Abstract
Ascorbate (vitamin C) deficiency leads to low immunity, scurvy, and other human diseases and is therefore a global health problem. Given that plants are major ascorbate sources for humans, biofortification of this vitamin in our foodstuffs is of considerable importance. Ascorbate is synthetized by a number of alternative pathways: (i) from the glycolytic intermediates D-glucose-6P (the key intermediates are GDP-D-mannose and L-galactose), (ii) from the breakdown of the cell wall polymer pectin which uses the methyl ester of D-galacturonic acid as precursor, and (iii) from myo-inositol as precursor via myo-inositol oxygenase. We report here the engineering of fruit-specific overexpression of a bacterial pyrophosphatase, which hydrolyzes the inorganic pyrophosphate (PPi) to orthophosphate (Pi). This strategy resulted in increased vitamin C levels up to 2.5-fold in ripe fruit as well as increasing in the major sugars, sucrose, and glucose, yet decreasing the level of starch. When considered together, these finding indicate an intimate linkage between ascorbate and sugar biosynthesis in plants. Moreover, the combined data reveal the importance of PPi metabolism in tomato fruit metabolism and development.
Keywords: tomato fruit, ripening, ascorbate, sugars, pyrophosphatase
Introduction
Nutrition is, by definition, aimed at maintaining human cell and organ homeostasis (Goodacre, 2007). In this context, a balance diet should be considered not just to include carbohydrates, proteins, and lipids, but also other physiologically active components such as certain amino acids and vitamins. Plants are the main dietary source in almost all trophic chains. Therefore, human nutritional health is ultimately dependent on the intake of mayor and minor nutrients from plants, especially given that humans are unable to synthesize certain organic compounds such as vitamins (Fitzpatrick et al., 2012).
L-ascorbate (AsA) is commonly called “vitamin C.” In plants, it acts as a scavenger of the free radicals generated by photosynthesis, cellular respiration, and abiotic stresses such as ozone and UV radiation (Conklin et al., 1996; Noctor and Foyer, 1998; Smirnoff and Wheeler, 2000). AsA has additionally been described to play an important role as an enzyme cofactor while participating in defense, cellular elongation, division and fruit ripening (Arrigoni and De Tullio, 2002; Pastori et al., 2003; Green and Fry, 2005). In humans, AsA has an integral role as cofactor of some dioxygenases enzymes which are involved in biosynthesis of carnitine and collagen (Padayatty et al., 2003). Therefore, its deficiency is associated with conditions such as scurvy and low immunity, which is mainly a consequence of the inactivation of these dioxygenases (De Tullio, 2012). AsA has also been associated to molecular events such as oxygen sensing, redox homeostasis, and carcinogenesis (Valko et al., 2006). In addition, different epidemiological studies have established a positive link between AsA content in food and health benefits such as the prevention of cardiovascular disease, cancer, and other inflammatory diseases (Blot et al., 1993; Steinmetz and Potter, 1996). Current approaches to relieve micronutrient deficiencies include the promotion of balanced diets, supplementation and food fortification, such as iodination of salt or fluoride fortification of toothpaste and tap water (Fletcher et al., 2004; Poletti et al., 2004). However, AsA is a difficult micronutrient for food fortification since it is oxidized very easily. AsA biofortification through metabolic engineering therefore represents an attractive alternative strategy to increase the intake of natural AsA in rich and poor countries alike (Muller and Krawinkel, 2005).
In plants, the biosynthetic pathway of AsA occurs by four different pathways, D-mannose/L-galactose (D-Man/L-Gal) or Smirnoff–Wheeler pathway, the major AsA biosynthetic route in plants, which involves GDP-D-mannose in the initial step (Wheeler et al., 1998). An alternative pathway with L-galacturonic acid as intermediate has been reported in strawberry, which proceeds via D-galacturonic acid to L-galactono-1,4-lactone (Agius et al., 2003) that serves as the linkage with the D-Man/L-Gal pathway. There are also alternative pathway of synthesizing AsA through the intermediates of L-gulose (Wolucka and Van Montagu, 2003) and myo-inositol (Lorence et al., 2004). However, the myo-inositol pathway remains controversial due to the lack of strong evidence. The AsA concentration remains the same in wild type and MiOX overexpression lines (Endres and Tenhaken, 2009).
Formation of GDP-D-mannose is the initial step in the D-Man/L-Gal pathway, which is synthesized from D-mannose-1P via GDP-mannose pyrophosphatase (Conklin et al., 1999) (Figure A1). This reaction generates inorganic pyrophosphate (PPi) as by-product. In plants, PPi plays a central role not only as by-product of activation and polymerization steps (Sonnewald, 1992; Geigenberger et al., 1998; Rojas-Beltran et al., 1999; Farre et al., 2001), if not as an energy donor per se (Stitt, 1998; Lopez-Marques et al., 2004). PPi is generally removed by inorganic pyrophosphatases, which hydrolyze PPi to orthophosphate (Pi). Heterologous expression of the Escherichia coli pyrophosphatase in an untargeted manner, conferring cytosolic localization of the encoded protein, showed an important role in the partitioning between sucrose (Suc) and starch (Sonnewald, 1992; Farre et al., 2001; Lee et al., 2005). In contrast, expression of the E. coli pyrophosphatase targeted to the plastid displayed only minor changes in metabolites levels (Farre et al., 2006). A transient down-regulation of plastid-targeted soluble pyrophosphatase in Nicotiana benthamiana, revealed an important role in photosynthesis as well as in the regulation of water exchanges under mild drought stress (George et al., 2010).
In this study, we generated Solanum lycopersicum cv MoneyMarker lines, which overexpress the gene encoding the inorganic pyrophosphatase from E. coli in an untargeted manner under the control of a fruit specific promoter. This strategy resulted in increased vitamin C levels up to 2.5-fold in ripe fruit as well as increasing in the major sugars, sucrose, and glucose, yet decreasing the level of starch. When considered together, these finding indicate an intimate linkage between ascorbate and sugar biosynthesis in plants.
Results
Generation of B33-PPi overexpression tomato lines
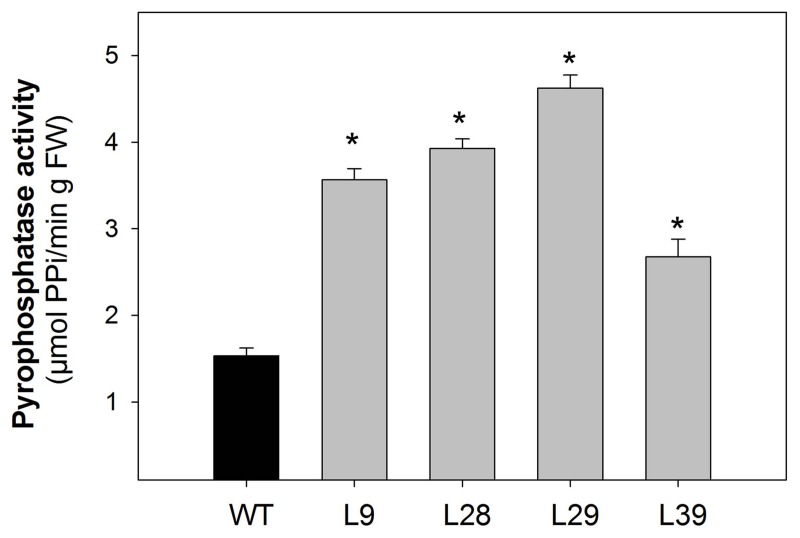
To assess the effect of over expression of a pyrophosphatase from E. coli (Sonnewald, 1992) in tomato fruit we introduced this gene in the sense orientation under the control of the patatin B33 promoter (Jelitto et al., 1992). This promoter has been shown to be ripening-specific promoter in tomato fruit (Frommer et al., 1994; Centeno et al., 2011). An initial screening was carried out on the basis of pyrophosphatase activity of ripe tomato fruits (data not shown). This screen allowed the identification of four lines displaying considerably elevated activity (L9, L28, L29, and L39), which were taken to the next generation. Eight T2 plants per line were grown in the greenhouse and young leaves (3 weeks old plants) as well as fruits at green (35 days after pollination; DAP) and red (60 DAP) stages were harvested. Assay of alkaline pyrophosphatase activity revealed that the selected lines displayed considerable increase in activity in red fruit (Figure 1). To ensure that this increase of target enzyme activity was restricted to fruits, the activity of the enzyme was additionally tested in young leaves where they were unaltered (10.8 ± 0.2; 11.1 ± 0.5; 11.4 ± 0.4; 10.4 ± 0.3; 10.5 ± 0.5 μmol min−1 g−1 FW in wild type, L9, L28, L29, and L39, respectively; values are mean ± SE).
Figure 1.
Pyrophosphatase activity of B33-PPi tomato lines. Pyrophosphatase activity in red tomato fruit (60 DAP). An asterisk indicates the values that were determined by the t-test to be significantly different (P < 0.05) from wild type.
