Introduction
There is a need for therapies that can effectively treat primary hepatocellular carcinoma (HCC) as well as metastases to the liver. HCC is the fifth most common cancer worldwide and the fourth most common cause for cancer death.1 Metastasis to the liver occurs commonly from primary cancers of the gastrointestinal track and other solid tumors. Surgical resection is an option for less than 30% of patients presenting with metastases.1,2 These patients are often not good surgical candidates, because of the location of the tumor or because of intercurrent disease, such as cirrhosis.2 However, thermal ablation is a cost-effective, nonsurgical option for treatment of patients with HCC and liver metastases3 and is currently practiced worldwide.
In this review, a new approach is discussed, combining thermal ablation with drug-carrying thermosensitive liposomes. The rationale for this approach is presented and discussed in light of current liposome formulations and methods to achieve thermal ablation. A summary of preclinical and clinical studies that are relevant to treatment of primary and metastatic liver cancer is also presented.
Thermal Ablation
There are many technologies capable of thermal ablation treatment of focal liver tumors. Power deposition may be localized in a liver target either with externally applied focused ultrasonography or invasively with 1 of several interstitial heating techniques. The underlying physics for 9 interstitial heating modalities has been reviewed previously.4 Clinical methodology and results from several of those invasive approaches were highlighted in a special issue on thermal ablation therapy of the International Journal of Hyperthermia.5 For liver tumors, the most common ablative approach involves use of radiofrequency (RF) electrodes inserted percutaneously to the liver target. RF electrodes are available that heat tissue from RF currents between an array of implanted electrodes, along the length of bipolar electrodes, or between implanted electrode(s) and a ground return pad on the skin. Some electrodes include internal cooling to reduce tissue desiccation around the needle, allowing increased power levels and effective treatment volume.6 To minimize the number of percutaneous insertions and expand the volume of tissue encompassed within an array, RF electrodes are available that deploy multiple tines curving out into surrounding tissue like an umbrella from a central larger electrode.7
Although there are fewer clinical systems available, interstitial microwave antennae are increasingly used for thermal ablation of liver tumors,8,9 to take advantage of increased penetration of power deposition around each implant.10,11 Laser interstitial thermal therapy is also commonly used for liver ablation,12 with either ultrasound13 or magnetic resonance (MR) image guidance.14 Use of high-intensity focused ultrasonography (HIFU) with MR imaging thermometry has also increased for liver ablation in recent years.15–17
Regardless of modality used, power is applied to reach ablative temperatures in the range of 50°C to 100°C. Because of rapid accumulation of lethal thermal dose in this temperature range, ablation generally occurs within minutes. However, this therapy is not without limitations. The risk for marginal recurrence increases when lesions are larger than 3 to 5 cm,1,18 and when they are located near large, thermally significant vessels, or visceral organs.18 In addition, long-term patient follow-up has shown high rates of local tumor progression after RF ablation treatment.3 Thus, there has been interest in combining thermal ablation with treatments that would augment cytotoxicity at the margin of the ablation zone.
Thermal Ablation and Chemotherapeutics
Several techniques have been assessed to overcome these limitations of ablation, including the combination of chemotherapy with RF ablation. Many chemotherapeutic agents, including doxorubicin, are known to interact synergistically with hyperthermia,19–23 so combining these agents with RF ablation would potentially be effective. For example, Mostafa and colleagues24 assessed the combination of RF ablation and a chemoembolic mixture, consisting of doxorubicin in iodized oil, in rabbits with hepatic tumors and showed that the combination of local drug delivery and hyperthermia resulted in larger coagulation areas relative to controls.
However, the main dose-limiting factor with chemotherapeutic agents, such as doxorubicin, is normal tissue toxicity, supporting the use of a less toxic liposomal formulation. The first doxorubicin HCl liposome formulation (Doxil, Janssen Products, LP, Horsham, PA, USA), was approved for several clinical indications by equal antitumor activity to free drug combined with reduced cardiotoxicity, which is a dose-limiting organ for free doxorubicin.25,26
Doxorubicin Liposome Formulation
Liposomes are spontaneously forming lipid bilayer vesicles containing an aqueous medium.27 They are nanoscale in size, in the range of 1 to a few hundred nanometers in diameter. They can be unilamellar or multilamellar. The most common types are unilamellar formulations, which are formed by passing larger liposomes at high pressure through filters of defined pore diameter or by ultrasonication. Several drugs have been encapsulated into liposomes.28 For oncologic applications, doxorubicin has been the most widely used drug, because it can be loaded at high concentration. By preloading the liposomes with acid, doxorubicin, which is a weak base, diffuses across the lipid bilayer and reaches such high concentrations inside the liposome that it crystallizes.29 This review focuses on doxorubicin-containing liposomes, because these are the best-developed formulations and they have been used in clinical trials.
Thermal Ablation and Liposomal Doxorubicin
Several studies have assessed the combination of RF ablation and the nonthermally sensitive liposomal doxorubicin, showing larger ablation zones compared with RF ablation alone, both at the preclinical and clinical levels.30–36 Goldberg and colleagues34 assessed the combination of RF ablation and Doxil in a rat mammary adenocarcinoma model and observed increased coagulation diameter in the solid tumors compared with RF ablation alone, and in a follow-up study, D'Ippolito and colleagues33 observed a decrease in tumor growth and potential increase in rat survival. In the same tumor model, Ahmed and colleagues32 reported that the combination of RF ablation and liposomal doxorubicin increased tumor uptake and accumulation of doxorubicin compared with liposomal doxorubicin alone, as well as increased tumor necrosis compared with ablation alone.31,32 Ahmed and colleagues30 also studied the combination of RF ablation followed by liposomal doxorubicin treatment in canine sarcomas, rabbit liver and kidneys, and in the thigh muscle of rats. These investigators observed increased coagulation and doxorubicin accumulation after the combined treatment, showing the effectiveness of this treatment in multiple tissue and tumor types. A clinical study conducted by Goldberg and colleagues35 treated 10 patients presenting with focal hepatic tumors and showed 25% to 30% greater tumor destruction when liposomal doxorubicin was combined with RF ablation compared with RF ablation alone.37
Several mechanisms have been suggested for the synergistic effect of liposomal doxorubicin and RF ablation.38 Solazzo and colleagues39 observed increased markers of DNA breakage, oxidative stress, and apoptosis, as well as increased heat-shock protein 70 in the areas surrounding the ablation zone after combination treatment. In addition, the changes in vasculature caused by ablation may affect liposome accumulation within the tumor.38 Ahmed and colleagues32 observed increased intratumoral drug uptake and found that less doxorubicin was necessary for tumor destruction after combined RF ablation and Doxil.
