Abstract
Objective(s)
Depletion of thymus derived naive T-cells is a feature of HIV infection. Here the impact of HIV infection on the compartmentalization of recent thymic emigrants of (RTE) and naive T-cells was examined.
Methods
Peripheral blood mononuclear cells (PBMC) and lymphoid tissue (LT) from 43 HIV-infected patients and 12 controls were examined for RTE distribution by measuring coding joint T-cell receptor excisional circles (cjTREC) by PCR and naive and memory T-cell subsets and adhesion molecules (L-selection, LFA-1) by flow cytometry.
Results
In HIV-infected patients, the RTE as quantified by cjTRECs in CD4 LT cells were significantly higher than in PBMC. Their values, however, were less than in control subjects, in both the LT and PBMC compartments. This was associated with an increase in L-selectin and LFA-1 expression on LT derived T cells. In PBMC, a significant positive relationship between TREC and naive CD4 cells and an inverse relationship between TREC and cellular viral load (CVL) was observed. Whereas in LT, there was a positive relationship between cjTREC and both naive CD4 cell percentage and CVL.
Conclusions
Collectively, the data suggests that LT is a significant reservoir for RTE. The RTE appeared to be entrapped in LT from HIV-infected subjects. Such entrapment is probably a response to the high viral load in these tissues. These observations may partially explain the decline in RTE observed in the peripheral blood of HIV-infected patients, and the delay in recovery of naive cells in blood after initiation of HAART.
Keywords: Naive T cells, recent thymic emigrants, TRECs, HIV, AIDS
Introduction
HIV-1 is known to alter the representation of memory and naive cells in the peripheral blood of infected individuals [1,2]. The depletion of thymus-derived naive CD4 and CD8 T cells is a fundamental feature of HIV-1 infection [1,2]. As naive CD4 cells are more resistant to productive infection by HIV-1 than memory cells [3–5], it is unlikely that they are being destroyed by direct viral infection. However, it is possible that they are not being produced due to thymic failure assuming that this is a primary pathologic lesion in HIV-1 disease as previously suggested [6–8]. Alternatively the loss of naive T cells may be due to enhanced differentiation to memory T cells or to enhanced programmed cell death of the activated CD45RACD62L subpopulation from exposure to proinflammatory cytokines. Increased apoptosis has been shown to occur in T cells from HIV-infected patients and particularly from those with AIDS [9]. Apoptosis occurs in both naive and memory CD4 and CD8 T-cells from HIV-infected patients [10], and this contributes to the HIV-driven immunosuppression.
Partial restoration of memory and naive T-cell sub-populations in patients on potent antiretroviral regimens has been reported [11–13]. The rise in naive CD4 cells, however, occurs at much later time in the course of treatment than that of memory cells [12]. Although the patients clinically improve rapidly, their T-cell subsets are not fully reconstituted. The thymus, where T lineage cells differentiate and mature to naive cells [14] maintains the peripheral T-cell pool to varying degrees through life. The thymus decreases substantially in size with age and has been felt to become functionally negligible by adulthood. A substantial number of HIV-infected subjects however, aged 20–59 years, have been shown by computed tomography to have thymic tissue [15,16]. The presence of detectable thymic tissue was significantly associated with younger age, a shorter duration of infection and a higher absolute number of naive CD4 T cells [15]. Individuals with abundant thymic tissue developed higher levels of naive cells after highly active antiretroviral therapy (HAART) than those with minimal tissue [6]. This suggests there is active thymic tissue in some of these individuals into adulthood.
Recently the functionality of the thymus has been evaluated by measuring recent thymic emigrants (RTE) in peripheral blood mononuclear cells (PBMC) using episomal DNA circles, the TCR excision circles (TREC), that result from T-cell receptor α β gene rearrangements as biomarkers [17–20]. The changes in TREC levels suggests that thymic output is maintained into late adulthood, decreases during HIV infection and rapidly increases in adults after HAART. It has also been shown that HIV-infected subjects who had baseline TREC levels within the normal range did not have increases in RTE following HAART, while those with preexisting impaired levels showed significant increases [18]. These increases were, however, numerically insufficient to account for the observed increases in naive CD4 T cell counts.
T cells migrate continuously between peripheral blood and secondary lymphoid tissue (LT) [21]. The patterns of migration of the memory and naive subpopulations have distinct patterns [22] that are influenced by the expression of homing molecules such as L-selectin [23] and adhesion receptors [24,25]. While memory cells tends to migrate to peripheral blood, naive cells tend to home to LT. In LT, naive cells after encountering the appropriate antigens are activated and may further differentiate to effector/memory cells. It has been shown previously that HIV viral burden in the LT microenvironment is several fold higher than in peripheral blood [26–28]. The impact of the infection on the homeostasis of naive and memory T-cell subpopulations and their entrapment in LT, has not been thoroughly examined. In this report the relationship of HIV infection to the compartmentalization of RTE and naive T cells and to markers of disease progression were examined.