Fruit size and yield
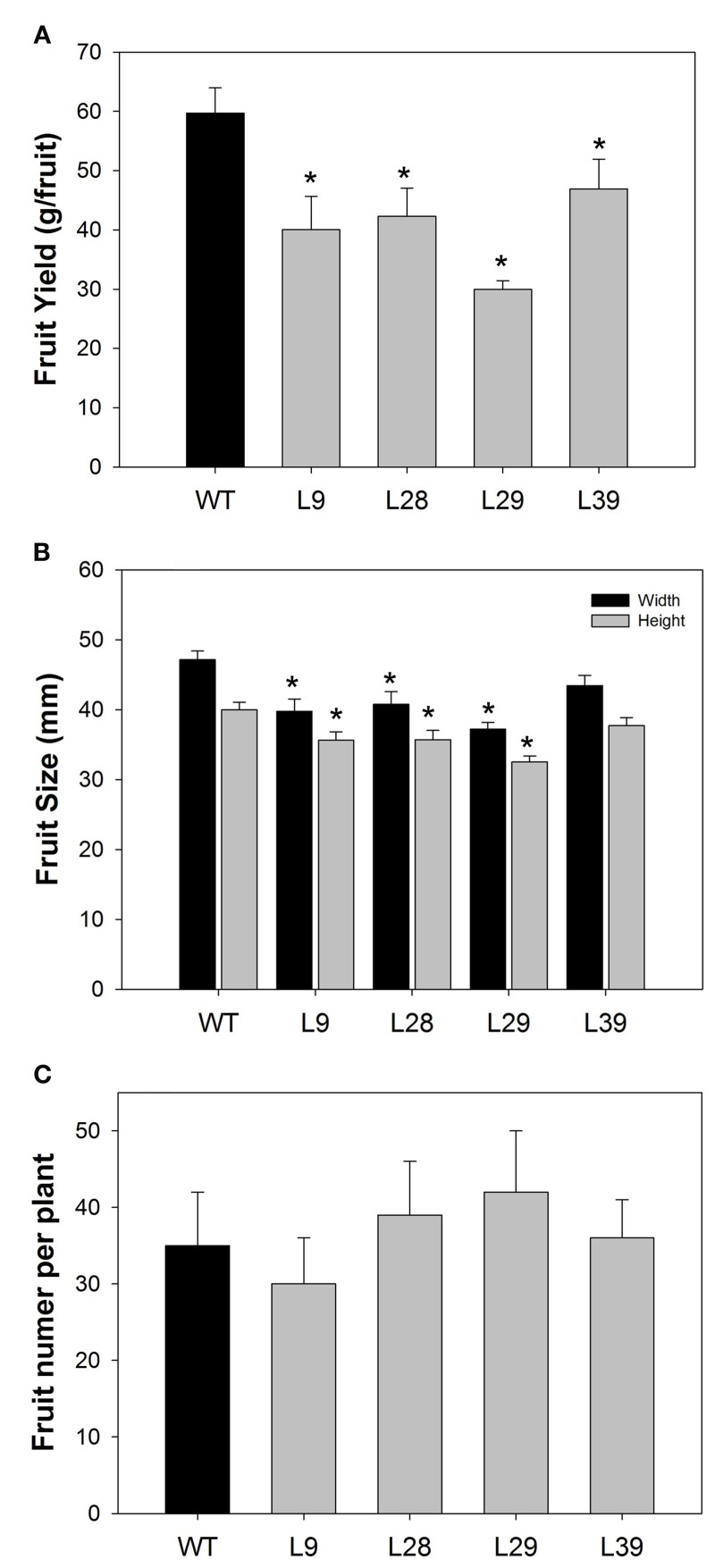
Fruit size and weight per fruit were determined in red fruit (60 DAP). All four lines exhibited a significantly lower weight per fruit (Figure 2A) as well as smaller fruit size in three of the four lines (L9, L28, and L29; Figure 2B). The total fruit number was, however, essentially unaltered (Figure 2C).
Figure 2.
Characterization of B33-PPi tomato lines. (A) Fruit yield (g per fruit), (B) fruit size, and (C) total fruit number of B33-PPi lines. All measurements were done in red stage (60 DAP). For all parameters, values are presented as the mean ± SE of eight biological replicates (one biological replicate is represented by one individual plant). An asterisk indicates the values that were determined by the t-test to be significantly different (P < 0.05) from wild type.
Pyrophosphate and inorganic phosphate levels
Having determined that the transformants displayed the desired alteration in enzyme activity, we next evaluated pyrophosphate levels themselves. For this purpose, pericarp tissues of red fruit at 60 DAP and young leaves (3 weeks old plants) were harvested and pyrophosphate levels were determined taking care to observe all control procedures required to minimize the influence of contaminants (Farre et al., 2001). These analyses revealed significant decreases in pyrophosphate levels in all lines ranking from 25 for L39 to 55% for L29 in red tomato fruit (Table 1). However, as anticipated both from the specificity of expression of the transgene and the lack of change in the activity no changes in pyrophosphate levels were observed in leaves (Table 1). Relatively consistent changes were also seen in the level of inorganic phosphate in red fruit. Inorganic phosphate level increased in three transgenic lines (L9, L28, and L39; Table 1).
Table 1.
Pyrophosphate and inorganic phosphate concentration in the B33-PPi red tomato fruits (60 DAP) and leaves (3 weeks old plants).
| WT | L9 | L28 | L29 | L39 | |
|---|---|---|---|---|---|
| PPi content (nmol g−1 FW−1) | |||||
| Fruit | 4.3 ± 0.8 | 2.5 ± 0.7 | 2.3 ± 0.4 | 1.8 ± 0.6 | 3.2 ± 0.5 |
| Leaves | 12.3 ± 2.5 | 10.8 ± 2.4 | 11.5 ± 2.2 | 13.4 ± 3.5 | 12.9 ± 2.4 |
| Pi content (μ mol g−1 FW−1) | |||||
| Fruit | 3.5 ± 0.5 | 7.1 ± 0.7 | 6.9 ± 0.5 | 8.5 ± 0.7 | 4.5 ± 0.5 |
Values are presented as the mean ± SE of 6 biological determinations. The values that are significantly different by t-test from the wild type are set in bold type (P < 0.05).
Metabolite profiling of green and red fruits of the B33-PPi lines
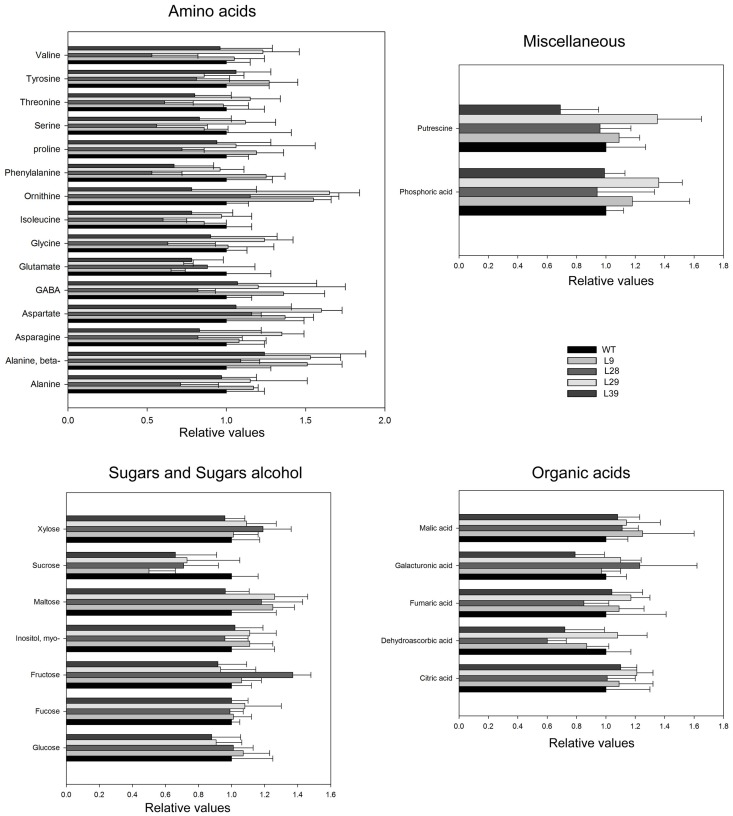
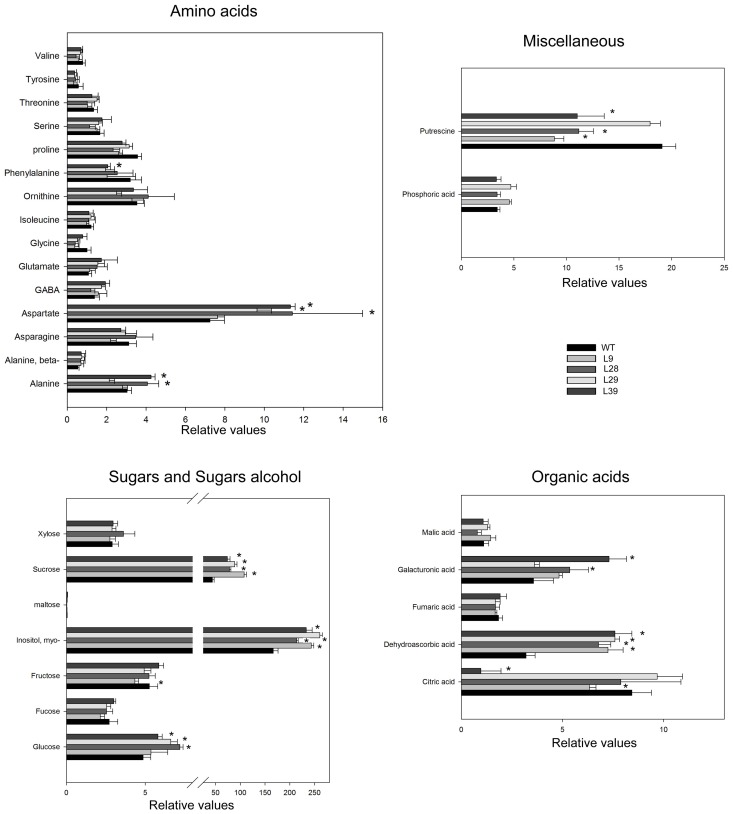
In order to further characterize the effects of the reduction of pyrophosphate content, we next applied and established gas chromatography (GC)-MS-based metabolite profiling method (Osorio et al., 2012) to pericarp tissue derived from green and red fruits. Surprisingly, at the green stage the metabolite profiles of the transgenic lines were remarkable similar to those of the WT (Figure 3). However, similar analysis in red stage revealed important changes in the levels of several few metabolites (Figure 4). In all four lines Suc was significantly increased in red tomato fruit by up to 2.5-fold in comparison to WT (Figure 4). This increase in Suc was accompanied by increases in Glc in three lines but no significant changes in Fru (only L9 showed slight decrease). The changes in these sugars were coupled to a decrease in starch content (Table 2) as well as an increase in the total soluble solids content (Brix) in all transgenic red fruit (6.2 ± 0.3; 7.4 ± 0.2; 8.4 ± 0.4; 6.9 ± 0.2 for the wild type, L9, L28, L29, and L39, respectively). To better understand these metabolic alterations, we next measured the AGPase in the red B33-PPi fruits. We observed a decrease in this activity in all transformants with the exception of the L39. However, the activation stage of this enzyme was invariant (Table 3).
