Preface
An emerging concept is that cellular communication in mammals can be mediated by exchange of genetic information, mainly microRNA. This can occur when extracellular vesicles, such as exosomes, secreted by a donor cell are taken up by an acceptor cell. Transfer of genetic material can also occur through intimate membrane contacts between donor and acceptor cells. Specialized cell-cell contacts, such as synapses, have the potential to combine these modes of genetic transfer.
Introduction
Cells of all multicellular organisms need to communicate with other cells in order to coordinate development and promote their environmental adaptation and function. Communication often involves soluble factors such as cytokines, chemokines, growth factors and neurotransmitters, and their specific recognition by cell-surface receptors. Recent evidence indicates that cells also communicate via the direct exchange of RNA. When eukaryotic cells encounter double-stranded RNA (dsRNA), genes carrying a matching sequence are silenced through RNA interference (RNAi). The surprising finding is that, in some animals and plants, the transport of a silencing signal between cells allows the same gene to be specifically silenced in cells that had not encountered the primary dsRNA. This process has been best characterized in plants and C. elegans 1. In plants, silencing RNAs move from cell to cell through plasmodesmata (PD), and over long distances through the phloem vascular tissue 2. When a leaf is infected with a plant virus, mobile signals transmitted to other leaves confer resistance to the spread of infection. Although viral-induced siRNAs and transgenes were known for many years to move through the plant, the movement of endogenous small RNAs (sRNAs) has been demonstrated only recently 3-5. The mobility of endogenous small RNAs, including microRNAs (miRNAs), generates morphogenic signalling gradients that guide the patterning of leaves and roots 3. Mobile sRNAs promote epigenetic modifications in the genome of recipient cells 5. Furthermore, when the recipient cells are seed or pollen, mobile sRNAs induce trans-generational epigenetic changes to enhance adaptation of progeny to future stresses 6 (Box 1). In C. elegans, silencing triggered by injected, ingested or locally-expressed dsRNA can spread throughout the organism to silence the targeted gene in all non-neuronal cells, including the germline, thus transmitting silencing to the next generation 7-10.
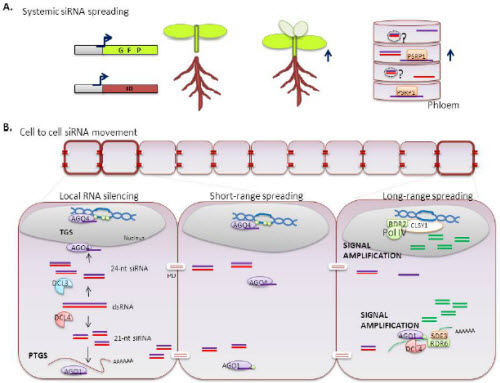
BOX1. Mechanisms of systemic RNA silencing in plants.
RNAi can move from cell to cell through plamodesmata (PD) and spread throughout the organism via the phloem 1, 2. Plants can transport RNA from older tissues to the root or shoot meristems along the direction of phloem flow (upper right). Long-distance spreading of RNA has been demonstrated by grafting rootstocks that express an inverted repeat (IR) that targets GFP mRNA. The recipient grafted tissues overexpress GFP (dark green). Emerging new growth is uniformly silenced (pale green) suggesting propagation of a GFP-specific silencing signal (upper left). The silencing signal might be transported as single-stranded RNA bound to phloem small RNA-binding protein (PSRP1), or possibly inside extracellular vesicles (EVs).
PDs are dynamic intercellular channels that establish symplasmic continuity between neighbouring cells. Double-stranded RNA (dsRNA, red/purple), derived from transgenic or endogenous loci, is processed by Dicer-like enzymes (DCLs). DCL4 and DCL3 produce primary siRNAs of 21 nt and 24 nt, respectively. The 24nt siRNA can be directed by Argonaute 4 (AGO4) to the transcriptional gene silencing (TGS) pathway whereas the 21 nt siRNA binds to AGO1, resulting in cleavage of complementary mRNAs via the post-transcriptional gene silencing (PTGS) pathway (local silencing). Both 21nt and 24nt duplexes can move from cell to cell through PD (short range spreading). It is unclear whether RNAs move through PD freely by diffusion, or through a regulated mechanism involving protein interactions. Silencing signals can travel through 10-15 contacting cells without amplification, but RNA-dependent RNA polymerases (RDRs) can direct the synthesis of secondary siRNAs to amplify and spread the silencing information (long range spreading). Both siRNA types can initiate signal amplification in recipient tissues via the action of RNA pol IV and RDRs.
The exchange of genetic information between mammalian cells is a more recent concept. Small RNAs have been detected in blood and other body fluids such as urine, saliva and milk. Most circulating small RNAs are contained within lipid or lipoprotein complexes, apoptotic bodies, or exosomes, which efficiently protect them from degradation by serum ribonucleases. Recent reports indicate that exosomes containing RNAs are transferred to recipient cells and modulate their function 11-14.