Although these studies on the combination of RF ablation and liposomal doxorubicin have shown improvement (ie, increased coagulation and antitumor effect), it has been suggested that optimization is still necessary to improve clinical outcome,35 particularly if advantage could be taken of the heat produced by ablation. One possible approach is through the use of thermosensitive liposomes, which would take advantage of the more moderate hyperthermic temperatures at the tumor margin, allowing for triggered release of drug at the edge of the heated zone. Gasselhuber and colleagues40 performed mathematical modeling of drug delivery from low-temperature-sensitive liposomes (LTSL) during RF ablation, which predicted higher drug accumulation with liposomal doxorubicin compared with free drug, as well as lower peak plasma concentration, supporting the use of temperature-sensitive liposomes in combination with RF ablation.
History of Thermosensitive Liposomes Leading to Development of Second-Generation Formulations
Dozens of studies have been published combining nonthermally sensitive liposomes with hyperthermia.41 The rationale for such combinations emanates from the observation that hyperthermia increases vascular pore sizes in tumor microvessels, leading to enhanced extravasation.42 Although tumor vasculature is typically more permeable to nanoparticles, this enhanced permeability and retention (EPR) effect is substantially increased with hyperthermia treatment. In this review, these studies are not discussed, because when compared head to head, thermally sensitive liposomes achieve higher drug delivery and better antitumor effect.42,43 Reviews of previous work with nonthermally sensitive liposomes have been published.41 Similarly, details of the chemical compositions and physical characterizations of thermosensitive liposomes have been reviewed elsewhere and are not discussed here.27,44
In a frozen state, the liposome membrane contains plates of frozen lipid that interface in a pattern that resembles a soccer ball.27 When the temperature is increased, the junctions between the frozen plates melt first, leading to enhanced permeability.27 The liposomes are not destroyed during the melting process; they merely go from a frozen to a melted state. Milton Yatvin was the first to recognize that the enhanced permeability of liposomes to aqueous media near their solid-liquid transition could be harnessed as a drug delivery method if it was combined with local application of heat.45,46The original formulation contained a mixture of 2 lipids of different melting temperatures; it showed a peak in permeability at 45°C. Yatvin and colleagues published several articles45,47,48 with this formulation, showing that it could improve antitumor effect of several drugs, when combined with hyperthermia. Yatvin and colleagues46 surmised that the increased antitumor effect seen with this formulation was the result of several mechanisms: (1) thermally increased perfusion and EPR, (2) enhanced release of bioavailable drug and (3) enhanced transendothelial drug transport.
Although the principle of thermally mediated drug delivery pioneered by Yatvin was brilliant, the earlier formulations46,49 had 3 important limitations:
The liposome was readily taken up by the reticuloendothelial system, as opposed to circulating long enough to be efficiently delivered to the heated tumor. The discovery that the circulation time of liposomes could be prolonged considerably by adding polyethylene glycol (PEG) to the surface was a major achievement; these liposomes could circulate for days, thereby maximizing the EPR effect.50 Gaber and colleagues49 PEGylated the Yatvin formulation to yield a long circulating thermosensitive liposome. Hyperthermia treatment induced enhanced liposome accumulation in tumors and increased drug release. The combination of these 2 effects enhanced delivery of drug to the tumor by nearly 50-fold, compared with administering the liposomes without heating.51
The temperature for drug release was too high (43°C–45°C). Typical temperatures that can be achieved in patients without pain or risk of thermal injury are in the range of 40°C to 43°C.52 Thus, there was a mismatch between what temperatures were achievable clinically and what was needed for maximum drug delivery.
The time to reach maximal drug release was too slow (>30 minutes) for routine clinical use.43 If a liposome entered the heated region and did not extravasate into the tumor, then it would not completely release its contents before the blood containing the liposome exited the tumor. As is shown later, the second-generation thermo-sensitive liposomes were designed to correct these deficiencies.
Second-Generation Thermosensitive Liposome Formulations
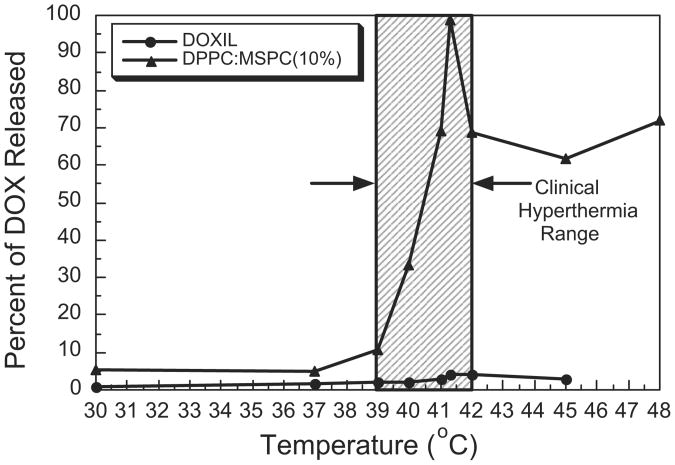
David Needham and Mark Dewhirst and colleagues collaborated to develop the first doxorubicin-containing thermosensitive liposome that showed release in the clinically acceptable hyperthermia range.42,43 In addition to a mixture of 2 double chain fatty acids, the formulation contained a small percentage of a single chain fatty acid, known as a lysolipid. It also contained PEG to extend the circulation time. The lysolipid provided for a very rapid drug release (<20 seconds).43 The maximum release rate temperature was 41.3°C (Fig. 1), but enhanced release occurred over a range from 39.5°C to 42°C. The circulation time was on the order of 2 hours in humans, which is considerably shorter than Doxil, but of sufficient length to work with a typical hyperthermia treatment, which lasts 30 to 60 minutes.1 The formulation was given the generic term LTSL. In this review, LTSL-Dox is used to represent the doxorubicin-containing formulation, which is the only drug formulation that has been studied extensively with this type of liposome. The generic term for the commercial doxorubicin-containing LTSL is lysothermosensitive liposomal doxorubicin (LTLD).
Fig. 1.
Percent of Dox released for Doxil and DPPC/MSPC (10%) at t = 4 minutes. Comparison of doxorubicin release after 4 minutes of heating across a range of temperatures for LTSL-Dox (DPPC/MSPC (10%)) versus Doxil. LTSL-Dox releases drug in the temperature range between 39.5°C and 42°C, which is in the range that can be achieved for routine application of hyperthermia. Maximal release occurs at the phase transition temperature of 41.3°C. DPPC - Dipalmitoylphosphatidylcholine; MSPC - Monostearoylphosphatidylcholine.
Other groups have also developed LTSL formulations. Lindner and colleagues53 described a long circulating formulation that contains a mixture of natural and synthetic lipids. This liposome achieves maximal release at 42°C. This group has extensively studied how plasma proteins affect the stability of LTSLs54 and has recently reported that the stability is affected by both albumin and IgG, the 2 most common proteins found in plasma.55 Both proteins tend to lower the temperature dependence of drug release from thermosensitive liposomes. The presence of PEG does not seem to protect the liposomes from these effects. It is important to keep such effects in mind in the engineering of LTSLs so that they still perform to expectation when used clinically. In this case, the goal is to have them maintain drug until heated so that release of drug is maximized in the area receiving hyperthermia.