Figure 3.
Primary metabolite levels in the receptacle of WT and B33-PPi lines at green stage. Data are normalized to the mean value of WT at the G stage. Values are means SE of six replicates. Asterisks indicate significant differences by t-test (P < 0.01) of the transgenic lines compared with WT at green stage.
Figure 4.
Primary metabolite levels in the receptacle of WT and B33-PPi lines at red stage. Data are normalized to the mean value of WT at the G stage. Values are means SE of six replicates. Asterisks indicate significant differences by t-test (P < 0.01) of the transgenic lines compared with WT at red stage.
Table 2.
Starch content in the B33-PPi red tomato fruits (60 DAP).
| WT | L9 | L28 | L29 | L39 | |
|---|---|---|---|---|---|
| Starch (nmol g−1 FW−1) | |||||
| Fruit | 438.3 ± 9.5 | 354.2 ± 13.1 | 316.4 ± 16.9 | 275.5 ± 20.5 | 384.6 ± 27.7 |
Values are presented as the mean ± SE of 6 biological determinations. The values that are significantly different by t-test from the wild type are set in bold type (P < 0.05).
Table 3.
Enzyme activities in the B33-PPi red tomato fruits (60 DAP).
| Enzymatic activities | WT | L9 | L28 | L29 | L39 |
|---|---|---|---|---|---|
| nmol min−1 g−1 FW−1 | |||||
| AGPase | 6.65 ± 0.75 | 3.98 ± 0.73 | 4.65 ± 0.42 | 3.97 ± 0.89 | 6.38 ± 1.02 |
| AGPase activation state (Vsel/Vred) | 0.67 ± 0.08 | 0.58 ± 0.09 | 0.73 ± 1.04 | 0.63 ± 0.76 | 0.76 ± 0.06 |
| MDHAR | 6.35 ± 0.31 | 12.34 ± 0.21 | 15.22 ± 0.46 | 11.10 ± 0.36 | 9.43 ± 0.42 |
| GalUR | 15.65 ± 0.86 | 14.89 ± 0.75 | 16.24 ± 0.65 | 15.23 ± 0.54 | 16.33 ± 0.76 |
| Aldonolactonase | 11.23 ± 1.34 | 10.34 ± 0.78 | 12.36 ± 1.06 | 13.23 ± 1.03 | 12.54 ± 0.87 |
| μ mol min−1 g−1 FW−1 | |||||
| MYOX | 12.43 ± 0.93 | 8.24 ± 0.85 | 7.77 ± 0.74 | 9.26 ± 0.82 | 10.52 ± 0.69 |
| GaLDH | 0.35 ± 0.09 | 0.78 ± 0.07 | 0.97 ± 0.04 | 1.12 ± 0.07 | 0.67 ± 0.05 |
Values are presented as the mean ± SE of 6 biological determinations. The values that are significantly different by t-test from the wild type are set in bold type (P < 0.05).
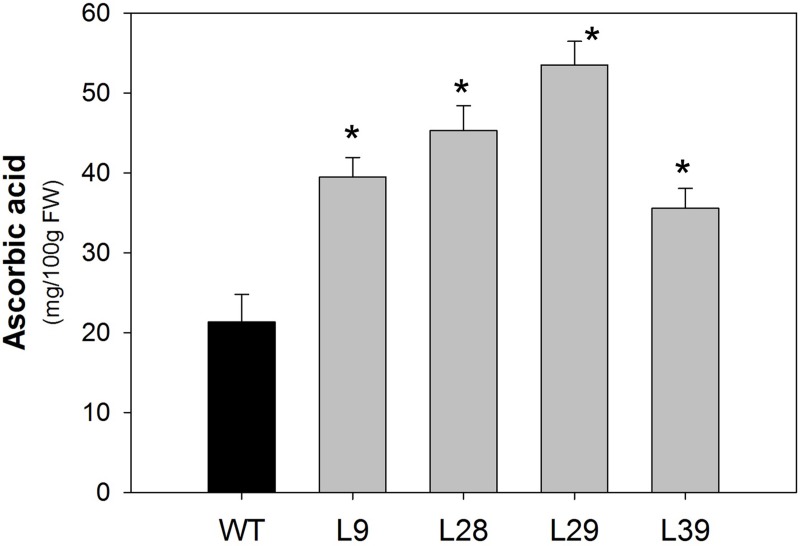
Interestingly, a strong increase in metabolites related to ascorbate biosynthesis, such as dehydroascorbic acid (all four lines), myo-inositol (all four lines), galacturonic acid (lines L28 and L39) (Figure 4) as well as a substantial increase of ascorbate (approximately between 2 and 3-fold) were observed (Figure 5).
Figure 5.
Ascorbate of B33-PPi tomato lines. Ascorbic acid was determined in red tomato fruit (60 DAP). The values are presented as the mean ± SE of six biological replicates. An asterisk indicates the values that were determined by the t-test to be significantly different (P < 0.05) from wild type.
Additionally, the transformants revealed an increase in two amino acids Ala (L28 and L39), and Asp (L28, L29, and L39) as well as a reduction in putrescine (L9, L28, L39) (Figure 4).
Expression of E. Coli pyrophosphatase in tomato fruits leads to alteration in ascorbic acid biosynthesis
Since some related ascorbate biosynthesis metabolites as well as ascorbate were modified in the red B33-PPi tomato fruits, we next evaluated if ascorbate biosynthesis and/or recycling were altered in these fruits. For this purpose we examined the different AsA biosynthetic pathways. First, we analyzed the transcript levels of some genes in the D-Man/L-Gal pathway. The expression of the two GDP-L-galactose phosphorylase (GGP) genes, a key point for the control of ascorbate pathway (Dowdle et al., 2007; Laing et al., 2007; Bulley et al., 2012) was up-regulated in all transgenic lines as well as the L-galactono-1,4-lactone dehydrogenase (GaLDH) gene, while the L-galactose dehydrogenase (GDH) gene showed a significant decrease only in one line (L9) (Figure 6). The higher activity of the last enzyme in this pathway, GaLDH, corroborated that D-Man/L-Gal pathway was up-regulated in red ripened B33-PPi fruits (Table 3). Second, related with the D-galacturonic acid pathway, we observed an increase in the level of its precursor, galacturonic acid, in two lines (Loewus and Kelly, 1961; Agius et al., 2003). However, the enzyme activities of the last two enzymes of this pathway, D-galacturonate reductase (GalUR) and aldonolactonase, were unaltered in these fruits (Table 3). Third, we observed that the myo-inositol level was altered in all transformants and considering that myo-inositol has been proposed as a precursor of ascorbate (Lorence et al., 2004), we determined the total myo-inositol oxygenase activity in red fruits (Table 3). Intriguingly, a significant decrease in the total myo-inositol oxygenase activity was observed in all transgenic lines (Table 3).
Figure 6.
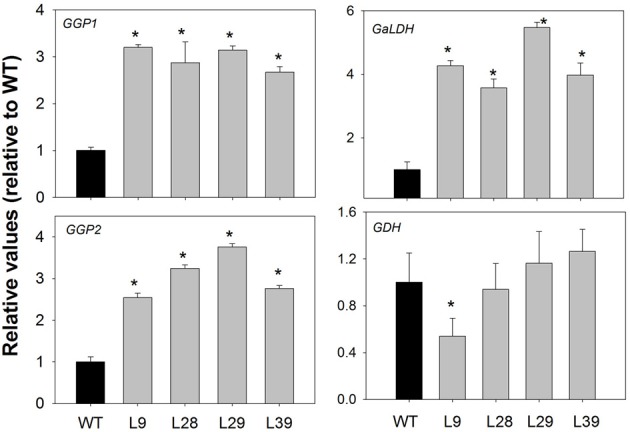
Expression of GGP, GaLDH, and GDH genes in red B33-PPi fruits. The abundance of GGP1(acc. number Solyc06g073320), GGP2 (acc. number Solyc02g091510), GaLDH (acc. number Solyc10g079470), and GDH (acc. number Solyc01g106450) mRNAs were measured by quantitative RT-PCR, respectively. An asterisk indicates the values that were determined by the t-test to be significantly different (P < 0.05) from wild type.
Additionally, we also determined the gene expression of three monodehydroascorbate reductase (MDHAR) and two dehydroascorbate reductase genes (DHAR) found in tomato. Both are involved in ascorbate recycling pathway. Interestingly, all transformats displayed a significant increase in MDHAR1, MDHAR2, and MDHAR3 transcript abundances (Figure 7). This result was in agreement with an increase in the MDHAR activity (Table 3). In contrast, we did not observed changes in the expression of the DHAR1 and DHAR2 genes (Figure 7).
Figure 7.