Materials and methods
Patient population
A total of 43 HIV-infected subjects participated in this study, 37 males and six females. The age range was 25–50 years (median, 38 years). The CD4 cell counts ranged from 11 × 106–1450 × 106 cells/l (median, 443 × 106 cells/l) and the HIV plasma viral load ranged from < 20 copies/ml to 7.5 × 105 copies/ml (median, 428 copies/ml). The patients were untreated (three subjects) or had been on dual or triple antiretroviral regimens for more than 3 months. LT was obtained from cervical excisional lymph node biopsies or tonsillar tissue. The procedures were performed in the General Clinical Research Center (GCRC) at the University of Texas Medical Branch, Galveston, Texas, USA. The LT cells were then mechanically dissociated in suspension before being analyzed for immunopheno-typic markers or extracted for cellular viral burden and TREC analysis. PBMC were obtained from blood collected from the patients at the time of the biopsy. Twelve control subjects ranged in age from 20 to 55 years (median, 35 years) participated in this study.
HIV viral burden
Plasma was separated from EDTA anti-coagulated blood and frozen for batch analysis. The quantification of HIV RNA copy number was performed by RT– PCR using the Amplicor HIV-1 Monitor Standard and UltraSensitive kits (Roche Diagnostic, Branchburg, New Jersey, USA). For the HIV cellular viral burden, 1 × 105 and 5 × 105 viable LN or PBMC were suspended in 200 μl cell phosphate buffer saline (PBS). The aliquots were then assayed by the Roche Monitor kit [28]. HIV RNA in all samples were measured initially by the Standard kits. If samples had <200 copies/ml, they were reevaluated by the Ultrasensitive kit; values < 20 copies/ml were considered undetectable.
Flow cytometry
Evaluation of naive versus memory subsets and adhesion molecules were done by fluorescence activated cell sorting (FACS) as described previously [10]. Briefly, 20 μl of anti-CD4 peridinin chlorophyll-a protein and 20 μl of either anti-CD45RO–phycoerythrin (PE)/ CD45RA–fluorescein isothiocyanate (FITC), CD45 RA–FITC/CD62L–PE, LFA-1–FITC or isotype matched control antibodies (Pharmingen, San Diego, California, USA) were mixed with 100 μl EDTA-treated whole blood. The cells were incubated in the dark for 30 min at 4°C. After incubation red blood cells were lysed by adding 2 ml 10% FACS Lysing Solution (Becton Dickinson, Mountanview, California, USA) to each sample. Samples were then incubated for 10 min at room temperature, centrifuged and washed once with cold PBS. After fixation in 2% paraformaldehyde the samples were stored at 4°C until analyzed. Three-color flow cytometric analysis was performed within 24 h of fixation on a FACSORT flow cytometer with collection and analysis of data using CellQuest software (Becton-Dickinson). The analysis was performed on 10 000 collected lymphocytes.
Measurement of RTE by TREC
solation of total CD4 cellular DNA
CD4 T lymphocytes were isolated by positive selection from PBMC or lymph node/tonsil LT cells using magnetic beads (Dynal, Inc., Lake Success, New York, USA) according to the manufacturer's recommendations. One million purified CD4 T cells were then lysed and total cellular DNA was isolated by a standard phenol–chloroform extraction procedure.
Measurement of TRECs
The copy number of cjTRECs in 1 μg of the cellular DNA was measured by PCR–ELISA as described previously [29]. The sequences of the primers used for amplifying VαJα coding joints were: 5′-CTAATAA TAAGATCCTCAAGGGTCGAGACTGTC-3′ (forward primer), and 5′-CCTGTTTGTTAAGGCACAT TAGAATCTCTCACTG-3′ (reverse primer). Briefly, 1 μg DNA was amplified in the presence of 1 × PCR buffer, 20 mM MgCl2, 20 mM dNTPs containing digoxigenin-labeled UTP, 2.2 mM each primer, and 1 U Taq DNA polymerase. The PCR was started at 95°C for 5 min, and consisted of 25 cycles of amplification (90°C for 30 s, 60°C for 30 s, 72°C for 30 s), and followed by extension at 72°C for 7 min. The PCR products were used for ELISA measurement using DIG-detection kit (Roche Molecular Inc./Boehringer Mannheim, Mannheim, Germany). For each sample, 10 μl PCR product was hybridized at 55°C for 3 h with biotin-labeled VαJα coding joint probe (5′-TCTGTGTCTAGCACGTAGCC-3′) in a streptavi-din-coated ELISA microplate. After washing the mi-croplate, anti-digoxigenin conjugated peroxidase was added and incubated for 30 min at 37°C, followed by addition of the colorimetric substrate ABTS. The colorimetric reaction was measured in a photometric ELISA reader at 405 nm. Plasmid DNA containing known copy numbers of the VαJα-coding joint were used as standard to determine the TREC copy numbers in the sample DNA.