In this review, we discuss recent studies that describe the trafficking of genetic material in mammals, focusing on extracellular vesicles (EVs) and the diverse structures that cells utilize for communication. We also discuss the potential of cellular synapses and other connective structures to act as specialized devices for mediating intercellular transfer of genetic material, and explore the potential biological relevance of this mode of communication.
Genetic transfer via EVs
Until recently, firm evidence for intercellular transfer of RNA in mammals remained elusive. The discovery that EVs contain genetic material that can be exchanged between cells, either directly or via body fluids, supports the notion that this form of exchange of genetic information is biologically significant in mammals.
Biogenesis of extracellular vesicles
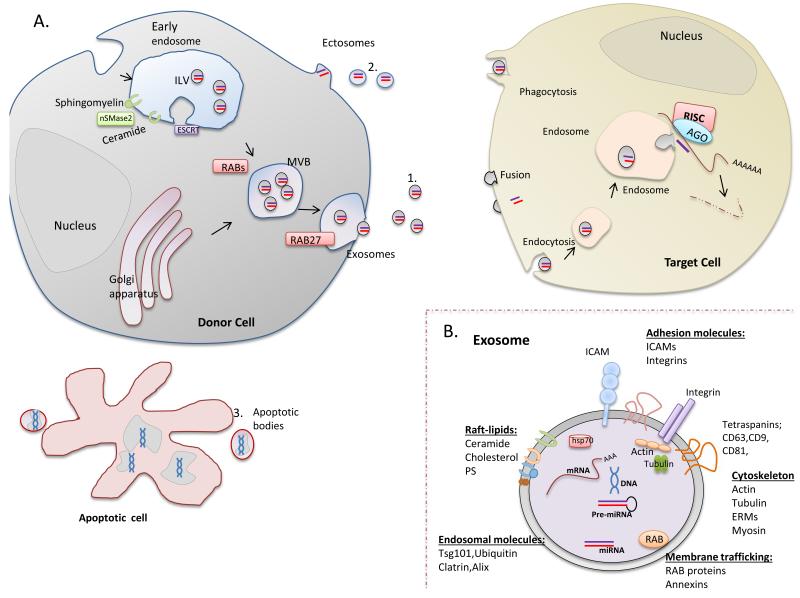
Depending on their origin, EVs are classified as exosomes, shedding vesicles or apoptotic bodies 15 (Fig. 1).
Figure 1. Long distance transfer of genetic material in extracellular vesicles (EVs).
A. EVs originate through at least three mechanisms. 1) Fusion of multivesicular bodies (MVBs) with the plasma membrane and release of their intraluminal vesicles (ILVs) as exosomes. Neutral sphingomyelinase 2 (nSMase2) is essential for formation of ILVs in the early endosome. Some proteins are channelled by the ESCRT machinery to the MVB route. Rab proteins such as RAB11, RAB27 and RAB35, known to participate in vesicle trafficking between intracellular compartments, have been shown to play a role in exosome secretion. 2) Blebbing of the cellular plasma membrane (ectosomes). 3) Breakdown of dying cells into apoptotic bodies. EVs, which are secreted into the extracellular environment, contain functional mRNA, microRNA and DNA molecules that can be taken up by recipient cells through mechanisms including fusion with the plasma membrane, phagocytosis, or endocytosis. B. All exosomes contain proteins involved in membrane transport and fusion (Rab proteins, Annexins). Cytoskeletal proteins, adhesion molecules and tetraspanins are also abundant. Exosome membranes are enriched in RAFT-lipids (cholesterol, ceramide, sphingolipids). ERM, ezrin–radixin– moesin; HSP, heat shock protein; ICAM, intercellular cell adhesion molecule. Exosomes also contain RNA, mainly microRNA.
EXOSOMES correspond to the intraluminal vesicles (ILVs) of late endosomes (multivesicular bodies, MVBs) 16. MVBs release exosomes by fusing with the plasma membrane. Their diameter is 30-100 nm. The endosomal origin of exosomes is reflected in their molecular composition, which includes Lamp1, CD63, and TSG101. All exosomes contain proteins involved in membrane transport and fusion (Rab GTPses, annexins and others). Cytoskeletal proteins, adhesion molecules and tetraspanin family proteins (e.g., tetraspanins CD81, CD82 and CD63) are also abundant. Exosome membranes also have a specific lipid composition, being enriched in lipids such as cholesterol, ceramide and sphingolipids. Ceramide is involved in the budding of ILVs into MVBs. Thus blockade of neutral sphingomyelinase 2 (nSMase2), an enzyme involved in ceramide synthesis, inhibits exosome production 17. In mammals, the ESCRT complex (endosomal sorting complex required for transport) might not control MVB formation, but could function to ensure proper ILV composition 18. Several Rab proteins (RAB11, RAB27 and RAB35) contribute to exosome secretion 19. The final step of exosome secretion, fusion of MVBs with the plasma membrane, likely involves a complex of SNARE proteins 19. Exosomes are released constitutively, although their secretion can be increased in response to cell activation or stress.