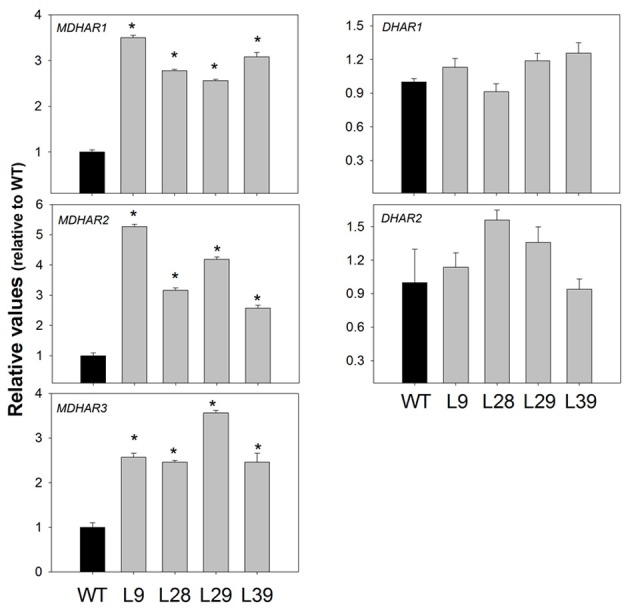
Expression of MDHAR, and DHAR genes in red B33-PPi fruits. The abundance of MDHAR1 (acc. number Solyc09g009390 ), MDHAR2 (acc. number Solyc02g086710), MDHAR3 (acc. number Solyc08g081530), DHAR1 (acc. number Solyc05g054760), and DHAR2 (acc. number Solyc11g011250). mRNAs were measured by quantitative RT-PCR, respectively. An asterisk indicates the values that were determined by the t-test to be significantly different (P < 0.05) from wild type.
Discussion
Until now, the breeding of tomato has been dominated by a focus on traits that benefit the grower, such as yield, plant and fruit size, and storage characteristics (Schuch, 1994; Giovannoni, 2006; Cong et al., 2008). As a result, there has been a loss of consumer quality traits such as flavor and nutritional value, and this has focused recent interest on the molecular genetics of such traits (Giovannoni, 2001; Causse et al., 2002, 2004; Fraser et al., 2009; Mounet et al., 2009; Enfissi et al., 2010; Centeno et al., 2011; Morgan et al., 2013). The accumulation of a range of soluble metabolites is important for both flavor and nutrition. In this paper, we characterized the consequences of over-expressing an E. coli pyrophosphatase gene under fruit-specific promoter. This manipulation had a broad impact on fruit development and ripening, emphasizing both the important role of pyrophosphatase in ascorbate and starch biosynthesis.
Effect of increasing pyrophosphatase activity on starch and sugars metabolism
Detailed analysis of sugars level revealed that starch content decreased while the major sugars, Suc and Glc increased in red ripe B33-PPi fruit. These data support the contention that active starch accumulation is an important contributory factor in determining the soluble solids content of mature fruit (Schaffer and Petreikov, 1997; Baxter et al., 2005). Here, we demonstrated that alterations in PPi metabolism have a strong effect on sugars metabolism and, hence, influence agronomic yield. Intriguingly, the data presented here are analogous to those previously described for transgenic potato plants in which higher PPi levels increased starch accumulation and decrease the level of Suc (Fernie et al., 2001a; Geigenberger et al., 2001), and decreased levels have been associated with lower starch biosynthetic rates (Geigenberger et al., 2001).
Different studies concerning starch metabolism in potato and tomato have suggested that AGPase activity plays an important role in its regulation (Geigenberger et al., 1999; Sweetlove et al., 1999). Regulation of the AGPase reaction has been very well characterized for several years. This enzyme is sensitive to allosteric regulation, being inhibited by inorganic phosphate and activated by 3PGA (3-phosphoglycerate) (Sowokinos, 1981; Sowokinos and Preiss, 1982). Additionally, it has been demonstrated to be transcriptionally regulated by sugars, nitrate, phosphate and trehalose-6-phosphate (Muller-Rober et al., 1990; Nielsen et al., 1998; Kolbe et al., 2005; Michalska et al., 2009). Moreover, it has been described that AGPase is also redox regulated (Tiessen et al., 2002; Centeno et al., 2011; Osorio et al., 2013) with malic acid potentially being a key component in this process at least in photosynthetically active tissues (Szecowka et al., 2012).
In this study, a strong correlation was found between starch concentration and AGPase activity in red ripe B33-PPi stage. Additionally, we observed that redox-state of AGPase was not altered in these transgenic fruit. This observation described here have lead us to propose that the activity of this enzyme was modified due to either a change in the rate of sugar influx into the tomato fruit and/or in the lower PPi levels found in these transgenic fruits.
Increased activity of pyrophosphatase correlates with increased ascorbate content in tomato fruit
There is a large potential for improving ascorbate content in food products by means of both genetic engineering and breeding. The exploitation of the large natural variation in ascorbate content in many fruit crops gives the opportunity of improving their nutritional value by classical breeding. The generation of linkage maps and the conduction of quantitative trait loci (QTL) analysis allow the identification of genomic regions associated with ascorbate content (Davey et al., 2006; Stevens et al., 2007; Zorrilla-Fontanesi et al., 2011). Such QTL analyses therefore increase our knowledge of the molecular mechanism by which ascorbate is regulated in plants.
The strategy to improve the amount of ascorbate by genetic engineering has been based on the up-regulation of genes encoding for enzymes of the biosynthetic or recovery pathways. In general, plants transformed with genes from different pathways have shown variable increases in ascorbate content in different plant tissues (Agius et al., 2003; Chen et al., 2003; Tokunaga et al., 2005; Eltayeb et al., 2007; Badejo et al., 2008, 2009; Bulley et al., 2009, 2012; Hemavathi et al., 2009; Qin et al., 2011; Zhang et al., 2011; Cronje et al., 2012). Mean increases in ascorbate content were usually two- to three-fold i.e., similar to those reported here. Other approaches have used genes that do not encode enzymes of the ascorbate biosynthetic pathway in plants. Thus, the ectopic expression of a rat L-gulonolactone oxidase, a gene involved in the synthesis of ascorbate in animals, produced an increase of about 7-fold in lettuce (Jain and Nessler, 2000). Similar levels were observed in potato plants ectopically expressing a bacterial pyrophosphorylase or a yeast invertase (Farre et al., 2008).
In this study, a strong correlation was displayed between the cellular PPi and ascorbate levels (up to 2.5-fold increase in red ripe transgenic fruits) and it was demonstrated that this was mechanistically linked to pyrophosphatase activity as previously was observed in potato tuber overexpressing a bacteria pyrophosphatase with a plastid targeting sequence (Farre et al., 2006). Additionally, an increase in some intermediates of ascorbate biosynthesis such as dehydroascorbate, galacturonate, and myo-inositol were also observed.
Formation of GDP-D-mannose is the initial step in the D-Man/L-Gal pathway of ascorbate biosynthesis, which is synthetized from D-mannose-1 phosphate via GDP-mannose pyrophosphatase (Conklin et al., 1999; Keller et al., 1999) (Figure A1). This reaction in the direction of ascorbate biosynthesis produces PPi as by-product. It is thus conceivable that the removal of PPi is favorable for ascorbate synthesis. Furthermore, the observed higher expression of GGP1, GGP2 genes, a key point for the control of ascorbate pathway (Dowdle et al., 2007; Laing et al., 2007; Bulley et al., 2012), and GalDH, the last gene in the AsA biosynthesis pathway, corroborates that D-Man/L-Gal pathway is activated in the B33-PPi fruits in comparison to WT fruits.
We also evaluated if other alternative pathways of AsA biosynthesis were altered in these fruits. When looked at the D-galacturonic acid pathway, an increase in the level of its precursor, galacturonic acid, was observed. However, the enzyme activities that catalyze the two last steps in this pathway, GalUR and aldonolactonase, were not altered. Increasing myo-inositol production has also shown varied results, with both increased (Lorence et al., 2004) and unaffected (Endres and Tenhaken, 2009) leaf AsA being reported. Together with an increase in myo-inositol levels, we observed a decrease in the myo-inositol oxygenase activity in the B33-PPi red fruits, suggesting that myo-inositol can act as a precursor for AsA biosynthesis as suggested by Lorence et al. (2004). Although, a previous study suggests that the L-galactose-1-phosphate phosphatase enzyme from the D-Man/L-Gal pathway, has a dual function that impacts both myo-inositol and AsA biosynthesis pathways (Torabinejad et al., 2009), further investigation are required to understand the impact of myo-inositol on AsA biosynthesis.
Also, our results revealed that ascorbate recycling pathway was altered in the B33-PPi red fruits since we found higher dehydroascorbic acid content and expression of the three tomato MDHAR genes.
Increased activity of pyrophosphatase also affects other metabolic changes
When other areas of metabolism are considered some interesting observations are apparent. Interestingly, the total level of organic acids and amino acids were largely invariant in the B33-PPi lines in comparison with WT. The exception to this was that we observed an accumulation in two amino acids, namely Ala and Asp. The accumulation of Asp can be explained because it acts as precursor in the synthesis of Asn via asparagine synthase. This reaction produces PPi as by-product that can be removed via pyrophosphatase. Therefore, we expect a shift in the reaction equilibrium to favor the Asn synthesis direction. Although significant differences were not found in the levels of Asn, this may be due to a co-ordinate up regulation of its metabolism. The reason for the increase in alanine is less clear but may merely reflect the additional availability of glucose for glycolytic reactions.
Morphological effects on B33-PPi fruits
In addition to the increase in soluble solids content, the fruit of the transgenic lines were compromised in size. This observation was also largely to be expected, since several direct genetic studies (Zrenner et al., 1996; Sonnewald et al., 1997; Sturm and Tang, 1999) have implicated Suc mobilization as a key determinant of sink strength in a broad range of species. As much as 10% (w/v) Suc has been reported in the phloem of plants (Hayashi and Chino, 1990), and the presence of AsA in the phloem sap was confirmed by radiolabeling (Franceschi and Tarlyn, 2002). It was also reported that the presence of sugar within the plant acts as potent signal that promotes AsA biosynthetic gene expression (Nishikawa et al., 2005). Within tomato fruit itself, a positive correlation between Suc feeding and the expression level in some genes of the D-Man/L-Gal pathway was described (Badejo et al., 2012), supporting the view that transportation of sugars from source tissues affect the AsA content in sink tissues through the up-regulation of AsA biosynthesis pathway genes. Despite the wide changes in morphological parameters, metabolic changes in the transgenic fruit were, by and large, confined to sugar and AsA metabolisms. We believed that fruit growth is largely dependent on the relationship between import of photoassimilate and AsA intake and/or biosynthesis.