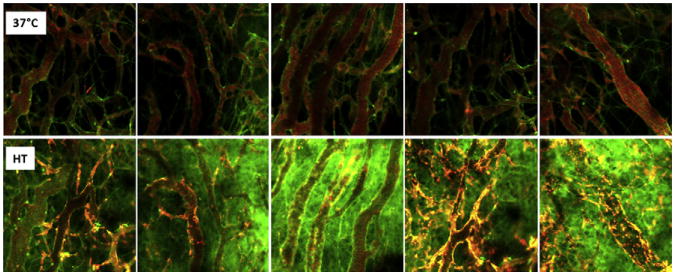
Dicheva and colleagues56 have recently reported on a new cationic LTSL formulation. The rationale for this design is based on a different principle than LTSL-Dox. In this case, the goal is to permit intracellular uptake of the cationic liposomes by vascular endothelium and tumor cells, before administration of heat. This concept comes from previous observations that cationic liposomes have a greater affinity for endothelial cells and tumor cells than other types of liposomes. There has been some speculation that damage to vascular endothelium by drug-containing cationic liposomes may lead to ischemia and tumor cell death, independent of any direct tumor cell killing caused by drug delivered by the liposomes. Thus, once accumulated, rapid drug release by intracellular cationic liposomes may achieve high intracellular concentrations of drug, thereby maximizing damage to both the endothelial cell and tumor cell compartments (Fig. 2). This factor is especially important for this approach, because reliance on the EPR effect alone does not yield uniform drug delivery throughout the tumor (also see Fig. 3).57
Fig. 2.
Performance of cationic thermosensitive liposome. Using the dorsal skin-fold window chamber, the localization and extravasation of the liposomes can be monitored over time. (Upper panel) Selected images of cationic thermosensitive liposome accumulation in endothelial cells lining vessel walls of B16 melanoma tumors, 2 hours after administration. Some evidence for extravasation is also observed. (Bottom panel) Appearance of window chamber after 1 hour of heating at 43°C. Green represents the presence of carboxyfluorescein, which was previously loaded into the liposomes. The appearance of green signal shows that the contents have been released. Note the lack of green signal in the images taken before heating, which reflects quenching of the fluorescence when calcein is encapsulated inside the liposome (upper panel). (Reprinted from Dicheva BM, Hagen TL, Li L, et al. Cationic thermosensitive liposomes: a novel dual targeted heat-triggered drug delivery approach for endothelial and tumor cells. Nano Lett 2012; [Epub ahead of print]. Copyright (2012) American Chemical Society; with permission.)
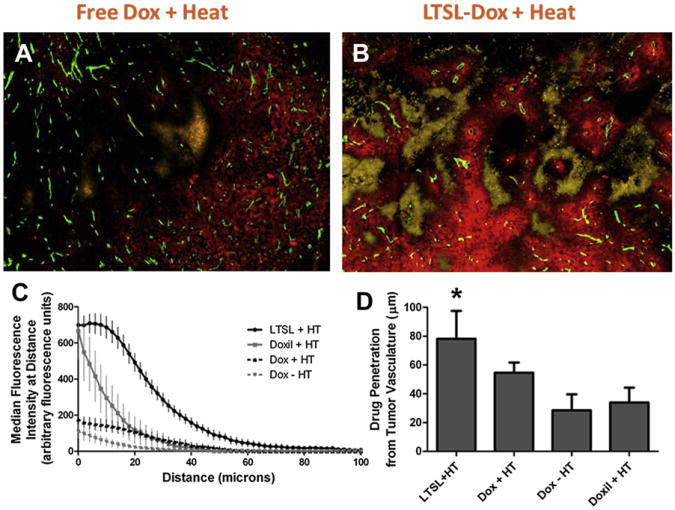
Fig. 3.
Comparison of drug penetration distances from nearest blood vessel for free drug ± 42°C heating for 1 hour, LTSL-Dox + heat and Doxil + heat (A, B). Green = CD31 for endothelial cells, red = doxorubicin, and yellow = EF5 hypoxia marker. The difference in total amount of drug delivered for free drug versus LTSL drug is obvious, but more importantly is the drug coverage around tumor blood vessels and the encroachment into hypoxic areas. The drug penetration distance was doubled for P = .0106, between LTSL + HT and Doxil + HT compared with the other treatment groups (C, D). The differences were highly significant. (From Manzoor AA, Lindner LH, Landon CD, et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res 2012;72(21):5573; with permission.)
Several other investigators have described liposomes containing polymers that show similar drug release characteristics.58–62 Thus, there is increasing interest in exploiting this type of drug delivery system.
Preclinical Studies with LTSL-Dox
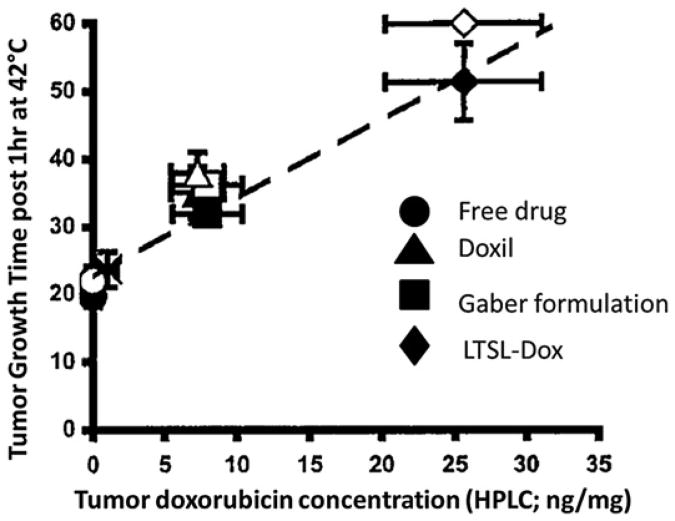
The LTSL-Dox formulation developed by Needham and Dewhirst has been tested extensively at the preclinical level. When FaDu head and neck cancer xenografts were heated to 42°C, LTSL-Dox showed 25-fold greater doxorubicin accumulation in the tumor tissue than free drug-treated tumors.42 It delivered 5-fold more drug than a Doxil formulation and the PEGylated thermosensitive liposome described by Gaber and colleagues49 (Fig. 4). Furthermore, the percentage of drug bound to DNA was substantially greater for LTSL-Dox, compared with the other treatment groups. DNA-bound drug is an important end point for this drug, because DNA damage is a primary mechanism for cell death with doxorubicin.
Fig. 4.
Relationship between the concentration of doxorubicin achieved in tumor tissue and the tumor growth time for free drug and 3 liposomal formulations. The Gaber formulation49 is a PEGylated version of the original Yatvin formulation.46 The open and closed symbols represent replicate experiments. Data were obtained by removing a cohort of animals at the end of treatment from each group and having the tumor analyzed for total doxorubicin concentration, using high-performance liquid chromatography. The remaining animals in each group were followed for tumor regrowth. (Adapted from Kong G, Anyarambhatla G, Petros WP, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res 2000;60:6954; with permission.)