ECTOSOMES, also termed shedding vesicles, which are usually larger than exosomes (diameter 100 nm – 1 μm ), are produced by direct plasma membrane blebbing 20. They are thought to arise from regions of the membrane enriched in lipid rafts, and they expose phosphatidylserine in the outer leaflet of their membrane. Except for tumour cells, which constitutively shed large numbers of ectosomes, the rate of release is low, and can be increased by cell activation or apoptosis.
APOPTOTIC BODIES are larger than ectosomes or exosomes (> 1μm in diameter), and are released as blebs of apoptotic cells. They are characterized by phosphatidylserine externalization and contain fragmented DNA.
Consequences of genetic transfer
EVs have emerged as potent vehicles for cell-cell communication since the discovery that they contain functional mRNA, microRNA and DNA molecules that can be taken up by target (acceptor) cells 12-14, 21, 22. The genetic information contained in EVs can influence or even direct the fate of the target cell, for example triggering its activation, migration, differentiation or de-differentiation, or promoting apoptosis or necrosis.
Early studies showed that secreted vesicles derived from embryonic stem cells or tumour cells contain mRNA 21, and that these transcripts can be delivered to target cells where they are translated into functional proteins 11. Subsequently, EVs were shown to also contain small RNA species 12. Even DNA can be transferred, for example to fibroblasts and endothelial cells through phagocytosis of tumour apoptotic bodies 22. In addition, viral microRNAs 23, 24 and retrotransposon RNA transcripts from human endogenous retroviruses 25 can be detected in EVs and can be transferred to acceptor cells 24. The genetic content of EVs is not simply a straightforward reflection of the genetic content of the cell of origin; specific populations of RNAs are selectively packaged into exosomes 11, 13, 14, indicating the existence of an as-yet unknown mechanism controlling the sorting of specific RNAs. A likely mediator of this process is the ESCRT protein complex, since ESCRT-II can directly bind RNA 26 and blockade of MVB formation by ESCRT depletion impairs miRNA silencing 27, 28. Another possibility is that the nucleic acid sequences themselves directly control their trafficking to EVs.
Once they are released, EVs can reach a nearby target cell, or a distant one by entering the bloodstream, but the mechanisms by which target cells take up and integrate RNA carried by EVs are poorly understood. The functional consequences of this transfer depend on the origin and status of the donor and recipient cells. For example, EVs derived from endothelial progenitors drive angiogenesis via the horizontal transfer of mRNAs to quiescent endothelial cells 29. Apoptotic bodies can transfer miR-126 to endothelial cells; this microRNA, by silencing the endogenous G protein signaling inhibitor RGS16, promotes an autoregulatory feedback loop in recipient cells that increases secretion of CXCL12, inducing endothelial repair and protecting against diet-induced atherosclerosis 30. Also in relation to atherosclerosis, EVs from atherosclerosis patients are enriched in miR-150 and promote endothelial cell migration by downregulating its target c-Myb 31.
This mechanism of cell-to-cell behavioural regulation is particularly important in cancer. Tumour cells produce abundant EVs, with the potential to influence the behaviour of surrounding healthy cells in order to facilitate tumour growth, metastasis or immune evasion. Tumour-cell derived EVs can induce angiogenesis by delivering RNA to endothelial cells 11, 32, and can also deliver DNA, including oncogenes such as c-myc, or retrotransposon RNA transcripts from human endogenous retroviruses to normal cells 25. In the immune system, exosomes from T or dendritic cells can fuse with the plasma membrane of target cells, releasing the cargo of functional miRNAs into their cytoplasm 13, 14.
Circulating RNA in vesicle-independent form
MicroRNA can also circulate in body fluids in a vesicle-independent form 33-36. Argonaute2, the key effector protein of miRNA-mediated silencing, forms circulating ribonucleoprotein complexes with microRNAs 33, 34. Other associated RNA-binding proteins, such as nucleophosmin 1, can form complexes with microRNA and protect them from degradation. Although the mechanism regulating export of circulating ribonucleoprotein complexes is unknown, it seems to be an active energy-dependent process.
Several screens have identified the genes involved in dsRNA uptake and spreading in C. elegans. Cellular uptake of dsRNA involves SID-1 (Systemic RNAi defective mutants). SID-1, a multipass transmembrane protein expressed by all non-neuronal cells, allows long dsRNAs and their derivatives to move passively from cell to cell across the cell membrane 10. The potential ability of SID-1 to transport endogenous dsRNA is supported by recent evidence showing that it imports synthetic miRNA precursors and long hairpin molecules 37. Another gene involved in dsRNA transfer in C. elegans is rsd-3, a homologue of the mammalian protein enthoprotin, which is involved in vesicle trafficking through its ENTH domain 9. In mammals, miRNAs can also be transported by high-density lipoproteins (HDLs) and delivered to recipient cells to modulate their function35. Inhibition of nSMase2, which blocks the release of microRNA-loaded exosomes 13, 17, 38, increases the amount of miRNA exported to HDLs. Thus, nSMase 2 and the ceramide pathway might regulate different but coordinated pathways of miRNA secretion. HDL-microRNA delivery to recipient cells is dependent on scavenger receptor class B type I (SR-BI) 35. Although mammalian homologues of C. elegans SID proteins exist and participate in lipid-modified siRNA uptake 39, the uptake of HDL-microRNA seems to be independent of SIDT1.