In summary, the results presented in this study provide direct evidence that the reduction in PPi content had strong effects on metabolism of sugar and ascorbate contents. Detailed analysis of starch metabolism revealed that this phenomenon was due to alteration in AGPase activity, caused by either a change in the rate of sugar influx into the tomato fruit and/or in the lower PPi levels found in these transgenic fruits. During ripening, the lack of accumulation of transitory starch was reflected by a decrease of soluble sugars. Moreover, we demonstrated that alterations in the level of PPi resulted in dramatic effect on ascorbate metabolism. These lines displaying low PPi content showed and increased flux to, and accumulation of, ascorbate. This occurred in spite of increases the ascorbate level via D-mannose-1P and via GDP-mannose pyrophosphatase. Further investigation is required to define this control, especially in fruit such as tomato, where it may contribute to taste (sugars and organic acids) and nutritional value (ascorbate) of the fruit which are important in determining fruit quality.
Materials and methods
Plant material
The gene encoding a pyrophosphatase from E. coli (Sonnewald, 1992) was introduced in the sense orientation into the vector pBinAR between the patatin B33 promotor (Rocha-Sosa et al., 1989) and the octopine synthase polyadenylation signal. This construct was introduced into tomato (Solanum lycopesicum, L.) cv Moneymaker plants by an Agrobacterium-mediated transformation protocol, and plants were selected and maintained as described in the literature (Tauberger et al., 2000). An initial screening was carried out on the basis of pyrophosphatase activity. This screen allowed the identification of four lines, which were taken to the next generation.
Metabolite determinations
PPi and Pi determination
PPi was extracted from tomato fruit by TCA/ether method (Jelitto et al., 1992). PPi was determined using the colorimetric PiPer Pyrophosphate assay kit (Invitrogen) according to the manufacturer's specifications. All glassware was pretreated overnight with 0.1 M HCl to remove residual phosphate. PPi levels were determined by a sample blank with or without sPPase, and total Pi was calculated by comparison at 595 nm with a linear Pi standard curve.
Pi was determined in the TCA extracts with a colorimetric assay as described by Taussky and Shorr (1953).
Primary metabolic profiling
Metabolite extraction derivatization, standard addition, and sample injection for GC-MS were performed according (Osorio et al., 2012). Both chromatograms and mass spectra were evaluated using TAGFINDER (Luedemann et al., 2008).
Ascorbic acid determination
Ascorbic acid extraction and analysis were performed as described (Lima-Silva et al., 2012). Ascorbic acid content was determined by comparison with a linear ascorbic acid standard curve.
Starch determination
The level of starch in the tissues were determined exactly as described previously (Fernie et al., 2001b).
Enzyme activities
Alkaline pyrophosphatase activity
The protein extraction and enzyme activity were analyzed as described Farre et al. (2001).
AGPase
AGPase activity was measured in the pyrophosphorolysis direction with a spectrophotometric assay, as described Tiessen et al. (2002, 2003). Frozen tissues were homogenized in liquid N2 and approx. 100 mg was extracted rapidly (1 min) with 1 ml of extraction buffer (50 mM Hepes-KOH, pH 7.8, and 5 mM MgCl2) at 4°C. After centrifugation (30 s at 13,000 g at 4°C), 10 μl of the supernatant was used for the AGPase assay. The reaction was performed in a total volume of 200 μl containing 50 mM Hepes-KOH, pH 7.8, 5 mM MgCl2, 10 μM Glc-1,6-bisP, 0.6 mM NADP+, 2.5 mM Na-PPi, 1 unit/ml phosphoglucomutase, 2.5 units/ml Glc-6-P dehydrogenase, and a range of concentrations of ADP-Glc (0.4−1 mM) in the absence of Pi, with or without DTT (10 mM) for activation assay. Reactions were followed on line at 340 nm and were linear up to 30 min. The activation state of AGPase is defined as the ratio of Vsel (−DTT) to Vred (+DTT).
Myo-inositol oxygenase
Two-hundred mg of tissue was incubated for 30 min at 30°C in a buffer containing 100 mM KPO4 (pH 7.2), 2 mM L-cysteine, 1 mM ammonium ferrous sulfate hexahydrate, and 60 mM myo-inositol. The reaction was stopped by boiling for 10 min and denatured protein removed by centrifugation (20,000 g, 15 min). Glucuronic acid was determined at 540 nm before and after samples developed a pink color with addition of a 3-hydroxybiphenylphenol color reagent (van den Hoogen et al., 1998).
D-galacturonate reductase
One gram of samples were homogenized in liquid nitrogen and extracted with 50 mM sodium phosphate buffer, pH 7.2, containing 2 mM EDTA, 2 mM dithiothreitol, 20% glycerol and PVPP. GalUR activity was measured by the decrease in absorbance at 340 nm at 25°C after the addition of 100 μ l of crude enzyme extract to the assay mixture (1 ml) consisted of 50 mM phosphate buffer (pH 7.2), 2 mM EDTA, 0.1 mM NADPH, 30 mM D-galacturonic acid and 2 mM dithiothreitol. The GalUR activity in the crude enzyme extract was recorded as nmol of NADPH oxidized min-1 mg-1 protein (Agius et al., 2003).
Aldonolactonase
The activity was measured by the change in absorbance of p-nitrophenol through acidification at 405 nm essentially as described Ishikawa and Shigeoka (2008).
L-galactono-1,4-lactone dehydrogenase
Samples were prepared as described by Mieda et al. (2004) and assayed at 340 nm by measuring the reduction of NAD+ in a reaction mixture containing 0.5 mM NAD+, 1 mM L-Gal, and the enzyme extract. L-Galactono-1,4-lactone dehydrogenase activity was assayed by the reduction of cytochrome c resulting in an increase in absorbance at 550 nm in a reaction mixture containing 50 mM TRIS-HCl, pH 8.5, 1 mM sodium azide, 42 mM L-Gal, 0.1% Triton X-100, 1.05 mg−1 ml cytochrome c, and the extract in a final volume of 1 ml as described by Yabuta et al. (2000).
Monodehydroascorbate reductase
The activity was measured according to the method of Hossain and Asada (1984).
Measurement of fruit brix
Ripe fruit tissue was homogenized with a razor blade, and the soluble solids (Brix) content of the resulting juice measured on a portable refractometer (Digitales Refrktometer DR6000; Krüss Optronic GmbH, Hamburg, Germany).
Analysis of gene expression by QRT-PCR
Total RNA was extracted according to Bugos et al. (1995) with minor modifications. Integrity of the extracted RNA was checked by electrophoresis under denaturing conditions after treating the RNA with RNase-free DNaseI (Roche). First-strand cDNA synthesis of 1 mg of RNA in a final volume of 20 mL was performed with Moloney murine leukemia virus reverse transcriptase, Point Mutant RNase H Minus (Promega), according to the supplier's protocol using oligo(dT) T19 primer.
Expression of the monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), L-galactono-1,4-lactone dehydrogenase (GaLDH), and L-galactose dehydrogenase (GDH) genes was analyzed by real-time qRT-PCR using the fluorescent intercalating dye SYBR Green in an iCycler detection system (Bio-Rad; http://www.bio-rad.com/). Relative quantification of the target expression level was performed using the comparative Ct method. The following primers were used: for analysis of MDHAR1 transcript levels (GenBank accession no. Solyc09g009390), forward, 5′-TCTACGGTGATAATGTGGGTGA-3′, reverse, 5′-ATTGCCTTGTTCTCTTCAGGTG-3′; for MDHAR2 (GenBank accession no. Solyc02g086710), forward, 5′-TTGAGTGATAAACCAGAGCCATC-3′, reverse, 5′-TTCTACGCCTCCTACCATACCA-3′; for MDHAR3 (GenBank accession no. Solyc08g081530), forward, 5′-ATTTCAAGGGTTTCGGTTCCT-3′, reverse, 5′-CATTTCCTCCTCCAACTACCAC-3′; for DHAR1 (GenBank accession no. Solyc05g054760), forward, 5′-TTTCCTACCTTCGTCTCATTTCTG-3′, reverse, 5′- GAACAAACATTCTGCCCATTGA -3′; for DHAR2 (GenBank accession no. Solyc11g011250), forward, 5′-GCTTCATTTGCGACTTCTATCAA-3′, reverse, 5′-AAAACCTCTTCTGGGTGCTCTG-3′; for GaLDH (GenBank accession no. Solyc10g079470), forward, 5′-GCTATTTCGGTATGCTCCGTTG-3′, reverse, 5′-CCTCACATTCGCTTCTTTCACT-3′; for GDH (GenBank accession no. Solyc01g106450), forward, 5′-TGTTTGTCAGTTCAACGAGGTC-3′, reverse, 5′-TTGTTTTAGATGTCCAAGTGCAA-3′ (Gilbert et al., 2009); for GGP1 (GenBank accession no.Solyc06g073320) forward, 5′-AGGGTGCAACTGAGGCAAATGC-3′, reverse, 5′-ATGGGCTGTGGAGGTGTGACA-3′; for GGP2 (GenBank accession no.Solyc02g091510) forward, 5′-GTCTTGGTTGGAGGTTGTAAT-3′, reverse, 5′-TGCACAAAAGTTGCTAGTCCT-3′. To normalize gene expression for differences in the efficiency of cDNA synthesis, transcript levels of the constitutively expressed elongation factor 1a of tomato (GenBank accession no. X14449) were measured using the following primers: forward, 5′-ACCACGAAGCTCTCCAGGAG-3′, reverse, 5′-CATTGAACCCAACATTGTCACC-3′ (Zanor et al., 2009).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are indebted to Dr. Lothar Willmitzer for his support. We acknowledge the excellent care of the plants by Helga Kulka (Max-Planck-Institut für Molekulare Pflanzenphysiologie). This work was partially supported by the ERAnet—financed project TomQML (Sonia Osorio and Alisdair R. Fernie). Sonia Osorio acknowledges the support by Ministerio de Ciencia e Innovación, Spain (Ramón and Cajal contract).