Statistical analysis
Comparisons of means of paired samples (PBMC versus LT from same subject) only were evaluated by paired Student's t test, for other evaluations unpaired Student's t test for difference of means was used. Means ± SE are stated throughout. For correlations between two parameters the predicted lines were determined by simple linear regression analysis. The P values and Pearson's correlation coefficients, (r), were calculated by using the Stat-100 statistical package (Biosoft, Cambridge, UK). Simple linear regression analysis was used for the evaluation of trend relationships of cjTRECs to CD4 and HIV viral load using the same statistical package.
Results
Distribution of RTE in PBMC and LT compartments of HIV-infected subjects
The LT microenvironment represents the site in which naive lymphocytes differentiate to the memory phenotype in response to appropriate antigen exposure. As it has been shown that in HIV-infected subjects the percentages of naive T cells in peripheral blood is diminished as compared to that in normal controls, the distribution of naive CD4 RTE, as measured by cjTREC, in LT and PBMC was examined. The cjTREC levels in the LT and PBMC, compartments of HIV-infected subjects were significantly lower than those of HIV-seronegative controls (Table 1). While the cjTREC levels in the LT and PBMC compartments from HIV-seronegative controls were not significantly different, the cjTREC levels in CD4 T cells in the LT of HIV-infected subjects were significantly higher than those in PBMC.
Table 1.
TREC levels in CD4 T cells from peripheral blood mononuclear cells (PBMC) and lymphoid tissue (LT) mononuclear cells from HIV-infected individuals. Data are mean ± SE.
| LT | PBMC | ||||
|---|---|---|---|---|---|
|
|
|
||||
| n | cjTREC levels (copies/μg DNA) | n | cjTREC levels (copies/μg DNA) | P (paired t test) | |
| HIV seronegative | 6 | 207 000 + 29978 | 12 | 144226 + 25885 | 0.066 (n = 6) |
| HIV seropositive | 19 | 82798 + 16840 | 43 | 41401 + 7876 | 0.0014 (n = 19) |
| P (unpaired t test) | 0.0015 | 0.003 | |||
Effect of age,absolute CD4 cell count and HIV plasma RNA on TREC levels from HIV-infected individuals
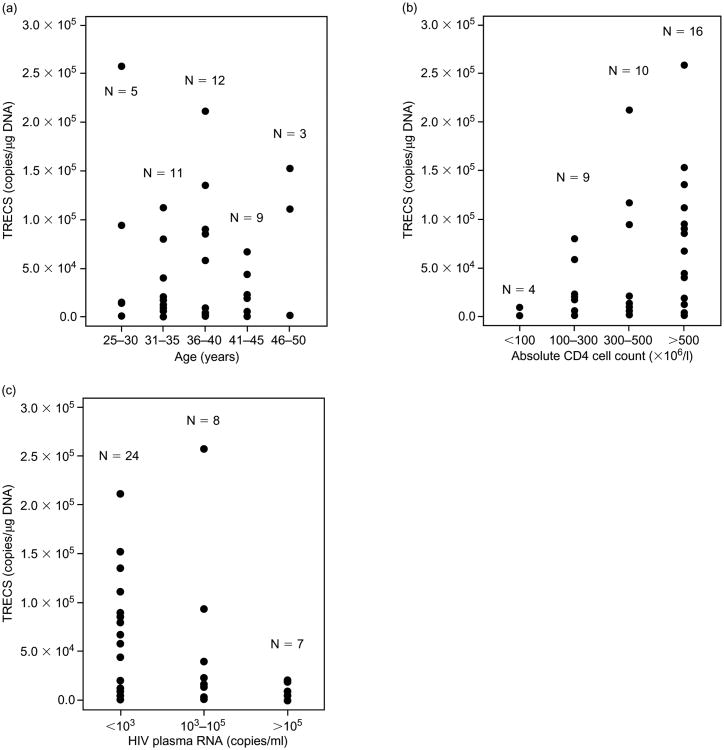
As cjTREC levels have been shown to be age dependent [17,18], we compared these levels among the age groups of the patients on the study. As shown in Fig. 1a, there was no statistically significant difference or appearance of a trend in cjTREC levels between the age groups examined. Others also, have not seen a difference in TREC levels between individuals in the 25–50-year age range [18].