In 2 separate tumor growth delay studies using the maximum tolerated dose (MTD) of doxorubicin in combination with local hyperthermia to the tumor-bearing limb, the LTSL-Dox formulation yielded a substantial proportion of long-term tumor control up to 60 days after treatment.42,43 Free drug showed little to no activity, whereas the other liposome formulations yielded some growth delay, but virtually no cures. LTSL-Dox showed superior antitumor activity in several xenograft and allograft tumor models.63
The extent of antitumor effect seen after LTSL-Dox treatment has been proportional to the concentration of drug delivered.42,63,64 Using noninvasive optical methods to measure doxorubicin concentrations and properties of hemoglobin in the SKOV3 ovarian cancer xenograft, Palmer and colleagues64 showed that the efficacy of LTSL-Dox with hyperthermia was also influenced by perfusion and hypoxia in addition to drug concentration achieved in each tumor (Fig. 5). It is not surprising to see the influence of hypoxia, because the efficacy of this drug has previously been reported to be reduced under hypoxic conditions in some tumor cell lines.65 The concentration dependence of the observed antitumor effect may reflect variations in the total amount of drug delivered, which is related to efficiency of perfusion. LTSL-Dox cannot show its antitumor activity if the drug is not delivered to all portions of the tumor. These results strongly suggest that measurements of perfusion and hypoxia before treatment with LTSL-Dox might yield important prognostic information about the usefulness of this formulation in individual patients. Alternatively, doxorubicin accumulation could be measured directly by using liposomes that are coloaded with MR contrast agents.66,67 The ability to measure drug accumulation in tumor tissue in real time while adjusting the heating pattern has been coined drug dose painting. This approach is being pursued by several groups that are involved in the development of HIFU as an alternative method to deliver drugs with thermally ablative temperatures in a variety of tumor sites.16,68–71
Fig. 5.
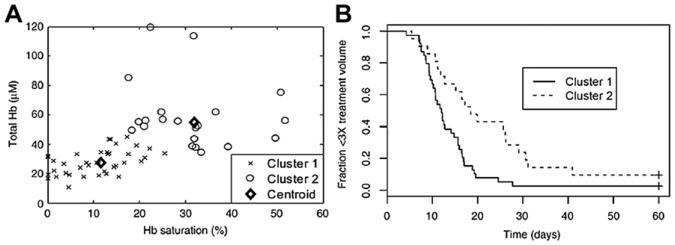
Relationship between perfusion and extent of tumor hypoxia and duration of local tumor control after treatment with LTSL-Dox and hyperthermia. (A) Total hemoglobin (Hb) and Hb saturation (Hbsat) reflect perfusion and extent of hypoxia in individual tumors as measured using a noninvasive optical spectroscopy method. These same tumors were followed for growth time. Cluster analysis revealed 2 separate populations of tumors, which were characterized by relatively low Hb and Hbsat versus the second group, which had higher values of these 2 parameters. (B) The time to reach 3 times treatment volume was linked to these values, indicating that the more poorly perfused and hypoxic tumors responded less favorably to the treatment. (Reproduced from Palmer GM, Boruta RJ, Viglianti BL, et al. Non-invasive monitoring of intra-tumor drug concentration and therapeutic response using optical spectroscopy. J Control Release 2010;142(3):463; with permission.)
The combination of hyperthermia with LTSL-Dox yielded higher drug concentrations than the Doxil and Gaber formulations, even although these were of equivalent size to LTSL-Dox. This finding led to the hypothesis that the difference in the achieved drug concentration was caused by intravascular drug release.42 If such a mechanism were operational, it would drive the drug out of the vasculature down its concentration gradient and into the interstitial space of the tumor. Recently, this theory has been proved.57 Using a combination of fluorescently labeled liposomes and taking advantage of the natural fluorescence of doxorubicin, Manzoor and colleagues57 used the dorsal skin-fold window chamber model to show that liposomal extravasation does not contribute to the enhanced drug delivery with LTSL-Dox when combined with 42°C heating. Instead, nearly 100% of the drug is released intravascularly. This situation creates a local continuous infusion of drug, exclusively in the heated tumor site. The properties of the original LTSL-Dox formulation of Needham and that of Lindner were similar in this respect. The presence of high intravascular drug concentration during heating yields greater perivascular penetration than can be achieved with either free drug or Doxil when they are combined with hyperthermia (see Fig. 3).
LTSL-Dox and the cationic thermosensitive formulations show maximal uptake in endothelial cell and pericyte nuclei.56,57 This uptake was not observed with free doxorubicin. It has previously been shown that LTSL-Dox treatment with 42°C heating can lead to vascular shutdown, whereas neither heat nor LTSL-Dox alone can achieve this type of effect.72 The antivascular effect may have been the result of endothelial cell damage and death after treatment, driven by the high concentrations of drug found in these cells. It would be interesting to compare the performance of the cationic liposomes, described earlier, with LTSL-Dox, to determine whether they show similar antivascular effects.
Canine Clinical Studies with LTSL
Before being used in human clinical trials, the LTSL-Dox formulation was tested in a phase I trial of companion dogs with spontaneous tumors.73 A total of 21 dogs were entered into this study at doses of 0.7, 0.93, and 1.0 mg/kg, with a planned course of 3 cycles every 3 weeks. Histologies included sarcomas and carcinomas. The median tumor volume was 90.6 cm3 (range, 3.1–1747.0 cm3).
The first 4 animals enrolled showed an anaphylactoid reaction during drug infusion. This reaction was typified by hypotension and an increase in end-respiratory pressure. Subsequent studies in normal dogs revealed this reaction to be a result of marked histaminemia. The remaining animals enrolled on the study were premedicated with steroids and antihistamines. No further reactions of this type were encountered. Similar premedication regimens are also being used in subsequent human trials. Dose-limiting toxicities (DLTs) included neutropenia (2 DLTs each at 0.93 and 1.0 mg/kg) and hepatic necrosis in 1 patient at 1.0 mg/kg after the second course. It is not clear whether the necrosis was caused by drug, as there were no increases in liver enzymes in this patient after the first treatment. The median temperature achieved for all cycles was 41.2°C, and the median 10th percentile of the temperature distribution was 39.5°C. Temperatures achieved in the tumors were adequate to initiate drug release from LTSL-Dox. A total of 20 of the dogs had at least 2 cycles of treatment. Of these dogs, 12 achieved stable disease, and 6 had a partial response to treatment.