Appendix
Figure A1.
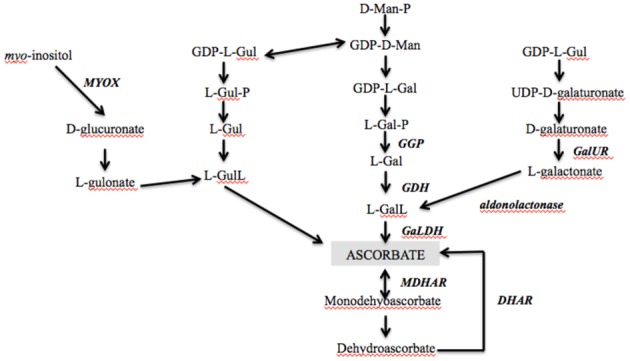
The proposed biosynthetic pathways for ascorbate in plants. L-Gal, L-galactose; L-GalL, l-galactono-1,4-lactone; -LGul, L-gulose; L-GuL, L-gulono-1,4-lactone; D-Man, d-mannose; UDP, uridine diphosphate; GGP, GDP-L-galactose phosphorylase; GDH, L-galactose dehydrogenase; GaLDH, L-galactono-1, 4-lactone dehydrogenase; GalUR, D-galacturonate reductase MYOX, myo-inositol oxidase; MDHAR, monodehydroascorbate reductase ; DHAR, dehydroascorbate reductase.
References
- Agius F., González-Lamothe R., Caballero J. L., Muñoz-Blanco J., Botella M. A., Valpuesta V. (2003). Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol. 21, 177–181 [DOI] [PubMed] [Google Scholar]
- Arrigoni O., De Tullio M. C. (2002). Ascorbic acid: much more than just an antioxidant. Biochim. Biophys. Acta 1569, 1–9 10.1016/S0304-416500235-416500235 [DOI] [PubMed] [Google Scholar]
- Badejo A. A., Eltelib H. A., Fukunaga K., Fujikawa Y., Esaka M. (2009). Increase in ascorbate content of transgenic tobacco plants overexpressing the acerola (Malpighia glabra) phosphomannomutase gene. Plant Cell Physiol. 50, 423–428 10.1093/pcp/pcn206 [DOI] [PubMed] [Google Scholar]
- Badejo A. A., Tanaka N., Esaka M. (2008). Analysis of GDP-D-mannose pyrophosphorylase gene promoter from acerola (Malpighia glabra) and increase in ascorbate content of transgenic tobacco expressing the acerola gene. Plant Cel Physiol. 49, 126–132 10.1093/pcp/pcm164 [DOI] [PubMed] [Google Scholar]
- Badejo A. A., Wada K., Gao Y., Maruta T., Sawa Y., Shigeoka S., et al. (2012). Translocation and the alternative D-galacturonate pathway contribute to increasing the ascorbate level in ripening tomato fruits together with the D-mannose/L-galactose pathway. J. Exp. Bot. 63, 229–239 10.1093/jxb/err275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter C. J., Carrari F., Bauke A., Overy S., Hill S. A., Quick P. W., et al. (2005). Fruit carbohydrate metabolism in an introgression line of tomato with increased fruit soluble solids. Plant Cell Physiol. 46, 425–437 10.1093/pcp/pci040 [DOI] [PubMed] [Google Scholar]
- Blot W. J., Li J. Y., Taylor P. R., Guo W., Dawsey S., Wang G. Q., et al. (1993). Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Natl. Cancer Inst. 85, 1483–1492 10.1093/jnci/85.18.1483 [DOI] [PubMed] [Google Scholar]
- Bugos R. C., Chiang V. L., Zhang X. H., Campbell E. R., Podila G. K., Campbell W. H. (1995). RNA isolation from plant tissues recalcitrant to extraction in guanidine. Biotechniques 19, 734–737 [PubMed] [Google Scholar]
- Bulley S., Wright M., Rommens C., Yan H., Rassam M., Lin-Wang K., et al. (2012). Enhancing ascorbate in fruits and tubers through over-expression of the l-galactose pathway gene GDP-l-galactose phosphorylase. Plant Biotechnol. J. 10, 390–397 10.1111/j.1467-7652.2011.00668.x [DOI] [PubMed] [Google Scholar]
- Bulley S. M., Rassam M., Hoser D., Otto W., Schünemann N., Wright M., et al. (2009). Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J. Exp. Bot. 60, 765–778 10.1093/jxb/ern327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causse M., Duffe P., Gomez M. C., Buret M., Damidaux R., Zamir D., et al. (2004). A genetic map of candidate genes and QTLs involved in tomato fruit size and composition. J. Exp. Bot. 55, 1671–1685 10.1093/jxb/erh207 [DOI] [PubMed] [Google Scholar]
- Causse M., Saliba-Colombani V., Lecomte L., Duffé P., Rousselle P., Buret M. (2002). QTL analysis of fruit quality in fresh market tomato: a few chromosome regions control the variation of sensory and instrumental traits. J. Exp. Bot. 53, 2089–2098 10.1093/jxb/erf058 [DOI] [PubMed] [Google Scholar]
- Centeno D. C., Osorio S., Nunes-Nesi A., Bertolo A. L., Carneiro R. T., Araujo W. L., et al. (2011). Malate plays a crucial role in starch metabolism, ripening, and soluble solid content of tomato fruit and affects postharvest softening. Plant Cell 23, 162–184 10.1105/tpc.109.072231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Young T. E., Ling J., Chang S., Gallie D. R. (2003). Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc. Natl. Acad. Sci. U.S.A. 100, 3525–3530 10.1073/pnas.0635176100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong B., Barrero L. S., Tanksley S. D. (2008). Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat. Genet. 40, 800–804 10.1038/ng.144 [DOI] [PubMed] [Google Scholar]
- Conklin P. L., Norris S. R., Wheeler G. L., Williams E. H., Smirnoff N., Last R. L. (1999). Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 96, 4198–4203 10.1073/pnas.96.7.4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin P. L., Williams E. H., Last R. L. (1996). Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc. Natl. Acad. Sci. U.S.A. 93, 9970–9974 10.1073/pnas.93.18.9970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronje C., George G. M., Fernie A. R., Bekker J., Kossmann J., Bauer R. (2012). Manipulation of L-ascorbic acid biosynthesis pathways in Solanum lycopersicum: elevated GDP-mannose pyrophosphorylase activity enhances L- ascorbate levels in red fruit. Planta 235, 553–564 10.1007/s00425-011-1525-1526 [DOI] [PubMed] [Google Scholar]
- Davey M. W., Kenis K., Keulemans J. (2006). Genetic control of fruit vitamin C contents. Plant Physiol. 142, 343–351 10.1104/pp.106.083279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Tullio M. C. (2012). Beyond the antioxidant: the double life of vitamin C. Subcell Biochem. 56, 49–65 10.1007/978-94-007-2199-9_4 [DOI] [PubMed] [Google Scholar]
- Dowdle J., Ishikawa T., Gatzek S., Rolinski S., Smirnoff N. (2007). Two genes in Arabidopsis thaliana encoding GDP-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 52, 673–689 10.1111/j.1365-313X.2007.03266.x [DOI] [PubMed] [Google Scholar]
- Eltayeb A. E., Kawano N., Badawi G. H., Kaminaka H., Sanekata T., Shibahara T., et al. (2007). Over-expression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 225, 1255–1264 10.1007/s00425-006-0417-0417 [DOI] [PubMed] [Google Scholar]
- Endres S., Tenhaken R. (2009). Myoinositol oxygenase controls the level of myoinositol in arabidopsis, but does not increase ascorbic acid. Plant Physiol. 149, 1042–1049 10.1104/pp.108.130948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enfissi E. M., Barneche F., Ahmed I., Lichtlé C., Gerrish C., McQuinn R. P., et al. (2010). Integrative transcript and metabolite analysis of nutritionally enhanced DE-ETIOLATED1 downregulated tomato fruit. Plant Cell 22, 1190–1215 10.1105/tpc.110.073866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farre E. M., Fernie A. R., Willmitzer L. (2008). Analysis of subcellular metabolite levels of potato tubers (Solanum tuberosum) displaying alterations in cellular or extracellular sucrose metabolism. Metabolomics 4, 161–170 10.1007/s11306-008-0107-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farre E. M., Tech S., Trethewey R. N., Fernie A. R., Willmitzer L. (2006). Subcellular pyrophosphate metabolism in developing tubers of potato (Solanum tuberosum). Plant Mol. Biol. 62, 165–179 10.1007/s11103-006-9011-9014 [DOI] [PubMed] [Google Scholar]
- Farre E. M., Tiessen A., Roessner U., Geigenberger P., Trethewey R. N., Willmitzer L. (2001). Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, organic acids, amino acids, and sugar alcohols in potato tubers using a nonaqueous fractionation method. Plant Physiol. 127, 685–700 10.1104/pp.010280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie A. R., Roessner U., Geigenberger P. (2001a). The sucrose analog palatinose leads to a stimulation of sucrose degradation and starch synthesis when supplied to discs of growing potato tubers. Plant Physiol. 125, 1967–1977 10.1104/pp.125.4.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie A. R., Roscher A., Ratcliffe R. G., Kruger N. J. (2001b). Fructose 2, 6-bisphosphate activates pyrophosphate: fructose-6-phosphate 1-phosphotransferase and increases triose phosphate to hexose phosphate cycling in heterotrophic cells. Planta 212, 250–263 10.1007/s004250000386 [DOI] [PubMed] [Google Scholar]
- Fletcher R. J., Bell I. P., Lambert J. P. (2004). Public health aspects of food fortification: a question of balance. Proc. Nutr. Soc. 63, 605–614 10.1079/PNS2004391 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick T. B., Basset G. J., Borel P., Carrari F., DellaPenna D., Fraser P. D., et al. (2012). Vitamin deficiencies in humans: can plant science help? Plant Cell 24, 395–414 10.1105/tpc.111.093120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V. R., Tarlyn N. M. (2002) l-Ascorbic acid is accumulated in source leaf phloem and transported to sink tissues in plants. Plant Physiol. 130, 649–656 10.1104/pp.007062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser P. D., Enfissi E. M., Bramley P. M. (2009). Genetic engineering of carotenoid formation in tomato fruit and the potential application of systems and synthetic biology approaches. Arch. Biochem. Biophys. 483, 196–204 10.1016/j.abb.2008.10.009 [DOI] [PubMed] [Google Scholar]