Fig. 1.
Relationship of TREC to age (a), absolute CD4 cell counts (b) and circulating HIV plasma RNA (c) of HIV-infected individuals. The trends for these relationships were evaluated statistically using simple linear regression analysis.
When the patients were stratified by their absolute CD4 T cell count, the cjTREC levels appeared to increase with the rise in the absolute counts (Fig. 1b). The mean cjTREC levels in the < 100, 100–300, 300–500 and the > 500 × 106 CD4 cells/l groups were 2525 ± 2173, 25 554 ± 8800, 47 952 ± 22 296 and 76 622 ± 17 261 copies/μg DNA respectively. The apparent trend for the increase of cjTREC concentration with the increase in CD4 cell counts was statistically significant (r, −0.37; P < 0.0188).
The relationship of TREC to the circulating HIV plasma RNA was also examined (Fig. 1c). The cjTREC levels in the subjects with < 103, 103 to 105, and > 105 copies/ml were 55 003 ± 11 911, 56 320 ± 30 600 and 7814 ± 3396 copies/μg DNA respectively. There was an apparent trend for an increase in cjTREC with the decrease in HIV viral load (r, −0.36; P = 0.0278). A significant difference however in the TREC levels from CD4 cells was observed between the patients groups with < 1000 and > 100 000 copies of HIV RNA/ml plasma (P < 0.0001). The fact that there was no difference in TREC levels between the subjects with < 1000 and those with 1000–100 000 copies of HIV RNA/ml plasma suggests that the functionality of the thymus is severely compromised only at late stages of HIV infection.
Distribution of naive and memory CD4+ T cells in LTand PBMC
The distribution of naive (CD45RACD62L) and memory (CD45RO+CD45RA−) CD4 T cells in the LT and PBMC compartments were examined next. The means of the percentages of naive and memory CD4 T cells in LT from HIV-seropositive patients were significantly different from those from HIV-seronegative controls (Table 2). The percentage of naive cells in LT was approximately half that in the PBMC in control subjects, and 1.3-fold that in PBMC from HIV-infected patients. This dramatic difference, in percentage of naive cells in LT, between infected and uninfected subjects suggests that naive cells are perhaps sequestered in the LT tissues in response to HIV infection.
Table 2.
Distribution of naive and memory CD4 T cells in peripheral blood mononuclear cells (PBMC) and lymphoid tissue (LT) mononuclear cells from HIV-infected individuals.
| HIV-seropositive (n = 19) | HIV-seropositive (n = 6) | P (unpaired t test) | |
|---|---|---|---|
| Naive cells (mean percentage ± SE) | |||
| LT | 49 ± 2 | 20 ± 5 | 0.008 |
| PBMC | 37 ± 5- | 42 ± 5 | 0.13 |
| P (paired t test) | 0.0009 | 0.016 | |
| Memory cells (mean percentage ± SE | |||
| ) | |||
| LT | 42 ± 3 | 74 ± 3 | 0.0001 |
| PBMC | 51 ± 4 | 63 ± 4 | 0.039 |
The relationship of TREC to percentage CD4 T cells and naive cells from HIV-infected patients
The relationship of the percentage of CD4 cells and their naive subpopulation to RTE in LT and PBMC was evaluated. A significant positive correlation between cjTREC levels and the percentage of CD4 cells in either LT or PBMC was observed (r, 0.72; P = 0.011 and r, 0.65; P = 0.016, respectively). A positive correlation between cjTREC and naive CD4 T cells in peripheral blood was also observed (r, 0.55; P = 0.04). This relationship however did not achieve statistical significance in the LT compartment (r, 0.5; P = 0.11; data not shown).