Human Clinical Studies with LTSL-Dox
The first clinical trials conducted with LTSL-Dox were in patients with liver metastases or HCC.1,74 Until now, we have focused on developing a drug formulation that performs well for an hour of heating in the temperature range from 39.5°C to 42°C, whereas thermal ablation temperatures are typically more than 50°C for a few minutes. After the publication of the antitumor effects of the LTSL-Dox,42 Dr Dewhirst was contacted by Bradford Wood, an interventional radiologist at the National Institutes of Health (NIH). Wood suggested that this formulation might have usefulness in the treatment of liver metastases and in primary liver cancer, if it were combined with RF-mediated thermal ablation. The rationale for the combination of thermal ablation with LTSL-Dox comes from the risk of marginal recurrence, as discussed earlier.1 Temperatures at the margins of lesions greater than 3 cm are not high enough to cause thermal coagulation, but they are in the range for drug release by LTSL-Dox. The concept is that any residual cells not killed directly by ablation would be killed by massive amounts of doxorubicin deposited there.
Subsequently, the first studies on humans were conducted at the NIH, in collaboration with the commercial developer. Later, the phase 1 study was expanded to include a site in Hong Kong, and additional Asian sites were added.74,75
Pharmacokinetics properties and MTD were determined in a phase 1 dose escalation study.75 Based on the plasma concentration–time curve, Poon and colleagues74 optimized the dose levels and treatment timing so that tumor ablation occurred during the peak plasma concentration. The phase 1 trials clearly showed that residual cancer cells remaining after ablation were killed with LTSL-Dox treatment. First, the imageable lesions tended to become larger after combined treatment (Fig. 6), whereas with RF ablation alone, they tended to shrink. In addition, the median time to progression for patients receiving the MTD (50 mg/m2) was 374 days, versus 80 days for patients receiving less than the MTD (P = .03). Lesions smaller than 3 cm are effectively treated with RF ablation alone, and tumors with metastases beyond the liver would not be suitable for a local treatment but are candidates for systemic chemotherapy.
Fig. 6.
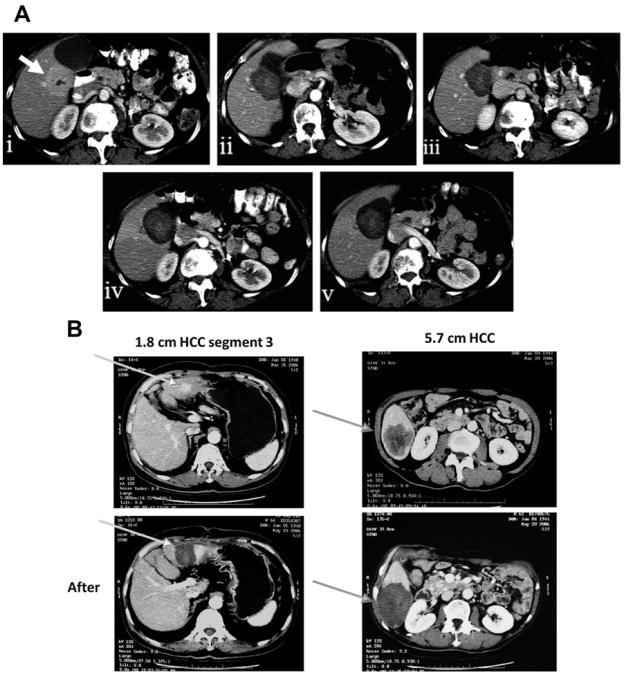
Contrast-enhanced computed tomography scans of hepatic lesions, before and after RF ablation, combined with LTLD. (A) Appearance of metastatic adrenal cell carcinoma, before and several months after thermal ablation. Note that the ablation zone enlarges and stabilizes after treatment. (i) Before treatment (arrow), (ii) 3 days after treatment, (iii) 4 weeks after treatment, (iv) 11 weeks after treatment, (v) 20 weeks after treatment. (B) Appearance of 2 primary HCCs, before and after thermal ablation. (Courtesy of Brad Wood and Celsion Corporation, Lawrenceville, NJ; Images (i), (ii), and (v) in (A) Reprinted from Wood BJ, Poon RT, Locklin JK, et al. Phase I study of heat-deployed liposomal doxorubicin during radiofrequency ablation for hepatic malignancies. J Vasc Interv Radiol 2012;23(2):248–55.e7, with permission from author and publisher; and Images (iii) and (iv) in (A) were kindly provided by the Celsion Corporation.)
Based on positive phase 1 results, the US Food and Drug Administration permitted the company to move directly to a randomized, double-blind phase 3 trial, which compared RF ablation alone with RF ablation with LTSL-Dox.76 This trial was completed recently, with 700 patients accrued (NCT00617981).
Future Directions
In addition to treating liver cancers, LTSL-Dox has been used in combination with local hyperthermia in 2 phase 1 trials of chest wall recurrences of breast cancer.77 One trial was conducted at Duke University, and the second was sponsored by the company that licensed the drug (NCT00826085). Results of those 2 trials are being combined, and a report will be submitted for publication soon. A phase 2 trial is being developed as a follow-on study. Other indications for this drug formulation are being considered. The company recently announced a collaborative agreement with Philips Corporation to test the drug in combination with HIFU for the treatment of bone metastases.
If the LTSL could be used to deliver other common anticancer agents or the newer targeted agents, the applicability of this technology could be expanded. Formulations are typically restricted to drugs that are relatively water-soluble so that the drug can remain encapsulated inside the liposome. We are currently developing LTSL-cisplatin, which could have broader applications in gastrointestinal cancer if it performs as well as LTSL-Dox.
Technologies are available that are capable of heating deep-seated tumors, such as pancreatic and colorectal cancer. The RF phased array systems of the BSD Corporation can heat these deep-seated tumors to the temperature ranges needed for local regional drug delivery with LTSLs.78–80 Trials could be envisioned that would combine LTSL-cisplatin with other chemotherapy and radiation for locally advanced rectal cancer and pancreatic cancer, for example. To take full advantage of such promising approaches requires substantial commercial support. This support has been sorely lacking for thermotherapy trials, aside from the phase 3 trial with LTSL-Dox referred to earlier. Lack of strong commercial support has made it challenging to conduct these types of clinical trials in the United States. On the other hand, insurance coverage for this modality is strong in Europe, and several well-organized trials have been completed there.81–83
Key Points.
Limitations of thermal ablation: this article explains available methods for thermal ablation of hepatocellular carcinomas and emphasizes the limitations, including marginal recurrence of large lesions and tumors near large vessels.
Combination therapy for thermal ablation and chemotherapeutics: combining thermal ablation with liposomal drugs such as doxorubicin, which behaves synergistically with heat, has shown improvement in coagulation diameter, drug accumulation, and necrosis.
Thermosensitive liposomes and thermal ablation: development of thermosensitive liposomes has provided a mechanism to combine thermal ablation and drug delivery to maximize drug release at the target site, showing benefits in both preclinical and clinical models.
Acknowledgments
Work supported by a grant from the NIH-NCI CA42745. The author thanks Celsion Corporation for the computed tomography images provided for Fig. 6.