- Frommer W., Mielchen C., Martin T. (1994). Metabolic control of patatin promoters from potato in transgenic tobacco and tomato plants. Plant Physiol. (Life Sci. Adv.) 13, 329–334 [Google Scholar]
- Geigenberger P., Hajirezeai M., Geiger M., Deiting U., Sonnewald U., Stitt M. (1998). Overexpression of pyrophosphatase leads to increased sucrose degradation and starch synthesis, increased activities of enzymes for sucrose-starch interconversions, and increased levels of nucleotides in growing potato tubers. Planta 205, 428–437 10.1007/s004250050340 [DOI] [PubMed] [Google Scholar]
- Geigenberger P., Muller-Rober B., Stitt M. (1999). Contribution of adenosine 5′-diphosphoglucose pyrophosphorylase to the control of starch synthesis is decreased by water stress in growing potato tubers. Planta 209, 338–345 10.1007/s004250050641 [DOI] [PubMed] [Google Scholar]
- Geigenberger P., Stamme C., Tjaden J., Schulz A., Quick P. W., Betsche T., et al. (2001). Tuber physiology and properties of starch from tubers of transgenic potato plants with altered plastidic adenylate transporter activity. Plant Physiol. 125, 1667–1678 10.1104/pp.125.4.1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George G. M., van der Merwe M. J., Nunes-Nesi A., Bauer R., Fernie A. R., Kossmann J., et al. (2010). Virus-induced gene silencing of plastidial soluble inorganic pyrophosphatase impairs essential leaf anabolic pathways and reduces drought stress tolerance in Nicotiana benthamiana. Plant Physiol. 154, 55–66 10.1104/pp.110.157776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L., Alhagdow M., Nunes-Nesi A., Quemener B., Guillon F., Bouchet B., et al. (2009). GDP-D-mannose 3, 5-epimerase (GME) plays a key role at the intersection of ascorbate and non-cellulosic cell-wall biosynthesis in tomato. Plant J. 60, 499–508 10.1111/j.1365-313X.2009.03972.x [DOI] [PubMed] [Google Scholar]
- Giovannoni J. (2001). Molecular biology of fruit maturation and ripening. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 725–749 10.1146/annurev.arplant.52.1.725 [DOI] [PubMed] [Google Scholar]
- Giovannoni J. J. (2006). Breeding new life into plant metabolism. Nat. Biotechnol. 24, 418–419 10.1038/nbt0406-418 [DOI] [PubMed] [Google Scholar]
- Goodacre R. (2007). Metabolomics of a superorganism. J. Nutr. 137, 259S–266S [DOI] [PubMed] [Google Scholar]
- Green M. A., Fry S. C. (2005). Apoplastic degradation of ascorbate: novel enzymes and metabolites permeating the plant cell wall. Plant Biosyst. 139, 2–7 10.1080/11263500500056849 [DOI] [Google Scholar]
- Hayashi H., Chino M. (1990). Chemical composition of phloem sap from the uppermost internode of the rice plant. Plant Cell Physiol. 31, 247–251 [Google Scholar]
- Hemavathi U. C., Young K., Akula N., Kim H. S., Heung J. J., Oh O. M., et al. (2009). Over-expression of strawberry D-galacturonic acid reductase in potato leads to accumulation of vitamin C with enhanced abiotic stress tolerance. Plant Sci. 177, 659–667 10.1016/j.plantsci.2009.08.004 [DOI] [Google Scholar]
- Hossain M. A., Asada K. (1984). Inactivation of ascorbate peroxidase in spinach chloroplast on dark addition of hydrogen peroxide: its protection by ascorbate. Plant Cell Physiol. 25, 1285–1295 [Google Scholar]
- Ishikawa T., Shigeoka S. (2008). Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci. Biotechnol. Biochem. 72, 1143–5114 10.1271/bbb.80062 [DOI] [PubMed] [Google Scholar]
- Jain A. K., Nessler C. L. (2000) Metabolic engineering of an alternative pathway for ascorbic acid biosynthesis in plants. Mol. Breed. 6, 73–78 10.1023/A:1009680818138 [DOI] [Google Scholar]
- Jelitto T., Sonnewald U., Willmitzer L., Hajirezeai M., Stitt M. (1992). Inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing E. coli pyrophosphatase in their cytosol. Planta 188, 238–244 10.1007/BF00216819 [DOI] [PubMed] [Google Scholar]
- Keller R., Springer F., Renz A., Kossmann J. (1999). Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to devel- opmental changes during senescence. Plant J. 19, 131–141 10.1046/j.1365-313X.1999.00507.x [DOI] [PubMed] [Google Scholar]
- Kolbe A., Tiessen A., Schluepmann H., Paul M., Ulrich S., Geigenberger P. (2005). Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc. Natl. Acad. Sci. U.S.A. 102, 11118–11123 10.1073/pnas.0503410102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A., Wright M. A., Cooney J., Bulley S. M. (2007). The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose guanyltransferase, increases leaf ascorbate content. Proc. Natl. Acad. Sci. U.S.A. 104, 9534–9539 10.1073/pnas.0701625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. W., Lee D. S., Bhoo S. H., Jeon J. S., Lee Y. H., Hahn T. R. (2005). Transgenic Arabidopsis plants expressing Escherichia coli pyrophosphatase display both altered carbon partitioning in their source leaves and reduced photosynthetic activity. Plant Cell Rep. 24, 374–382 10.1007/s00299-005-0951-y [DOI] [PubMed] [Google Scholar]
- Lima-Silva V., Rosado A., Amorim-Silva V., Munoz-Merida A., Pons C., Bombarely A., et al. (2012). Genetic and genome-wide transcriptomic analyses identify co-regulation of oxidative response and hormone transcript abundance with vitamin C content in tomato fruit. BMC Genomics 13:187 10.1186/1471-2164-13-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewus F. A., Kelly S. (1961). Identity of l-ascorbic acid formed from d-glucose by the strawberry (Fragaria). Nature 191, 1059–1061 10.1038/1911059a0 [DOI] [PubMed] [Google Scholar]
- Lopez-Marques R. L., Perez-Castineira J. R., Losada M., Serrano A. (2004). Differential regulation of soluble and membrane-bound inorganic pyrophosphatases in the photosynthetic bacterium Rhodospirillum rubrum provides insights into pyrophosphate-based stress bioenergetics. J. Bacteriol. 186, 5418–5426 10.1128/JB.186.16.5418-5426.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorence A., Chevone B. I., Mendes P., Nessler C. L. (2004). Myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 134, 1200–1205 10.1104/pp.103.033936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedemann A., Strassburg K., Erban A., Kopka J. (2008). TagFinder for the quantitative analysis of gas chromatography–mass spectrometry (GC-MS)-based metabolite profiling experiments. Bioinformatics 24, 732–737 10.1093/bioinformatics/btn023 [DOI] [PubMed] [Google Scholar]
- Morgan M. J., Osorio S., Gehl B., Baxter C. J., Kruger N., Ratcliffe., et al. (2013). Metabolic engineering of tomato fruit organic acid content guided by biochemical analysis of an introgression line. Plant Physiol. 161, 397–407 10.1104/pp.112.209619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounet F., Moing A., Garcia V., Petit J., Maucourt M., Deborde C., et al. (2009). Gene and metabolite regulatory network analysis of early developing fruit tissues highlights new candidate genes for the control of tomato fruit composition and development. Plant Physiol. 149, 1505–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska J., Zauber H., Buchanan B. B., Cejudo F. J., Geigenberger P. (2009). NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc. Natl. Acad. Sci. U.S.A.106, 9908–9913 10.1073/pnas.0903559106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda T., Yabuta Y., Rapolu M., Motoki T., Takeda T., Yoshimura K., et al. (2004). Feedback inhibition of spinach l-galactose dehydrogenase by l-ascorbate. Plant Cell Physiol. 45, 1271–1279 10.1093/pcp/pch152 [DOI] [PubMed] [Google Scholar]
- Muller O., Krawinkel M. (2005). Malnutrition and health in developing countries. CMAJ 173, 279–286 10.1503/cmaj.050342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Rober B. T., Kossmann J., Hannah L. C., Willmitzer L., Sonnewald U. (1990). One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol. Gen. Genet. 224, 136–146 10.1007/BF00259460 [DOI] [PubMed] [Google Scholar]
- Nielsen T. H., Krapp A., Roper-Schwarz U., Stitt M. (1998). The sugar-mediated regulation of genes encoding the small subunit of Rubisco and the regulatory subunit of ADP glucose pyrophosphorylase is modified by phosphate and nitrogen. Plant Cell Environ. 21, 443–454 10.1046/j.1365-3040.1998.00295.x [DOI] [Google Scholar]
- Nishikawa F., Kato M., Hyodo H., Ikoma Y., Sugiura M., Yano M. (2005). Effect of sucrose on ascorbate level and expression of genes involved in the ascorbate biosynthesis and recycling pathway in harvested broccoli florets. J. Exp. Bot. 56, 65–72 [DOI] [PubMed] [Google Scholar]
- Noctor G., Foyer C. H. (1998). Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Biol. 49, 249–279 10.1146/annurev.arplant.49.1.249 [DOI] [PubMed] [Google Scholar]