The relationship of TREC to the viral load in LT and PBMC compartments
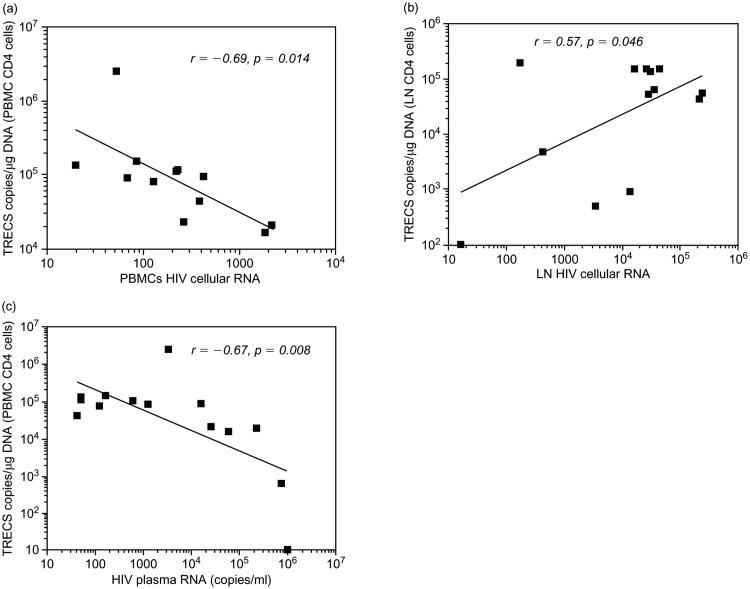
Fourteen HIV-infected subjects with paired samples from peripheral blood and LT were examined for their cjTREC levels in relationship to plasma and cell associated HIV RNA. The viral load in LT cells ranged from 16 to 239 880 copies/105 cells (median, 27 459 copies/105 cells) and in PBMC from undetectable to 2154 copies/105 cells (median 229 copies/105 cells). The cell associated viral load in LT was approximately 120-fold that in PBMC. There was an apparent inverse correlation between cjTREC levels and HIV cell associated (r, −0.69; P = 0.014) and plasma RNA (r, −0.67; P = 0.008) respectively (Fig. 2a and c). In contrast, the TREC levels in LT (Fig. 2b) positively correlated with the viral load in LT cells (r, 0.57; P < 0.046). These data suggest that the increase in viral load in LT is instrumental in attracting and retaining RTE in this compartment.
Fig. 2.
The relationship of TREC to cellular and plasma viral load in an HIV-infected cohort. The cellular viral load was measured in lymph node (LN) cells and PBMC of HIV-infected subjects. (a) n = 12, (b) n = 13, (c) n = 14.
The distribution of L-selectin and LFA-1 in LTand PBMC
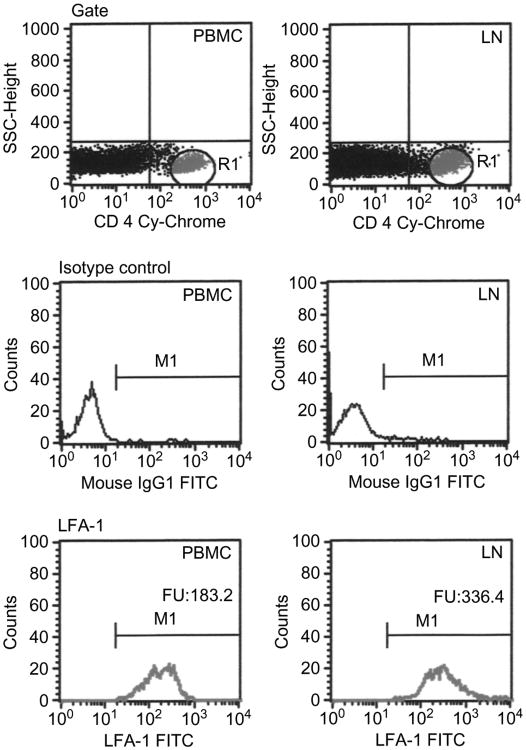
The pattern of the adhesion molecules, L-selectin and LFA-1 expression on T cells was examined. These molecules are known to be involved in facilitating the recruitment of T cells to LT. The percentage of the CD4 T-cell subpopulation expressing LFA-1 was approximately 95% or higher in both the PBMC or LN compartments (data not shown). However, the mean fluorescence intensity (MFI) for LFA-1 on CD4 in the LT, 298 ± 67 fluorescent units (FU; n = 9), was significantly higher than in PBMC, 139 ± 19 FU (P = 0.03, paired t test). The pattern of LFA-1 expression in T-cell subsets from a representative patient is shown in Fig. 3. This suggests that a higher number of LFA-1 molecules per cell are expressed in LT CD4 cells than in peripheral blood CD4 T cells.
Fig. 3.
FACS analysis for the expression of LFA-1 on CD4 T cells. The panels showa comparison for the expression of LFA-1 in peripheral blood and lymph nodes obtained from a representative patient infected with HIV.
L-selectin, however, displayed a different pattern. In control HIV-seronegative subjects, the percentage of CD4 T cells expressing L-selectin in LT (60%) was significantly less than in PBMC (83%) from the same subjects (P = 0.017; n = 16; paired t test). In contrast, in HIV-infected subjects the percentage CD4 cells expressing L-selectin in LT (82%) was significantly higher than in controls (P = 0.018; unpaired t test) and approached a value similar to that observed in PBMC (data not shown).