Cell-to-cell genetic transfer
Although transfer of genetic material does not require cell-to-cell contact, it has been suggested that, as occurs in plants, small RNAs move between mammalian cells through highly-organized cell-cell structures such as gap junctions, intercellular bridges or synapses (Fig. 2 and 3).
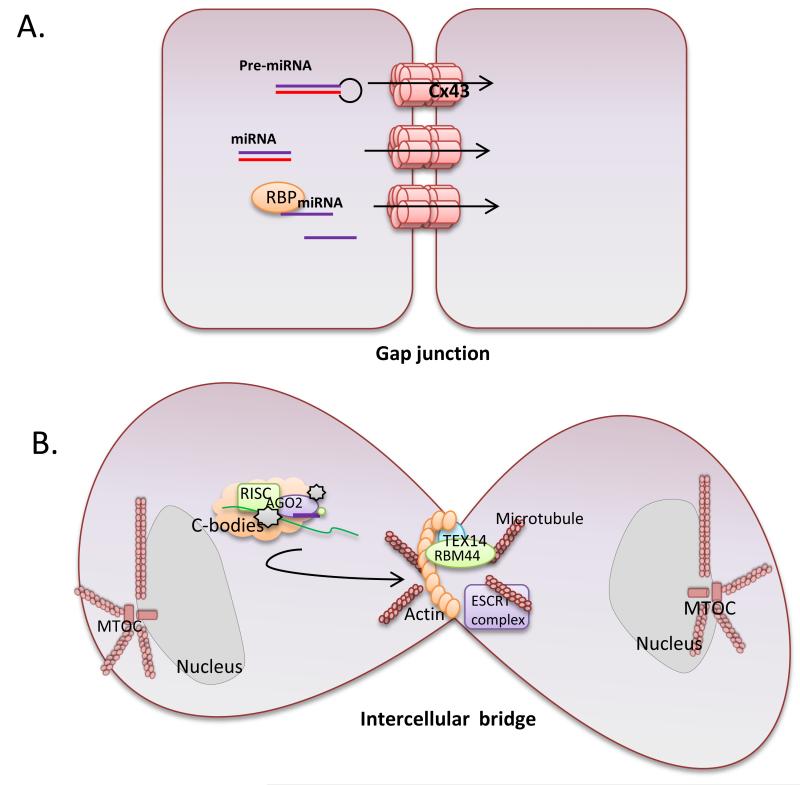
Figure 2. Connective structures for short-distance transfer of genetic material.
Intercellular communication can occur over short distances through the establishment of gap junctions or germ cells intercellular bridges. A. Gap junctions are composed of hexameric connexin oligomers that allow trafficking of small molecules between adjacent cells. The silencing signal might be transported as single-stranded RNA associated with RNA-binding proteins (RBP) or as double-stranded small RNA. B. Intercellular bridges are formed in the germline by incomplete cytokinesis and contain an actin ring. TEX14 and the RNA binding motif protein 44 (RBM44) locates at intercellular bridges. These bridges support cell-to-cell transfer of chromatoid bodies (c-bodies). C-bodies are cytoplasmic granules enriched in microRNA, mRNAs, and proteins of the miRNA-RISC complex.
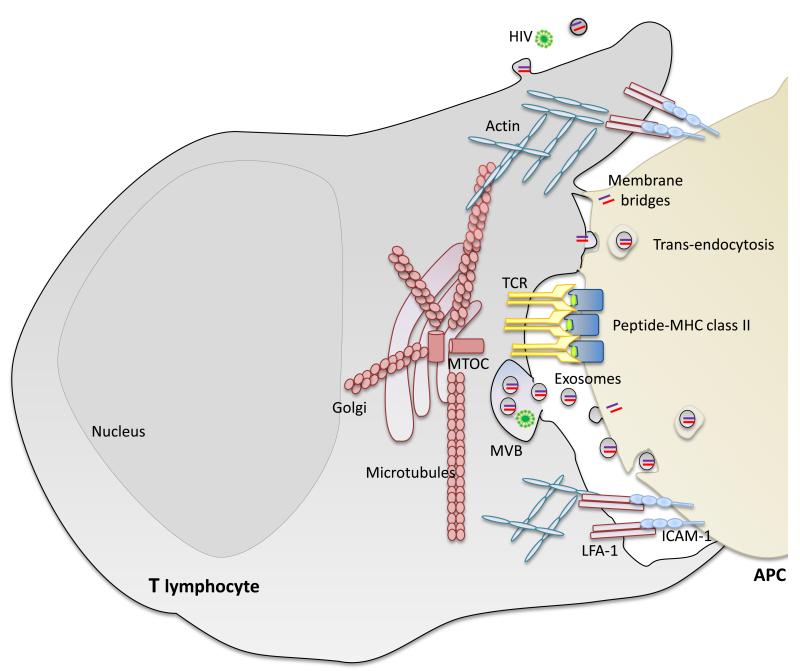
Figure 3. The immune synapse (IS) acts as a platform facilitating the passage of genetic material between cells.