- Osorio S., Alba R., Nikoloski N., Kochevenko A., Fernie A. R., Giovannoni J. J. (2012). Integrative comparative analyses of transcript and metabolite profiles from pepper and tomato ripening and development stages uncovers species-specific patterns of network regulatory behavior. Plant Physiol. 159, 1713–1729 10.1104/pp.112.199711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio S., Vallarino J. G., Szecowka M., Ufaz S., Tzin V., Angelovici R., et al. (2013). Alteration of the interconversion of pyruvate and malate in the plastid or cytosol of ripening tomato fruit invokes diverse consequences on sugar but similar effects on cellular organic acid, metabolism, and transitory starch accumulation. Plant Physiol. 161, 628–643 10.1104/pp.112.211094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padayatty S. J., Katz A., Wang Y., Eck P., Kwon O., Lee J., et al. (2003). Vitamin C as an antioxidant: evaluation of its role in disease prevention. J. Am. Coll. Nutr. 22, 18–35 10.1080/07315724.2003.10719272 [DOI] [PubMed] [Google Scholar]
- Pastori G. M., Kiddle G., Antoniw J., Bernard S., Veljovic-Jovanovic S., Verrier P. J., et al. (2003). Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15, 939–951 10.1105/tpc.010538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti S., Gruissem W., Sautter C. (2004). The nutritional fortification of cereals. Curr. Opin. Biotechnol. 15, 162–165 10.1016/j.copbio.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Qin A., Shi O., Yu X. (2011). Ascorbic acid contents in transgenic potato plants overexpressing two dehydroascorbate reductase genes. Mol. Biol. Rep. 38, 1557–1566 10.1007/s11033-010-0264-0262 [DOI] [PubMed] [Google Scholar]
- Rocha-Sosa M., Sonnewald U., Frommer W., Stratmann M., Schell J., Willmitzer L. (1989). Both developmental and metabolic signals activate the promoter of a class I patatin gene. EMBO J. 8, 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Beltran J. A., Dubois F., Mortiaux F., Portetelle D., Gebhardt C., Sangwan R. S., et al. (1999). Identification of cytosolic Mg2+-dependent soluble inorganic pyrophosphatases in potato and phylogenetic analysis. Plant Mol. Biol. 39, 449–461 10.1023/A:1006136624210 [DOI] [PubMed] [Google Scholar]
- Schaffer A. A., Petreikov M. (1997). Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol. 113, 739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch W. (1994). Improving tomato fruit quality and the European regulatory framework. Euphytica 79, 287–291 10.1007/BF00022529 [DOI] [Google Scholar]
- Smirnoff N., Wheeler G. L. (2000). Ascorbic acid in plants: biosynthesis and function. Crit. Rev. Biochem. Mol. 35, 291–314 10.1080/10409230008984166 [DOI] [PubMed] [Google Scholar]
- Sonnewald U. (1992). Expression of E. coli inorganic pyrophosphatase in transgenic plants alters photoassimilate partitioning. Plant J. 2, 571–581 [PubMed] [Google Scholar]
- Sonnewald U., Hajirezaei M. R., Kossmann J., Heyer A., Trethewey R. N., Willmitzer L. (1997). Increased potato tuber size resulting from apoplastic expression of a yeast invertase. Nat. Biotechnol. 15, 794–797 10.1038/nbt0897-0794 [DOI] [PubMed] [Google Scholar]
- Sowokinos J. R. (1981). Pyrophosphorylases in Solanum tuberosum: II. Catalytic properties and regulation of ADP-glucose and UDP-glucosepyrophosphorylase activities in potatoes. Plant Physiol. 68, 924–929 10.1104/pp.68.4.924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos J. R., Preiss J. (1982). Pyrophosphorylases in Solanum tuberosum: III. Purification, physical, and catalytic properties of ADP-glucose pyrophosphorylase in potatoes. Plant Physiol. 69, 1459–1466 10.1104/pp.69.6.1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz K. A., Potter J. D. (1996). Vegetables, fruit, and cancer prevention. J. Am. Diet. Assoc. 96, 1027–1039 10.1016/S0002-822300273-822300278 [DOI] [PubMed] [Google Scholar]
- Stevens R., Buret M., Duffé P., Garchery C., Baldet P., Rothan C., et al. (2007). Candidate genes and quantitative trait loci affecting fruit ascorbic acid content in three tomato populations. Plant Physiol. 143, 1943–1953 10.1104/pp.106.091413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. (1998). Pyrophosphate as an energy donor in the cytosol of plant cells: an enigmatic alternative to ATP. Bot. Acta 111, 167–175 [Google Scholar]
- Sturm A., Tang G.Q. (1999). The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci. 4, 401–407 [DOI] [PubMed] [Google Scholar]
- Sweetlove L. J., Muller-Rober B., Willmitzer L., Hill S. A. (1999). The contribution of adenosine 5′-diphosphoglucose pyrophosphorylase to the control of starch synthesis in potato tubers. Planta 209, 330–337 10.1007/s004250050640 [DOI] [PubMed] [Google Scholar]
- Szecowka M., Osorio S., Obata T., Araujo W., Rohrmann J., Nunes-Nesi A., et al. (2012). Decreasing the mitochondrial synthesis of malate in potato tubers does not affect plastidial starch synthesis suggesting that the physiological regulation of ADPglucose pyrophosphorylase is context-dependent. Plant Physiol. 160, 2227–2238 10.1104/pp.112.204826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauberger E., Fernie A. R., Emmermann M., Renz A., Kossmann J., Willmitzer L., et al. (2000). Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplasts import carbon from the cytosol in the form of glucose-6-phosphate. Plant J. 23, 43–53 10.1046/j.1365-313x.2000.00783.x [DOI] [PubMed] [Google Scholar]
- Taussky H. H., Shorr E. (1953). A microcolorimetric method for the determination of inorganic phosphorus. J. Biol. Chem. 202, 675–685 [PubMed] [Google Scholar]
- Tiessen A., Hendriks J. H., Stitt M., Branscheid A., Gibon Y., Farre E. M., et al. (2002). Starch synthesis in potato tubers is regulated by post-translational redox modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell 14, 2191–2213 10.1105/tpc.003640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiessen A., Prescha K., Branscheid A., Palacios N., McKibbin R., Halford N. G., et al. (2003). Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. Plant J. 35, 490–500 10.1046/j.1365-313X.2003.01823.x [DOI] [PubMed] [Google Scholar]
- Tokunaga T., Miyahara K., Tabata K., Esaka M. (2005). Generation and properties of ascorbic acid-overproducing transgenic tobacco cells expressing sense RNA for l-galactono-1, 4-lactone dehydrogenase. Planta 220, 854–863 10.1007/s00425-004-1406-1403 [DOI] [PubMed] [Google Scholar]
- Torabinejad J., Donahue J. L., Gunesekera B. N., Allen-Daniels M. J., Gillaspy G. E. (2009). VTC4 is a bifunctional enzyme that affects myoinositol and ascorbate biosynthesis in plants. Plant Physiol. 150, 951–961 10.1104/pp.108.135129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M., Rhodes C. J., Moncol J., Izakovic M., Mazur M. (2006). Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160, 1–40 10.1016/j.cbi.2005.12.009 [DOI] [PubMed] [Google Scholar]
- van den Hoogen B. M., van Weeren P. R., Lopes-Cardozo M., van Golde L. M., Barneveld A., van de Lest C. H. (1998). A microtiter plate assay for the determination of uronic acids. Anal. Biochem. 257, 107–111 10.1006/abio.1997.2538 [DOI] [PubMed] [Google Scholar]
- Wheeler G. L., Jones M. A., Smirnoff N. (1998). The biosynthetic pathway of vitamin C in higher plants. Nature 393, 365–369 10.1038/30728 [DOI] [PubMed] [Google Scholar]
- Wolucka B. A., Van Montagu M. (2003). GDP-mannose 3′,5′-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J. Biol. Chem. 278, 47483–47490 10.1074/jbc.M309135200 [DOI] [PubMed] [Google Scholar]
- Yabuta Y., Yoshimura K., Takeda T., Shigeoka S. (2000). Molecular characterization of tobacco mitochondrial l-galactono-gamma-lactone dehydrogenase and its expression in Escherichia coli. Plant Cell Physiol. 41, 666–675 10.1093/pcp/41.6.666 [DOI] [PubMed] [Google Scholar]
- Zanor M. I., Osorio S., Nunes-Nesi A., Carrari F., Lohse M., Usadel B., et al. (2009). RNA interference of LIN5 in tomato confirms its role in controlling Brix content, uncovers the influence of sugars on the levels of fruit hormones, and demonstrates the importance of sucrose cleavage for normal fruit development and fertility. Plant Physiol. 150, 1204–1218 10.1104/pp.109.136598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Liu J., Zhang Y., Cai X., Gong P., Zhang J., et al. (2011). Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep. 30, 389–398 10.1007/s00299-010-0939-0930 [DOI] [PubMed] [Google Scholar]
- Zorrilla-Fontanesi Y., Cabeza A., Domínguez P., Medina J. J., Valpuesta V., Denoyes-Rothan B., et al. (2011). Quantitative trait loci and underlying candidate genes controlling agronomical and fruit quality traits in octoploid strawberry (Fragaria × ananassa). Theor. Appl. Genet. 123, 755–778 10.1007/s00122-011-1624-1626 [DOI] [PubMed] [Google Scholar]
- Zrenner R., Schuler K., Sonnewald U. (1996). Soluble acid invertase determines the hexose-to-sucrose ratio in cold-stored potato tubers. Planta 198, 246–252 10.1007/BF00206250 [DOI] [PubMed] [Google Scholar]