Discussion
In the HIV-infected cohort examined in this study, the level of RTE in peripheral blood as determined by measuring cjTREC, was found to be significantly less than in uninfected control subjects, in agreement with previously reported studies [17,18]. The wide range of variation in TREC levels among the patients was independent of their age (range, 25–50 years), but was more related to the severity of their disease. The concentration of RTE in the blood was severely diminished in patients with > 100 000 copies HIV RNA/ml plasma. The cjTREC levels however, were more associated with changes in the absolute CD4 cell counts than with changes in the plasma viral load. Significant levels of cjTREC were observed in patients with viral loads up to 100 000 copies/ml and some had levels that were within the normal range. This suggests that in HIV infection, although thymic function declines, substantial output of naive cells is maintained through later stages of infection.
The data presented show that LT is a significant reservoir for RTE in both HIV-seronegative as well as -seropositive subjects. This is not surprising as naive T cells after exiting the thymus migrate to secondary LT such as peripheral lymph nodes and Peyer's patches in search for their predetermined antigen [30]. This is in contrast to the previous suggestions that lymph nodes had extremely low amounts of RTE [17]. We performed our studies on viable CD4 T cells from LT cell suspensions in real time, whereas the study by Douek et al. [17] measured the TREC in DNA extracted from formaldehyde-fixed, paraffin-embedded lymph nodes. Interestingly, in HIV-infected patients the level of RTE in LT was significantly higher than in peripheral blood. The TREC levels however, in both the LT and PBMC compartments were lower than from uninfected control subjects. TREC levels in peripheral blood tend to decrease if thymic output is impaired [17,18] or if there is peripheral expansion of new naive T cells in the circulation [17,31]. As there were more RTE in LT than in PBMC, it is possible that the observed decline of RTE in the peripheral blood could partially result from selective trapping of these cells in the lymph nodes.
The cellular viral load from LT cells was significantly higher than in PBMC as previously reported [26–28]. Among the patients examined, both the PBMC and plasma viral load inversely correlated with the PBMC/ TREC levels. In LT on the other hand, the cellular viral load positively correlated with the LT/TREC levels. This correlation leads us to suggest that the increased concentration of RTE in LT as compared to PBMC in infected individuals may be a response to the high viral load in these tissues. The increased RTE and naive CD4 cells in LT may perhaps indicate their entrapment or sequestration in these tissues. The naive cells are more resistant to HIV infection than memory cells [3,4,32,33]. Thus the entrapment may be needed to compensate for the depletion of the memory cells resulting from infection. Consequently naive cells will clonally expand and differentiate to effector and memory cells.
Entrapment of naive cells in LT can be facilitated by expression of L-selectin, that is required for homing of RTE, and by activation-dependent adhesion mechanisms, that are mediated by leukocyte integrins [22–24, 30]. L-selectin is expressed on all circulating leukocytes except memory cells [34]. In the present study, the frequency of expression of L-selectin on CD4 cells from LT was enhanced as compared to controls. Moreover, the expression of LFA-1, an adhesion marker, was higher in LT than in PBMC from the same subject. The functional activation of LFA-1 on the surface of lymphocytes mediates firm transient attachment to widespread cellular and extra-cellular matrix components in LT [35–38]. The functional activation of the integrins occurs when lymphocytes encounter their appropriate antigen and become activated. We and others have shown previously that the levels of T-cell activation in LN cells were higher than in PBMC from the same HIV-infected subjects [28,39].
The initial increase in CD4 cells in patients treated with antiretroviral therapy was suggested to occur as a result of de novo synthesis from improved thymic function [17]. This was based on the assumption that HIV-infection suppressed thymic function and that TREC levels increased much earlier [17] than naive cells in patients on HAART [12,40]. Others attributed the CD4 increase in peripheral blood to redistribution of lymphocytes from secondary lymphoid tissues to the circulation [39]. This was based on the observation that T-cell activation markers and the frequency of cells expressing the adhesion markers, intracellular-, and vascular cell adhesion molecules (ICAM and VCAM, respectively) were dramatically reduced in lymph nodes following HAART. We believe that the increase in CD4 cells occurs by both mechanisms – de novo thympoiesis as well as redistribution. The fact that RTE levels positively correlated with the CD4 cell percentage, absolute CD4 cell counts and percentage naive cells and their inverse relation to the viral load in the peripheral blood argues for de novo thympoiesis, particularly in patients with advanced HIV disease. Alternatively the high concentration of RTE and enhanced expression of L-selectin and LFA-1 on T cells in association with the high cellular viral load in LT favors the redistribution view. The data from the present study suggests that entrapment of RTE in LN facilitates their differentiation to memory cells. The memory cell would then, upon significant resolution of the local infection during HAART, be released to the circulation. The memory, rather than the naive, T-cell subpopulation was shown to be predominantly responsible for the early increase in CD4 during HAART [12,13]. Once equilibrium has occurred, and HIV mediated cell death has ceased, migrating RTE and naive cells may also be released from the LT and contribute to the restoration of the CD4 cell levels.