During immune synapsis, the molecules involved in antigen recognition (TCR and peptide-loaded-MHC class II) locate at a central cluster surrounded by a peripheral ring enriched in adhesion molecules (integrin LFA-1 and ICAMs) and the actin cytoskeleton. The T lymphocyte orients its MTOC and secretory compartments (Golgi apparatus and MVBs) toward the APC. We propose that the IS provides a more efficient path for the exchange of genetic material through the combination of different mechanisms, including the polarized secretion of microRNA-loaded exosomes, trans-endocytosis and membrane bridges. Pathogens, including bacteria and viruses, hijack biological synapses to spread from cell to cell. APC, antigen presenting cell; MVB, multivesicular body.
Gap junctions are formed by hexameric connexin oligomers that allow transfer of small molecules (<1.2 kD), such as ions and small metabolites, required for electromechanical connections between neuronal, smooth-muscle, and epithelial cells. Gap junctions are true gates, existing in an open or a closed state. This state is regulated by post-translational modifications of connexins (e.g., redox state or phosphorylation) or variations in transmembrane physical-chemical conditions (e.g., transmembrane voltage, pH and extracellular cation concentration). siRNAs have been suggested to move through connexin-43-based gap junctions 40. Shuttling of microRNA through gap junctions has been described between cardiac cells 41, 42, bone-marrow stromal and tumour cells 43, and glioma cells 44. Transfer of siRNAs and miRNAs is impaired by overexpressing a dominant-negative connexin43 mutant 41 and by gap junction channel uncouplers 42-44. In these studies the passage of fluorescently tagged siRNA analogues or overexpressed microRNAs was observed. Further studies are needed to confirm physiological transfer of RNAs. The molecular pathways regulating the movement of sRNA through gap junctions are as yet unknown.
At the end of cytokinesis, daughter mammalian cells are transiently connected by an intercellular bridge. But in the germline, these transient structures are transformed into stable intercellular bridges interconnecting hundreds of daughter cells in a syncytium. Intercellular bridges are essential for male fertility. They are composed of general cytokinesis molecules and additional germ cell-specific factors, such as TEX14. TEX14 is required for the intercellular bridge stability in gametes of both sexes. The RNA binding motif protein 44 (RBM44) locates at intercellular bridges and interacts with TEX14, and may participate in RNA transport. Intercellular bridges allow sharing of mRNA between post-meiotic haploid spermatids, keeping them phenotypically diploid 45. Cytoplasmic granules loaded with RNA and RNA binding proteins (that in germ cells have been called chromatoid bodies and are related to p-bodies) can move between spermatids through the intercellular bridge in a microtubule-dependent manner 46. Nonetheless, evidence supporting cell-cell movement of sRNA through gap junctions or intercellular bridges is scarce, and other mechanisms, such as nanotubes 47 cannot be excluded.
Synapses as platforms for genetic exchange
Recent evidence indicates that specialized junctional structures such as synapses constitute efficient communication gateways. The intrinsic stability of these junctions promotes the exchange of vesicles and the formation of additional structures, providing an appropriate environment for the exchange of genetic material. Immune synapses (ISs) are formed at the T cell-antigen-presenting cell (APC) interface during antigen recognition, and play a central role in T-cell activation and in the polarized delivery of effector molecules, such as cytokines and lytic granules 48. The T cell receptor (TCR) and associated signalling protein complexes accumulate in a central cluster (cSMAC, central supramolecular activating complex) surrounded by a peripheral ring of adhesion molecules (pSMAC). Signalling triggers massive reorganization of the actin and microtubule cytoskeletons, with the T cell microtubule-organizing centre (MTOC) moving toward the plasma membrane at the cSMAC 49. Both the Golgi and MVBs localize at the synapse 13, 50, where exocytosis and endocytosis occur (Fig. 3) 51.
We propose that ISs facilitate the passage of genetic molecules between cells. The immune synapse promotes exchange of microRNA-loaded exosomes between a T lymphocyte and its cognate APC 13. Transfer of plasma membrane-associated proteins among interacting immune cells is well established 52. Besides promoting the exchange of exosomes, ISs also might facilitate trans-endocytosis and the formation of nanotubes, gap junctions, and membrane bridges between the two cell membranes that mediate direct exchange of proteins 53-57 (Fig. 3). Whether regulatory RNA transfer between immune cells through these structures needs to be addressed.