Footnotes
Conflict of Interest Statement: Dr Dewhirst holds stock in the Celsion Corporation
References
- 1.Poon R, Borys N. Lyso-thermosensitive liposomal doxorubicin: a novel approach to enhance efficacy of thermal ablation of liver cancer. Expert Opin Pharmacother. 2009;10(2):333–43. doi: 10.1517/14656560802677874. [DOI] [PubMed] [Google Scholar]
- 2.Lencioni R, Crocetti L, Cioni D, et al. Percutaneous radiofrequency ablation of hepatic colorectal metastases–technique, indications, results, and new promises. Invest Radiol. 2004;39(11):689–97. doi: 10.1097/00004424-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221(1):159–66. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 4.Stauffer PR, Diederich CJ, Seegenschmiedt MH. Interstitial heating technologies. In: Seegenschmiedt MH, Fessenden P, Vernon CC, editors. Thermoradiotherapy and thermochemotherapy: volume 1, biology, physiology and physics. Berlin, New York: Springer-Verlag; 1995. pp. 279–320. [Google Scholar]
- 5.Stauffer PR, Goldberg SN. Introduction: thermal ablation therapy. Int J Hyperthermia. 2004;20(7):671–7. doi: 10.1080/02656730400007220. [DOI] [PubMed] [Google Scholar]
- 6.Schirmang TC, Dupuy DE. Image-guided thermal ablation of nonresectable hepatic tumors using the Cool-Tip radiofrequency ablation system. Expert Rev Med Devices. 2007;4(6):803–14. doi: 10.1586/17434440.4.6.803. [DOI] [PubMed] [Google Scholar]
- 7.Koda M, Tokunaga S, Matono T, et al. Comparison between different thickness umbrella-shaped expandable radiofrequency electrodes (SuperSlim and CoAccess): experimental and clinical study. Exp Ther Med. 2011;2(6):1215–20. doi: 10.3892/etm.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lencioni R, Crocetti L. Image-guided ablation for hepatocellular carcinoma. Recent Results Cancer Res. 2013;190:181–94. doi: 10.1007/978-3-642-16037-0_12. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Zheng Y, Li S, et al. Percutaneous microwave ablation of larger hepatocellular carcinoma. Clin Radiol. 2013;68(1):21–6. doi: 10.1016/j.crad.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Andreano A, Huang Y, Meloni MF, et al. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2010;37(6):2967–73. doi: 10.1118/1.3432569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38(3):135–43. doi: 10.1067/j.cpradiol.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pacella CM, Francica G, Di Costanzo GG. Laser ablation for small hepatocellular carcinoma. Radiol Res Pract. 2011;2011:595627. doi: 10.1155/2011/595627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francica G, Petrolati A, Di Stasio E, et al. Influence of ablative margin on local tumor progression and survival in patients with HCC </=4 cm after laser ablation. Acta Radiol. 2012;53(4):394–400. doi: 10.1258/ar.2012.110471. [DOI] [PubMed] [Google Scholar]
- 14.Vogl TJ, Jost A, Nour-Eldin NA, et al. Repeated transarterial chemoembolisation using different chemotherapeutic drug combinations followed by MR-guided laser-induced thermotherapy in patients with liver metastases of colorectal carcinoma. Br J Cancer. 2012;106(7):1274–9. doi: 10.1038/bjc.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung TT, Chu FS, Jenkins CR, et al. Tolerance of high-intensity focused ultrasound ablation in patients with hepatocellular carcinoma. World J Surg. 2012;36(10):2420–7. doi: 10.1007/s00268-012-1660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staruch R, Chopra R, Hynynen K. Localised drug release using MRI-controlled focused ultrasound hyperthermia. Int J Hyperthermia. 2011;27(2):156–71. doi: 10.3109/02656736.2010.518198. [DOI] [PubMed] [Google Scholar]
- 17.Wijlemans JW, Bartels LW, Deckers R, et al. Magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU) ablation of liver tumours. Cancer Imaging. 2012;12(2):387–94. doi: 10.1102/1470-7330.2012.9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Widmann G, Schullian P, Haidu M, et al. Stereotactic radiofrequency ablation (SRFA) of liver lesions: technique effectiveness, safety, and interoperator performance. Cardiovasc Intervent Radiol. 2012;35(3):570–80. doi: 10.1007/s00270-011-0200-4. [DOI] [PubMed] [Google Scholar]
- 19.Hahn GM, Strande DP. Cytotoxic effects of hyperthermia and adriamycin on Chinese hamster cells. J Natl Cancer Inst. 1976;57(5):1063–7. doi: 10.1093/jnci/57.5.1063. [DOI] [PubMed] [Google Scholar]
- 20.Overgaard J. Combined adriamycin and hyperthermia treatment of a murine mammary carcinoma in vivo. Cancer Res. 1976;36(9 Pt 1):3077–81. [PubMed] [Google Scholar]
- 21.Rotstein LE, Daly J, Rozsa P. Systemic thermochemotherapy in a rat model. Can J Surg. 1983;26(2):113–6. [PubMed] [Google Scholar]
- 22.Haas GP, Klugo RC, Hetzel FW, et al. The synergistic effect of hyperthermia and chemotherapy on murine transitional cell-carcinoma. J Urol. 1984;132(4):828–33. doi: 10.1016/s0022-5347(17)49882-x. [DOI] [PubMed] [Google Scholar]
- 23.Dahl O. Hyperthermic potentiation of doxorubicin and 4′-epi-doxorubicin in a transplantable neurogenic rat tumor (BT4A) in BD IX rats. Int J Radiat Oncol Biol Phys. 1983;9(2):203–7. doi: 10.1016/0360-3016(83)90100-1. [DOI] [PubMed] [Google Scholar]
- 24.Mostafa EM, Ganguli S, Faintuch S, et al. Optimal strategies for combining trans-catheter arterial chemoembolization and radiofrequency ablation in rabbit V×2 hepatic tumors. J Vasc Interv Radiol. 2008;19(12):1740–8. doi: 10.1016/j.jvir.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 25.Gabizon A, Peretz T, Sulkes A, et al. Systemic administration of doxorubicin-containing liposomes in cancer patients: a phase I study. Eur J Cancer Clin Oncol. 1989;25(12):1795–803. doi: 10.1016/0277-5379(89)90350-7. [DOI] [PubMed] [Google Scholar]
- 26.Safra T, Muggia F, Jeffers S, et al. Pegylated liposomal doxorubicin (Doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m(2) Ann Oncol. 2000;11(8):1029–33. doi: 10.1023/a:1008365716693. [DOI] [PubMed] [Google Scholar]