Collectively, the data suggest that thymic output is maintained in a substantial number of HIV-infected patients despite the high viral load and that LT is a significant reservoir for RTE. The RTE, as determined by cjTREC, and naive CD4 cells appeared to be entrapped or sequestered in LT from HIV-infected subjects. Such entrapment is probably a response to the high viral load in these tissues. These observations may explain in part the decline in RTE observed in the peripheral blood of HIV-infected patients, and the delay in recovery of NV cells in blood after initiation of antiretroviral therapy.
Acknowledgments
The authors thank Z. McVey, M. Mallen and J. Niles for their technical assistance, L. Careaga for preparing this manuscript, the nursing staff of the GCRC and the HIV patients who volunteered to participate in the study.
Sponsorship: Partially supported by the Immunology Support laboratory of the AIDS Clinical Trial Group (NIH, 2U01 A132782-05) and by the GCRC at the University of Texas Medical Branch at Galveston (NIH, M01 RR-0073), and by an amFAR grant (02682-28GI) for LA and AL.
References
- 1.Rabin RL, Roedereer M, Maldonado T, Petry A, Herzenberg LA, Herzenberg LA. Altered representations of naive and memory CD8T -cells subsets in HIV-1 infected children. J Clin Invest. 1995;95:2054–2060. doi: 10.1172/JCI117891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roedereer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA, Herzenberg LA. CD8 naive T-cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnittman SM, Lane HC, Greenhouse J, Justement JS, Baseler M, Fauci AS. Preferential infection of CD4 memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci USA. 1990;87:6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roederer M, Raju P, Mitra D, Herzenberg LA, Herzenberg LA. HIV does not replicate in naïve CD4 T cells stimulated with CD3/CD28. J Clin Invest. 1997;99:1555–1564. doi: 10.1172/JCI119318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods T, Roberts B, Butera S, Folks T. Loss of inducible virus in CD45RA naive cells after human immunodeficiency virus-1 entry accounts for preferential viral replication in CD45RO memory cells. Blood. 1997;89:1635–1641. [PubMed] [Google Scholar]
- 6.McCune JM. HIV-1: The infective process in vivo. Cell. 1991;64:351–363. doi: 10.1016/0092-8674(91)90644-e. [DOI] [PubMed] [Google Scholar]
- 7.Su K, Kaneshima H, Bnonyhadi M, Salimi S, Kraft D, Rabin L, et al. HIV-1 induced thymocyte depletion is associated with indirect cytopathicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 8.Hellerstein MK, McCune MK. T-cell Turnover in HIV-1 Disease. Immunity. 7:1997. 583–589. doi: 10.1016/s1074-7613(00)80379-9. [DOI] [PubMed] [Google Scholar]
- 9.Gougeon ML, Garcia S, Heeney J, et al. PCD in AIDS-related HIV and SIV infections. AIDS Res Hum Retrovirus. 1993;9:553–563. doi: 10.1089/aid.1993.9.553. [DOI] [PubMed] [Google Scholar]
- 10.Nokta M, Rossero R, Nichols J, Rosenbaum M, Pollard RB. Effect of didanosine stavudine and hydroxyurea therapy on apoptosis in CD45RA and CD45RO T-lymphocyte subpopulations. AIDS Res Hum Retrovirus. 1999;15(3):255–264. doi: 10.1089/088922299311439. [DOI] [PubMed] [Google Scholar]
- 11.Lederman M, Connick E, Landay A, et al. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine,lamivudine,and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J Infect Dis. 1998;178:70–79. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 12.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T-cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 13.Al-Harthi L, Siegel J, Spritzler J, Pottage J, Agnoli M, Landay A. Maximum suppression of HIV replication leads to the restoration of HIV-specific responses in early HIV disease. AIDS. 2000;14:761–770. doi: 10.1097/00002030-200005050-00001. [DOI] [PubMed] [Google Scholar]
- 14.Nikolic-Zugic J. Phenotypic and functional stages in the intrathy-mic development of αβ T cells. Immunol Today. 1991;65:65–70. doi: 10.1016/0167-5699(91)90160-u. [DOI] [PubMed] [Google Scholar]
- 15.McCune JM, Loftus R, Schmidt DK, et al. High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J Clin Invest. 1998;101(11):2301–2308. doi: 10.1172/JCI2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith KY, Valdez H, Landay A, et al. Thymic size and lymphocyte restoration in-patients with human immunodeficiency virus infection after 48 weeks of zidovudine, lamivudine ,and ritonavir therapy. J Infect Dis. 2000;181:141–147. doi: 10.1086/315169. [DOI] [PubMed] [Google Scholar]