In the neural system, intercellular communication is primarily mediated by the release from the afferent neuron’s axon of neurotransmitters into the synaptic cleft, and their capture by the dendritic spines of the efferent neuron or by direct conduction through gap junctions. Within neurons, large amounts of mRNA, tRNA, rRNA and sRNA are transported from soma to distal growth cones or distal dendrites for local translation. In addition, Schwann cells can deliver ribosomes, and probably mRNA, to injured axons 58. MVBs are detected in dendrites and presynaptic terminals, where they can fuse with the plasma membrane to release exosomes into the synaptic cleft under the control of glutamatergic synaptic activity 59. Exosomes mediate trans-synaptic protein transfer at Drosophila neuromuscular junctions 60, raising the intriguing possibility that neurons might also exchange information in the form of RNA 61.
Pathogens hijack biological synapses to spread efficiently from cell to cell, reflecting the relevance of synapses as an entry ports also for exogenous genetic material. Some viruses use existing synapses to promote viral spreading, and viruses can also promote the establishment of new contacts (viral synapses) between infected and uninfected cells 62, 63. The exosomal machinery is also exploited by pathogens. For example, HIV particles hijack dendritic cell exosomes to release virus and infect T cells 64, and B cells infected with Epstein-Barr virus transfer exosomes containing viral microRNA to target cells, where it is functional 23 (Fig. 3).
Conclusions/perspective
Accumulating evidence suggests that genetic material, mainly in the form of regulatory RNAs, can be exchanged between cells as extracellular information. Movement of viral-induced siRNAs and transgenes in plants has been known since 1997. However, the movement of endogenous small RNAs and its functional implications has been demonstrated only recently 3-5. In C. elegans, transfer of exogenous and transgenic RNA is well supported, but evidence for functional transfer of endogenous RNA is still lacking. In Drosophila, uninfected cells can take up viral dsRNA, a mechanism of virus-specific intracellular immunity that prevents subsequent infection and virus spread65.The evidence discussed here indicates that movement and intercellular transfer of regulatory RNA also occurs between mammalian cells. Transfer of genetic material adds an exciting and novel dimension to the cell-cell communication modes in complex organisms. However, important questions remain. Defining the routes through which cell-to-cell genetic transfer occurs is a major area of research. EVs were among the first vehicles confirmed to transfer genetic material, and some of the mechanisms through which EVs are shuttled from cell to cell have been elucidated. However, the biological significance of this transfer remains unclear. In vitro approaches based on overexpression of mRNA or microRNA have shown that these molecules are functional in their new location. This phenomenon is extremely interesting from the point of view of gene therapy. The outstanding challenges now are to define whether transfer occurs at endogenous levels of these molecules and what is the physiological importance of this movement.
Substantial progress has been made in plants regarding the kind of information transferred. 21nt and 24nt RNA duplexes have been shown to move from cell to cell through plasmodesmata (PD), and silencing signals may also be transported as single-stranded RNA through the phloem. In C. elegans, SID1 and SID2 are thought to meditate the cellular uptake of double-stranded RNA. Definition of the nature of the small RNAs that can be transferred in mammals is still pending.
A phenomenon apparently exclusive to plants and C. elegans is that the silencing signal can be amplified, inducing systemic silencing. Amplification of the RNAi signal involves RNA-dependent RNA polymerases (RDRs), which copy secondary siRNAs corresponding to flanking sequences upstream or downstream of the originally targeted sequence. The secondary siRNAs are thus able to target parts of the mRNA not targeted by the original siRNA 66, 67. The first functional mammalian homologue of RDRs was recently identified in human cells 68. Future challenges include determining whether amplification of RNAi signals occurs in mammals and if RDR-related molecules are involved.
Depending on the pathway used, genetic communication can occur over a distance or be confined to the local microenvironment. The exchange of genetic material mainly takes two forms. In one, particles secreted by the donor cell are taken up by an acceptor cell. These particles include EVs such as exosomes and apoptotic bodies or ribonucleoproteins. The other mode of transfer involves the formation of intimate membrane contacts between donor and acceptor cell, such as gap junctions in animal cells, and plasmodesmata in plants. Specialized contacts such as cellular synapses combine all these modes of genetic transfer. Cellular synapses and other cellular connective structures can provide specialized platforms for the intercellular transfer of genetic material. These structures could potentially be manipulated to achieve specific and efficient delivery of regulatory RNAs to either potentiate or suppress processes in the acceptor cell. The design of therapeutic delivery systems to take advantage of connective intercellular structures such as synapses holds the promise of specific delivery of ‘therapeutic’ exosomes to selected target cells. The therapeutic and diagnostic potential of RNA-based communication is just emerging (Box 2).
BOX 2. The therapeutic and diagnostic potential of RNA-based communication.
Circulating cell-free nucleic acids have promising potential as non-invasive diagnostic markers for pathological processes such as chronic inflammation, cancer and cardiovascular disease 69, 70. Detection of genetic biomarkers in the blood of cancer patients might provide insight into the genetic status of individual tumours and the tissue origin of cancers of unclear primary origin 71. Another exciting area is the existence of cell-free fetal DNA and mRNA in the blood of pregnant women, which opens the possibility of extending the current armoury of non-invasive methods for prenatal diagnosis 72.
