial layer from the vasa vasorum of the aortas from PRKG1-mutation-positive individuals compared to controls (Figure 3). Interestingly, similar invasion of the vasa vasorum into the medial layer was previously observed in aortas of individuals with MYH11 and MYLK mutations.6,21
Figure 3.

Aortic Pathology from Individuals TAA216 III:10 and TAA292 II:4, with the PRKG1 Mutation Resulting in p.Arg177Gln, and an Unaffected Control
Compared with the control aorta, aortas from cases showed medial degeneration upon Movat staining, as shown by increased proteoglycan deposition (blue), elastic-fiber fragmentation and loss (black), and decreased cells (red). Immunostaining for Von Willebrand factor (vWF, marker of endothelial cells; brown) showed increased vascularity (arrows) in the medial layer of aortas from individuals with the PRKG1 mutation. In the control aortas, only the vasa vasorum in the adventitial layer or at the boundary (arrowhead) of the medial and adventitial layers stained with vWF. Note that the aortic specimen from TAA292 II:4 is from a dissected segment of the aorta and the images only show the medial layer of aorta.
The data presented here demonstrate that a single missense mutation in PRKG1 leads to thoracic aortic disease. Although rare variants in PRKG1 are present in the population, only the p.Arg177Gln alteration was confirmed to be disease causing, most likely as a result of its unique gain-of-function effect on PKG-1α activity. Therefore, only PRKG1 rare variants that were demonstrated to increase PKG-1α activity should be considered disease causing until further data are available. Similar to previously identified mutations in genes encoding proteins involved in SMC contraction, PRKG1 c.530G>A leads to PKG-1α alteration p.Arg177Gln, which increases PKG-1α activity and causes decreased phosphorylation of the RLC and thus should decrease vascular SMCs contraction. The mechanism for the increased activation of the altered protein and how the altered protein partially disrupts the regulation of the wild-type protein should be the focus of future studies. Additionally, the PKG-1α p.Arg177Gln alteration should lead to SMC relaxation and hypotension on the basis of the biochemical analysis, and additional studies are needed for determining why hypertension is present in many individuals who have the mutation. Finally, these data illustrate the complexity of associating rare variants with complex, adult-onset diseases. Studies testing the association between rare variants in PRKG1 and thoracic aortic disease by using burden tests would not demonstrate an association, and studies with the specific PRKG1 mutation resulting in p.Arg177Gln would only show an association in a large cohort of affected individuals or if family-based studies were pursued.
Acknowledgments
The authors are extremely grateful to the families involved in this study, as well as the physicians and genetic counselors who aided in the collection of clinical data from the families (available in the Supplemental Data). We would like to thank the National Heart, Lung, and Blood Grand Opportunity Exome Sequencing Project and its ongoing studies, which produced and provided exome variant calls for comparison: the Lung Cohorts Sequencing Project (HL-102923), the Women’s Health Initiative Sequencing Project (HL-102924), the Heart Cohorts Sequencing Project (HL-103010), the Broad Institute Sequencing Project (HL-102925), the GenTAC Registry Consortium (HHSN268200648199C and HHSN268201000048C), the Northwest Genomics Center Sequencing Project (HL-102926, D.A.N, M.J.R, and J.S.), and the Family Studies Project Team. We thank Z. Ren for technical assistance. The following sources provided funding for these studies: RO1 HL62594 (D.M.M.), P50HL083794-01 (D.M.M.), P01HL110869-01 (D.M.M.), UL1 RR024148 (University of Texas Health Science Center at Houston), the Vivian L. Smith Foundation (D.M.M.), the TexGen Foundation (D.M.M.), Richard T. Pisani funds (D.M.M.), RO1 GM090161 (C.K.), Groupements d’Intérêt Scientifique Maladies Rares (C.B.), PHRC AOM09093 (G.J.), PHRC AOM 10108 (C.B.), ANR 2010 BLAN 1129 from the French National Research Agency (G.J.), and the European Union 7th Framework Program integrated project “Fighting Aneurysmal Disease” (www.fighting-aneurysm.org) (J.B.M.).