- 27.Landon CD, Park JY, Needham D, et al. Nanoscale drug delivery and dyperthermia: the materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed J. 2011;3:38–64. doi: 10.2174/1875933501103010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregoriadis G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995;13(12):527–37. doi: 10.1016/S0167-7799(00)89017-4. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Hirsh DJ, Cabral-Lilly D, et al. Doxorubicin physical state in solution and inside liposomes loaded via a pH gradient. Biochim Biophys Acta. 1998;1415(1):23–40. doi: 10.1016/s0005-2736(98)00175-8. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed M, Liu Z, Lukyanov AN, et al. Combination radiofrequency ablation with intratumoral liposomal doxorubicin: effect on drug accumulation and coagulation in multiple tissues and tumor types in animals. Radiology. 2005;235(2):469–77. doi: 10.1148/radiol.2352031856. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed M, Lukyanov AN, Torchilin V, et al. Combined radiofrequency ablation and adjuvant liposomal chemotherapy: effect of chemotherapeutic agent, nanoparticle size, and circulation time. J Vasc Interv Radiol. 2005;16(10):1365–71. doi: 10.1097/01.RVI.0000175324.63304.25. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed M, Monsky WE, Girnun G, et al. Radiofrequency thermal ablation sharply increases intratumoral liposomal doxorubicin accumulation and tumor coagulation. Cancer Res. 2003;63(19):6327–33. [PubMed] [Google Scholar]
- 33.D'Ippolito G, Ahmed M, Girnun GD, et al. Percutaneous tumor ablation: reduced tumor growth with combined radio-frequency ablation and liposomal doxorubicin in a rat breast tumor model. Radiology. 2003;228(1):112–8. doi: 10.1148/radiol.2281020358. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg SN, Girnan GD, Lukyanov AN, et al. Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intravenous liposomal doxorubicin in a rat breast tumor model. Radiology. 2002;222(3):797–804. doi: 10.1148/radiol.2223010861. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg SN, Kamel IR, Kruskal JB, et al. Radiofrequency ablation of hepatic tumors: increased tumor destruction with adjuvant liposomal doxorubicin therapy. Am J Roentgenol. 2002;179(1):93–101. doi: 10.2214/ajr.179.1.1790093. [DOI] [PubMed] [Google Scholar]
- 36.Solazzo S, Mertyna P, Peddi H, et al. RF ablation with adjuvant therapy: comparison of external beam radiation and liposomal doxorubicin on ablation efficacy in an animal tumor model. Int J Hyperthermia. 2008;24(7):560–7. doi: 10.1080/02656730802070768. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed M, Goldberg SN. Combination radiofrequency thermal ablation and adjuvant IV liposomal doxorubicin increases tissue coagulation and intratumoural drug accumulation. Int J Hyperthermia. 2004;20(7):781–802. doi: 10.1080/02656730410001711655. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed M, Moussa M, Goldberg SN. Synergy in cancer treatment between liposomal chemotherapeutics and thermal ablation. Chem Phys Lipids. 2012;165(4):424–37. doi: 10.1016/j.chemphyslip.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solazzo SA, Ahmed M, Schor-Bardach R, et al. Liposomal doxorubicin increases radiofrequency ablation-induced tumor destruction by increasing cellular oxidative and nitrative stress and accelerating apoptotic pathways. Radiology. 2010;255(1):62–74. doi: 10.1148/radiol.09091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gasselhuber A, Dreher MR, Negussie A, et al. Mathematical spatio-temporal model of drug delivery from low temperature sensitive liposomes during radiofrequency tumour ablation. Int J Hyperthermia. 2010;26(5):499–513. doi: 10.3109/02656731003623590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong G, Dewhirst MW. Hyperthermia and liposomes. Int J Hyperthermia. 1999;15(5):345–70. doi: 10.1080/026567399285558. [DOI] [PubMed] [Google Scholar]
- 42.Kong G, Anyarambhatla G, Petros WP, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res. 2000;60(24):6950–7. [PubMed] [Google Scholar]
- 43.Needham D, Anyarambhatla G, Kong G, et al. A new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60(5):1197–201. [PubMed] [Google Scholar]
- 44.Koning GA, Eggermont AM, Lindner LH, et al. Hyperthermia and thermosensitive liposomes for improved delivery of chemotherapeutic drugs to solid tumors. Pharm Res. 2010;27(8):1750–4. doi: 10.1007/s11095-010-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinstein JN, Magin RL, Yatvin MB, et al. Liposomes and local hyperthermia: selective delivery of methotrexate to heated tumors. Science. 1979;204(4389):188–91. doi: 10.1126/science.432641. [DOI] [PubMed] [Google Scholar]
- 46.Yatvin MB, Weinstein JN, Dennis WH, et al. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202(4374):1290–3. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 47.Yatvin MB, Muhlensiepen H, Porschen W, et al. Selective delivery of liposome-associated cis-dichlorodiammineplatinum(II) by heat and its influence on tumor drug uptake and growth. Cancer Res. 1981;41(5):1602–7. [PubMed] [Google Scholar]
- 48.Yatvin MB, Cree TC, Tegmo-Larsson IM, et al. Liposomes as drug carriers in cancer therapy: hyperthermia and pH sensitivity as modalities for targeting. Strahlentherapie. 1984;160(12):732–40. [PubMed] [Google Scholar]
- 49.Gaber MH, Hong K, Huang SK, et al. Thermosensitive sterically stabilized liposomes: formulation and in vitro studies on mechanism of doxorubicin release by bovine serum and human plasma. Pharm Res. 1995;12(10):1407–16. doi: 10.1023/a:1016206631006. [DOI] [PubMed] [Google Scholar]
- 50.Papahadjopoulos D, Allen TM, Gabizon A, et al. Sterically stabilized liposomes– improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci U S A. 1991;88(24):11460–4. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaber MH, Wu NZ, Hong K, et al. Thermosensitive liposomes: extravasation and release of contents in tumor microvascular networks. Int J Radiat Oncol Biol Phys. 1996;36(5):1177–87. doi: 10.1016/s0360-3016(96)00389-6. [DOI] [PubMed] [Google Scholar]
- 52.Dewhirst MW, Vujaskovic Z, Jones E, et al. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia. 2005;21(8):779–90. doi: 10.1080/02656730500271668. [DOI] [PubMed] [Google Scholar]
- 53.Lindner LH, Eichhorn ME, Eibl H, et al. Novel temperature-sensitive liposomes with prolonged circulation time. Clin Cancer Res. 2004;10(6):2168–78. doi: 10.1158/1078-0432.ccr-03-0035. [DOI] [PubMed] [Google Scholar]
- 54.Hossann M, Wiggenhorn M, Schwerdt A, et al. In vitro stability and content release properties of phosphatidylglyceroglycerol containing thermosensitive liposomes. Biochim Biophys Acta. 2007;1768(10):2491–9. doi: 10.1016/j.bbamem.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 55.Hossann M, Syunyaeva Z, Schmidt R, et al. Proteins and cholesterol lipid vesicles are mediators of drug release from thermosensitive liposomes. J Control Release. 2012;162(2):400–6. doi: 10.1016/j.jconrel.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 56.Dicheva BM, Hagen TL, Li L, et al. Cationic thermosensitive liposomes: a novel dual targeted heat-triggered drug delivery approach for endothelial and tumor cells. Nano Lett. 2012 doi: 10.1021/nl3014154. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 57.Manzoor AA, Lindner LH, Landon CD, et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72(21):5566–75. doi: 10.1158/0008-5472.CAN-12-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kono K. Thermosensitive polymer-modified liposomes. Adv Drug Deliv Rev. 2001;53(3):307–19. doi: 10.1016/s0169-409x(01)00204-6. [DOI] [PubMed] [Google Scholar]