Delivery of nucleic acids to target cells is a major challenge for clinical medicine, offering a possible therapeutic strategy for regenerative medicine and the treatment of cancer and viral infection. The main obstacle to achieving gene silencing by RNAi technologies in vivo is specific delivery. Several strategies have been suggested for systemic delivery of siRNA. One involves chemically modified siRNAs, synthetic nanoparticles or exosomes 73. An advantage of exosomes as RNA delivery vehicles is that they do not activate the interferon response and are, in principle, safer and more manageable than cell therapy. Unlike soluble factors, exosomes are protected from the environment by their lipid bilayer, and can reach their target cells. A second advantage is that extracellular vesicles can deliver multiple messages simultaneously, including specific subsets of mRNA, microRNA or proteins. This factor highlights the need to expand our knowledge of how specific genetic messages are selected for incorporation into exosomes, and how vesicles containing mRNA and miRNA are released and circulate in the bloodstream.
Acknowledgements
M.M. is supported by Instituto de Salud Carlos III (Spanish Ministry of Science and Innovation, Spain). F.S.-M. is supported by grants SAF2011-25834, and ERC-2011-AdG 294340-GENTRIS. Editorial support was provided by S. Bartlett. The authors thank Pablo Vera, Sergio Moreno, Miguel Vicente-Manzanares, Miguel Manzanares, Francesc Baixauli, Cristina Gutierrez-Vazquez and Carolina Villarroya for critical reading of the manuscript. The Centro Nacional de Investigaciones Cardiovasculares (CNIC) is supported by the Spanish Ministry of Science and Innovation and the Pro-CNIC Foundation.
Biographies
Maria Mittelbrunn Maria Mittelbrunn did her training in immunology at the Hospital Universitario de la Princesa, Madrid. Since 2007 she has worked as a Research Associate at the Centro Nacional de Investigaciones Cardiovasculares (CNIC). Her research focuses on the molecular basis of communication processes during the formation of the immune synapse between antigen presenting cells and T cells.
Francisco Sánchez-Madrid Francisco Sánchez-Madrid did his postdoctoral training at Harvard Medical School. He established his laboratory in 1985 at the Hospital Universitario de la Princesa, Madrid. He is Professor of Immunology at the Universidad Autónoma de Madrid and the Scientific Director of the Princesa Research Institute. His research interests include the molecular mechanisms of leukocyte adhesion, polarization, migration and activation, especially in relation to interactions with endothelium and the formation of the immune synapse, and their relevance to inflammatory diseases.
REFERENCES
- 1.Melnyk CW, Molnar A, Baulcombe DC. Intercellular and systemic movement of RNA silencing signals. EMBO J. 2011;30:3553–63. doi: 10.1038/emboj.2011.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brosnan CA, Voinnet O. Cell-to-cell and long-distance siRNA movement in plants: mechanisms and biological implications. Curr Opin Plant Biol. 2011;14:580–7. doi: 10.1016/j.pbi.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Carlsbecker A, et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature. 2010;465:316–21. doi: 10.1038/nature08977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunoyer P, et al. Small RNA duplexes function as mobile silencing signals between plant cells. Science. 2010;328:912–6. doi: 10.1126/science.1185880. [DOI] [PubMed] [Google Scholar]
- 5.Molnar A, et al. Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science. 2010;328:872–5. doi: 10.1126/science.1187959. [DOI] [PubMed] [Google Scholar]
- 6.Slotkin RK, et al. Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell. 2009;136:461–72. doi: 10.1016/j.cell.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 8.Jose AM, Garcia GA, Hunter CP. Two classes of silencing RNAs move between Caenorhabditis elegans tissues. Nat Struct Mol Biol. 2011 doi: 10.1038/nsmb.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whangbo JS, Hunter CP. Environmental RNA interference. Trends Genet. 2008;24:297–305. doi: 10.1016/j.tig.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science. 2002;295:2456–9. doi: 10.1126/science.1068836. [DOI] [PubMed] [Google Scholar]
- 11.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 13.Mittelbrunn M, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montecalvo A, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–66. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 16.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–81. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–7. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 18.Babst M. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr Opin Cell Biol. 2011;23:452–7. doi: 10.1016/j.ceb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–68. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 20.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Baj-Krzyworzeka M, et al. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother. 2006;55:808–18. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehnfors J, et al. Horizontal transfer of tumor DNA to endothelial cells in vivo. Cell Death Differ. 2009;16:749–57. doi: 10.1038/cdd.2009.7. [DOI] [PubMed] [Google Scholar]
- 23.Pegtel DM, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–33. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meckes DG, Jr., et al. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci U S A. 2010;107:20370–5. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balaj L, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irion U, St Johnston D. bicoid RNA localization requires specific binding of an endosomal sorting complex. Nature. 2007;445:554–8. doi: 10.1038/nature05503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143–9. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 28.Lee YS, et al. Silencing by small RNAs is linked to endosomal trafficking. Nat Cell Biol. 2009;11:1150–6. doi: 10.1038/ncb1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deregibus MC, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–8. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 30.Zernecke A, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–44. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Grange C, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–56. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 33.Arroyo JD, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–33. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–33. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–59. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shih JD, Hunter CP. SID-1 is a dsRNA-selective dsRNA-gated channel. RNA. 2011;17:1057–65. doi: 10.1261/rna.2596511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101:2087–92. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolfrum C, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–57. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 40.Valiunas V, et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568:459–68. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kizana E, Cingolani E, Marban E. Non-cell-autonomous effects of vector-expressed regulatory RNAs in mammalian heart cells. Gene Ther. 2009;16:1163–8. doi: 10.1038/gt.2009.64. [DOI] [PubMed] [Google Scholar]
- 42.Hosoda T, et al. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123:1287–96. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Lim PK, et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550–60. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 44.Katakowski M, Buller B, Wang X, Rogers T, Chopp M. Functional microRNA is transferred between glioma cells. Cancer Res. 2010;70:8259–63. doi: 10.1158/0008-5472.CAN-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braun RE, Behringer RR, Peschon JJ, Brinster RL, Palmiter RD. Genetically haploid spermatids are phenotypically diploid. Nature. 1989;337:373–6. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- 46.Morales CR, et al. A TB-RBP and Ter ATPase complex accompanies specific mRNAs from nuclei through the nuclear pores and into intercellular bridges in mouse male germ cells. Dev Biol. 2002;246:480–94. doi: 10.1006/dbio.2002.0679. [DOI] [PubMed] [Google Scholar]
- 47.Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9:431–6. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- 48.Huse M, Quann EJ, Davis MM. Shouts, whispers and the kiss of death: directional secretion in T cells. Nat Immunol. 2008;9:1105–11. doi: 10.1038/ni.f.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vicente-Manzanares M, Sanchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol. 2004;4:110–22. doi: 10.1038/nri1268. [DOI] [PubMed] [Google Scholar]
- 50.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–27. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griffiths GM, Tsun A, Stinchcombe JC. The immunological synapse: a focal point for endocytosis and exocytosis. J Cell Biol. 2010;189:399–406. doi: 10.1083/jcb.201002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7:238–43. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 53.Chauveau A, Aucher A, Eissmann P, Vivier E, Davis DM. Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells. Proc Natl Acad Sci U S A. 2010;107:5545–50. doi: 10.1073/pnas.0910074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mendoza-Naranjo A, et al. Functional gap junctions accumulate at the immunological synapse and contribute to T cell activation. J Immunol. 2011;187:3121–32. doi: 10.4049/jimmunol.1100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qureshi OS, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–3. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–61. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 57.Ueda H, Morphew MK, McIntosh JR, Davis MM. CD4+ T-cell synapses involve multiple distinct stages. Proc Natl Acad Sci U S A. 2011;108:17099–104. doi: 10.1073/pnas.1113703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Court FA, Hendriks WT, MacGillavry HD, Alvarez J, van Minnen J. Schwann cell to axon transfer of ribosomes: toward a novel understanding of the role of glia in the nervous system. J Neurosci. 2008;28:11024–9. doi: 10.1523/JNEUROSCI.2429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lachenal G, et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46:409–18. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 60.Korkut C, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dinger ME, Mercer TR, Mattick JS. RNAs as extracellular signaling molecules. J Mol Endocrinol. 2008;40:151–9. doi: 10.1677/JME-07-0160. [DOI] [PubMed] [Google Scholar]
- 62.Igakura T, et al. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–6. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- 63.Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283–93. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Izquierdo-Useros N, et al. HIV and mature dendritic cells: Trojan exosomes riding the Trojan horse? PLoS Pathog. 2010;6:e1000740. doi: 10.1371/journal.ppat.1000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saleh MC, et al. Antiviral immunity in Drosophila requires systemic RNA interference spread. Nature. 2009;458:346–50. doi: 10.1038/nature07712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sijen T, et al. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–76. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- 67.Vaistij FE, Jones L, Baulcombe DC. Spreading of RNA targeting and DNA methylation in RNA silencing requires transcription of the target gene and a putative RNA-dependent RNA polymerase. Plant Cell. 2002;14:857–67. doi: 10.1105/tpc.010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maida Y, et al. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature. 2009;461:230–5. doi: 10.1038/nature08283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cortez MA, et al. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8:467–77. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469:336–42. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenfeld N, et al. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol. 2008;26:462–9. doi: 10.1038/nbt1392. [DOI] [PubMed] [Google Scholar]
- 72.Hung EC, Chiu RW, Lo YM. Detection of circulating fetal nucleic acids: a review of methods and applications. J Clin Pathol. 2009;62:308–13. doi: 10.1136/jcp.2007.048470. [DOI] [PubMed] [Google Scholar]
- 73.Alvarez-Erviti L, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–5. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]