Supplemental Data
Web Resources
The URLs for data presented herein are as follows:
dbSNP Annotation, www.ncbi.nlm.nih.gov/projects/SNP/
Ensembl Genome Browser, http://www.ensembl.org/index.html
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
Online Mendelian Inheritance in Man (OMIM), http://www.omim.org
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
UCSC Genome Browser, http://www.genome.ucsc.edu/
References
- 1.Pape L.A., Tsai T.T., Isselbacher E.M., Oh J.K., O’gara P.T., Evangelista A., Fattori R., Meinhardt G., Trimarchi S., Bossone E., International Registry of Acute Aortic Dissection (IRAD) Investigators Aortic diameter >or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD) Circulation. 2007;116:1120–1127. doi: 10.1161/CIRCULATIONAHA.107.702720. [DOI] [PubMed] [Google Scholar]
- 2.Milewicz D.M., Guo D.C., Tran-Fadulu V., Lafont A.L., Papke C.L., Inamoto S., Kwartler C.S., Pannu H. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu. Rev. Genomics Hum. Genet. 2008;9:283–302. doi: 10.1146/annurev.genom.8.080706.092303. [DOI] [PubMed] [Google Scholar]
- 3.Loeys B.L., Schwarze U., Holm T., Callewaert B.L., Thomas G.H., Pannu H., De Backer J.F., Oswald G.L., Symoens S., Manouvrier S. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N. Engl. J. Med. 2006;355:788–798. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 4.Zhu L., Vranckx R., Khau Van Kien P., Lalande A., Boisset N., Mathieu F., Wegman M., Glancy L., Gasc J.M., Brunotte F. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat. Genet. 2006;38:343–349. doi: 10.1038/ng1721. [DOI] [PubMed] [Google Scholar]
- 5.Guo D.C., Pannu H., Tran-Fadulu V., Papke C.L., Yu R.K., Avidan N., Bourgeois S., Estrera A.L., Safi H.J., Sparks E. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat. Genet. 2007;39:1488–1493. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- 6.Wang L., Guo D.C., Cao J., Gong L., Kamm K.E., Regalado E., Li L., Shete S., He W.Q., Zhu M.S. Mutations in myosin light chain kinase cause familial aortic dissections. Am. J. Hum. Genet. 2010;87:701–707. doi: 10.1016/j.ajhg.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regalado E.S., Guo D.C., Villamizar C., Avidan N., Gilchrist D., McGillivray B., Clarke L., Bernier F., Santos-Cortez R.L., Leal S.M., NHLBI GO Exome Sequencing Project Exome sequencing identifies SMAD3 mutations as a cause of familial thoracic aortic aneurysm and dissection with intracranial and other arterial aneurysms. Circ. Res. 2011;109:680–686. doi: 10.1161/CIRCRESAHA.111.248161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boileau C., Guo D.C., Hanna N., Regalado E.S., Detaint D., Gong L., Varret M., Prakash S.K., Li A.H., d’Indy H., National Heart, Lung, and Blood Institute (NHLBI) Go Exome Sequencing Project TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat. Genet. 2012;44:916–921. doi: 10.1038/ng.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Laar I.M., Oldenburg R.A., Pals G., Roos-Hesselink J.W., de Graaf B.M., Verhagen J.M., Hoedemaekers Y.M., Willemsen R., Severijnen L.A., Venselaar H. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat. Genet. 2011;43:121–126. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 10.Pannu H., Fadulu V.T., Chang J., Lafont A., Hasham S.N., Sparks E., Giampietro P.F., Zaleski C., Estrera A.L., Safi H.J. Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation. 2005;112:513–520. doi: 10.1161/CIRCULATIONAHA.105.537340. [DOI] [PubMed] [Google Scholar]
- 11.Devereux R.B., de Simone G., Arnett D.K., Best L.G., Boerwinkle E., Howard B.V., Kitzman D., Lee E.T., Mosley T.H., Jr., Weder A., Roman M.J. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am. J. Cardiol. 2012;110:1189–1194. doi: 10.1016/j.amjcard.2012.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran-Fadulu V., Chen J.H., Lemuth D., Neichoy B.T., Yuan J., Gomes N., Sparks E., Kramer L.A., Guo D., Pannu H. Familial thoracic aortic aneurysms and dissections: three families with early-onset ascending and descending aortic dissections in women. Am. J. Med. Genet. A. 2006;140:1196–1202. doi: 10.1002/ajmg.a.31236. [DOI] [PubMed] [Google Scholar]
- 13.Silberstein M., Tzemach A., Dovgolevsky N., Fishelson M., Schuster A., Geiger D. Online system for faster multipoint linkage analysis via parallel execution on thousands of personal computers. Am. J. Hum. Genet. 2006;78:922–935. doi: 10.1086/504158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis S.H., Corbin J.D. Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit. Rev. Clin. Lab. Sci. 1999;36:275–328. doi: 10.1080/10408369991239213. [DOI] [PubMed] [Google Scholar]
- 15.Francis S.H., Busch J.L., Corbin J.D., Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J.J., Casteel D.E., Huang G., Kwon T.H., Ren R.K., Zwart P., Headd J.J., Brown N.G., Chow D.C., Palzkill T., Kim C. Co-crystal structures of PKG Iβ (92-227) with cGMP and cAMP reveal the molecular details of cyclic-nucleotide binding. PLoS ONE. 2011;6:e18413. doi: 10.1371/journal.pone.0018413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brickman E., Soll L., Beckwith J. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J. Bacteriol. 1973;116:582–587. doi: 10.1128/jb.116.2.582-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moll D., Prinz A., Gesellchen F., Drewianka S., Zimmermann B., Herberg F.W. Biomolecular interaction analysis in functional proteomics. J. Neural Transm. 2006;113:1015–1032. doi: 10.1007/s00702-006-0515-5. [DOI] [PubMed] [Google Scholar]
- 19.Inamoto S., Kwartler C.S., Lafont A.L., Liang Y.Y., Fadulu V.T., Duraisamy S., Willing M., Estrera A., Safi H., Hannibal M.C. TGFBR2 mutations alter smooth muscle cell phenotype and predispose to thoracic aortic aneurysms and dissections. Cardiovasc. Res. 2010;88:520–529. doi: 10.1093/cvr/cvq230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roh S., Choi S., Lim I. Involvement of protein kinase A in nitric oxide stimulating effect on a BK(Ca) channel of human dermal fibroblasts. J. Invest. Dermatol. 2007;127:2533–2538. doi: 10.1038/sj.jid.5700907. [DOI] [PubMed] [Google Scholar]
- 21.Pannu H., Tran-Fadulu V., Papke C.L., Scherer S., Liu Y., Presley C., Guo D., Estrera A.L., Safi H.J., Brasier A.R. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum. Mol. Genet. 2007;16:2453–2462. doi: 10.1093/hmg/ddm201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