- 17.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Lewin SR, Markowitz M, et al. Measuring recent thymic emigrants in blood of normal and HIV-1 infected individuals before and after effective therapy. J Exp Med. 1999;190:725–732. doi: 10.1084/jem.190.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steffens CM, Al-Harthi L, Shott S, Yogev R, Landay A. Evaluation of thymopoiesis using T cell receptor excision circles (TRECs): differential correlation between adult and pediatric TRECs and naive phenotypes. Clin Immunol. 2000;97:95–101. doi: 10.1006/clim.2000.4938. [DOI] [PubMed] [Google Scholar]
- 20.Poulin JF, Viswanathan MN, Harris JM, et al. Direct evidence for thymic function in adult humans. J Exp Med. 1999;190:479–486. doi: 10.1084/jem.190.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butcher EC. The regulation of lymphocyte traffic. Curr Topics Microbiol Immunol. 1986;128:85–122. doi: 10.1007/978-3-642-71272-2_3. [DOI] [PubMed] [Google Scholar]
- 22.Mackay CR. Migration pathways and immunologic memory among T lymphocytes. Semin Immunol. 1992;4:51–58. [PubMed] [Google Scholar]
- 23.Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- 24.Hamann A, Westrick DJ, Duijevstijn A, et al. Evidence for an accessory role of LFA-1 in lymphocyte-high endothelium interaction during homing. J Immunol. 1988;140:693–699. [PubMed] [Google Scholar]
- 25.Camp RL, Scheynius A, Johansson C, Pure E. CD44 is necessary for optimal contact allergic responses but is not required for normal leukocyte extravasation. J Exp Med. 1993;178:497–507. doi: 10.1084/jem.178.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pantaleo G, Graziosi C, Demarest JF, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 27.Pantaleo G, Graziosi C, Fauci AS. The immunopathogenesis of human immuno-deficiency virus infection. N Engl J Med. 1993;328:327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- 28.Nokta MA, Li XD, Nichols J, et al. Chemokine/CD4 receptor density ratios correlate with HIV replication in lymph node and peripheral blood of HIV infected individuals. AIDS. 2001;15:161–169. doi: 10.1097/00002030-200101260-00004. [DOI] [PubMed] [Google Scholar]
- 29.Al-Harthi L, Marchetti G, Steffens CM, Poulin J, Sekaly R, Landay A. Detection of T cell receptor circles (TRECs) as biomarkers for de novo T cell synthesis using a quantitative polymerase chain reaction-enzyme linked immunosorbent assay (PCR-ELISA) J Immunol Methods. 2000;237:187–197. doi: 10.1016/s0022-1759(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 30.Picker LJ. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- 31.Haxenberg MD, Otto SA, Cohen-Stuart JW, JC, et al. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naïve T cell population in HIV-1 infection. Nature Med. 2000;6:1036–1042. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 32.Spina CA, Prince HE, Richman DD. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Invest. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woods TC, Roberts BD, Butera ST, Folks TM. Loss of inducible virus in CD45RA naive cells after human immunodeficiency virus-1 entry accounts for preferential viral replication in CD45RO memory cells. Blood. 1997;89:1635–1641. [PubMed] [Google Scholar]
- 34.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 35.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:426–433. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 36.Dustin ML, Springer TA. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Ann Rev Immunol. 1991;9:27–66. doi: 10.1146/annurev.iy.09.040191.000331. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu Y, Shaw S. Lymphocyte interactions with extracellular matrix. FASEB J. 1991;5:2292–2299. doi: 10.1096/fasebj.5.9.1860621. [DOI] [PubMed] [Google Scholar]
- 38.Arnaout MA. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–1050. [PubMed] [Google Scholar]
- 39.Bucy RP, Hockett RD, Derdeyn CA, et al. Initial increase in blood CD4+ lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J Clin Invest. 1999;103(10):1391–1398. doi: 10.1172/JCI5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang ZQ, Notermans DW, Sedgewick G, et al. Kinetics of CD4+ T cell repopulation of lymphoid tissues after treatment of HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:1154–1159. doi: 10.1073/pnas.95.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]