- 59.Ruel-Gariepy E, Leclair G, Hildgen P, et al. Thermosensitive chitosan-based hydrogel containing liposomes for the delivery of hydrophilic molecules. J Control Release. 2002;82(2–3):373–83. doi: 10.1016/s0168-3659(02)00146-3. [DOI] [PubMed] [Google Scholar]
- 60.Kono K, Nakai R, Morimoto K, et al. Thermosensitive polymer-modified liposomes that release contents around physiological temperature. Biochim Biophys Acta. 1999;1416(1–2):239–50. doi: 10.1016/s0005-2736(98)00226-0. [DOI] [PubMed] [Google Scholar]
- 61.Pradhan P, Giri J, Rieken F, et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2010;142(1):108–21. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Unezaki S, Maruyama K, Takahashi N, et al. Enhanced delivery and antitumor activity of doxorubicin using long-circulating thermosensitive liposomes containing amphipathic polyethylene-glycol in combination with local hyperthermia. Pharm Res. 1994;11(8):1180–5. doi: 10.1023/a:1018949218380. [DOI] [PubMed] [Google Scholar]
- 63.Yarmolenko PS, Zhao Y, Landon C, et al. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. Int J Hyperthermia. 2010;26(5):485–98. doi: 10.3109/02656731003789284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palmer GM, Boruta RJ, Viglianti BL, et al. Non-invasive monitoring of intra-tumor drug concentration and therapeutic response using optical spectroscopy. J Control Release. 2010;142(3):457–64. doi: 10.1016/j.jconrel.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung EU, Yoon JH, Lee YJ, et al. Hypoxia and retinoic acid-inducible NDRG1 expression is responsible for doxorubicin and retinoic acid resistance in hepatocellular carcinoma cells. Cancer Lett. 2010;298(1):9–15. doi: 10.1016/j.canlet.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 66.Ponce AM, Viglianti BL, Yu D, et al. Magnetic resonance imaging of temperature-sensitive liposome release: drug dose painting and antitumor effects. J Natl Cancer Inst. 2007;99(1):53–63. doi: 10.1093/jnci/djk005. [DOI] [PubMed] [Google Scholar]
- 67.Viglianti BL, Ponce AM, Michelich CR, et al. Chemodosimetry of in vivo tumor liposomal drug concentration using MRI. Magn Reson Med. 2006;56(5):1011–8. doi: 10.1002/mrm.21032. [DOI] [PubMed] [Google Scholar]
- 68.Negussie AH, Yarmolenko PS, Partanen A, et al. Formulation and characterisation of magnetic resonance imageable thermally sensitive liposomes for use with magnetic resonance-guided high intensity focused ultrasound. Int J Hyperthermia. 2011;27(2):140–55. doi: 10.3109/02656736.2010.528140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Smet M, Heijman E, Langereis S, et al. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: an in vivo proof-of-concept study. J Control Release. 2011;150(1):102–10. doi: 10.1016/j.jconrel.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 70.Peller M, Schwerdt A, Hossann M, et al. MR characterization of mild hyperthermia-induced gadodiamide release from thermosensitive liposomes in solid tumors. Invest Radiol. 2008;43(12):877–92. doi: 10.1097/RLI.0b013e31818768cd. [DOI] [PubMed] [Google Scholar]
- 71.Ranjan A, Jacobs GC, Woods DL, et al. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit V×2 tumor model. J Control Release. 2012;158(3):487–94. doi: 10.1016/j.jconrel.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Q, Tong S, Dewhirst MW, et al. Targeting tumor microvessels using doxorubicin encapsulated in a novel thermosensitive liposome. Mol Cancer Ther. 2004;3(10):1311–7. [PubMed] [Google Scholar]
- 73.Hauck ML, LaRue SM, Petros WP, et al. Phase I trial of doxorubicin-containing low temperature sensitive liposomes in spontaneous canine tumors. Clin Cancer Res. 2006;12(13):4004–10. doi: 10.1158/1078-0432.CCR-06-0226. [DOI] [PubMed] [Google Scholar]
- 74.Poon RT, Borys N. Lyso-thermosensitive liposomal doxorubicin: an adjuvant to increase the cure rate of radiofrequency ablation in liver cancer. Future Oncol. 2011;7(8):937–45. doi: 10.2217/fon.11.73. [DOI] [PubMed] [Google Scholar]
- 75.Wood BJ, Poon RT, Locklin JK, et al. Phase I study of heat-deployed liposomal doxorubicin during radiofrequency ablation for hepatic malignancies. J Vasc Interv Radiol. 2012;23(2):248–255. e7. doi: 10.1016/j.jvir.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Celsion. Phase 3 study of ThermoDox with radiofrequency ablation (RFA) in treatment of hepatocellular carcinoma (HCC) Bethesda (MD): National Library of Medicine (US); 2011. [Accessed June 15, 2012]. Available at: ClinicalTrials.gov [Internet] [Google Scholar]
- 77.Celsion. Phase 1/2 study of ThermoDox with approved hyperthermia in treatment of breast cancer recurrence at the chest wall (DIGNITY) Bethesda (MD): National Library of Medicine (US); 2012. [Accessed June 15, 2012]. Available at: ClinicalTrials.gov [Internet] [Google Scholar]
- 78.Jones EL, Samulski TV, Dewhirst MW, et al. A pilot Phase II trial of concurrent radiotherapy, chemotherapy, and hyperthermia for locally advanced cervical carcinoma. Cancer. 2003;98(2):277–82. doi: 10.1002/cncr.11475. [DOI] [PubMed] [Google Scholar]
- 79.Rau B, Wust P, Hohenberger P, et al. Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer: a phase II clinical trial. Ann Surg. 1998;227(3):380–9. doi: 10.1097/00000658-199803000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Wit R, van der Zee J, van der Burg ME, et al. A phase I/II study of combined weekly systemic cisplatin and locoregional hyperthermia in patients with previously irradiated recurrent carcinoma of the uterine cervix. Br J Cancer. 1999;80(9):1387–91. doi: 10.1038/sj.bjc.6690533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000;355(9210):1119–25. doi: 10.1016/s0140-6736(00)02059-6. [DOI] [PubMed] [Google Scholar]
- 82.Issels RD, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11(6):561–70. doi: 10.1016/S1470-2045(10)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Overgaard J, Gonzalez Gonzalez D, Hulshof MC, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet. 1995;345(8949):540–3. doi: 10.1016/s0140-6736(95)90463-8. [DOI] [PubMed] [Google Scholar]