Abstract
In this issue of Blood, Linette et al report a cautionary tale—a lethal off-target toxicity following T-cell receptor (TCR) optimization to enhance affinity against a tumor-associated antigen, MAGE-A3.1
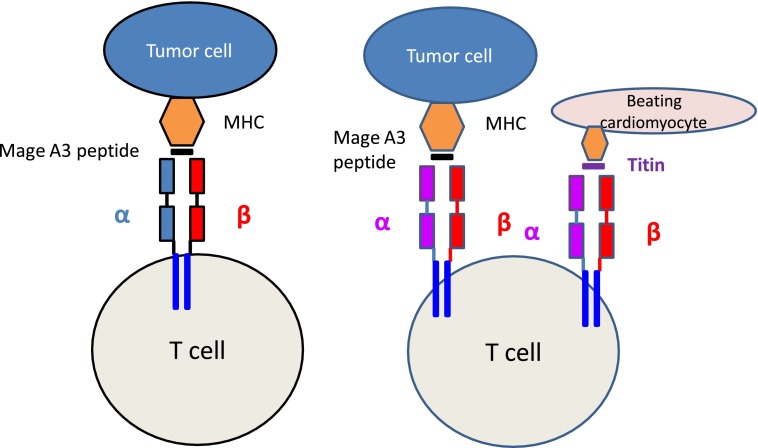
A native TCR recognizes a MAGE-A3 peptide expressed by major histocompatibility complex (MHC) molecules on tumor cells. After affinity enhancement by mutations to the α chain, the TCR now recognizes the tumor cell with enhanced affinity but also recognizes another peptide derived from titin expressed by beating cardiomyocytes that is not recognized by the native TCR.
Two patients with MAGE-A3–expressing tumors received T cells genetically modified to express an affinity-enhanced TCR against an HLA-A*01–restricted MAGE-A3 peptide. As the infused T cells expanded in vivo, both patients developed fevers and progressive cardiogenic shock, dying within a week of infusion. Their autopsies showed severe myocardial damage with T-cell infiltration.1 While no MAGE-A3 expression was detected in heart autopsy tissues, a follow-up study (BJ Cameron, A Gerry, and J Dukes, manuscript submitted 2013) showed that the T cells expressing the affinity-enhanced TCR could be activated by exposure to a beating cardiomyocyte culture generated from HLA-A*01–positive induced pluripotent stem cells (iPSCs), due to recognition of an unrelated peptide derived from titin, a protein that is a component of striated muscle.
A limitation of T-cell cancer immunotherapy is that the affinity of native TCRs for antigens expressed in tumors is generally low. To improve the activity of tumor-directed T cells, investigators have therefore isolated high-affinity TCRs specific for tumor peptides from the T cells of patients who responded to immunotherapy and have used these constructs to genetically modify T cells.2 When T cells expressing these TCRs specific for tumor antigens such as MART are adoptively transferred, they have induced dramatic clinical responses in a subset of patients, with no evidence of off-target activity.3 Several investigators have tried to further improve T-cell activity by artificially enhancing the affinity of the transferred tumor antigen–directed TCRs. This has been accomplished by engineering the TCRs by substituting codons in the α or β chain of the TCR (see figure) and selecting the receptors with the highest affinity. This approach can augment affinity by several logs.4 Unfortunately, while these maneuvers enhance tumor recognition, they may also mean that the receptors can now recognize additional and unrelated peptides expressed by normal tissues, albeit at lower affinity than the originally targeted antigen. This effect may be accentuated if there is mispairing of introduced and endogenous TCR α/β chains, since this will introduce yet more novel specificities.2
MAGE-A3, the tumor antigen targeted in the study by Linette et al, is a high-priority immunotherapy target since it is frequently expressed by many cancers but is usually absent from adult cells.5 The parental TCR from which the investigators obtained their optimized MAGE TCR was isolated from a patient with a MAGE-A3-positive melanoma; it was then affinity enhanced by introducing 4 substitutions in the α chain of the CDR2 region.1 Preclinical in vitro functional analysis of this optimized MAGE-A3 TCR did not revealed any cross reactivity. It was only when the investigators used beating cardiomyocytes derived from iPSCs to investigate the serious adverse events that HLA-A*01–restricted recognition of a titin-derived peptide was observed. Importantly, T cells expressing the wild-type MAGE-A3 TCR were not able to recognize the same cardiomyocytes, confirming that the ability to recognize titin had been conferred by the affinity enhancement steps (see figure). Therefore, this report has an important message about the potential of affinity-enhanced TCRs to produce off-target toxicities that may not be predictable by standard toxicology studies.
Of note, a National Cancer Institute group using a different optimized TCR specific for MAGE-A3 peptides presented on HLA-A*02 has also reported severe off-tumor target adverse events, but in this case, the toxicity was neurologic and was due to cross-reactivity with other members of the MAGE cancer testis family, which were unexpectedly expressed in the central nervous system.6
Although significant toxicity has been seen in these two studies using different affinity-optimized TCRs, use of the same approach but with TCRs directed to the NY ESO-1 antigen have been both safe and clinically efficacious.7 How can this potentially promising strategy be safely applied to other tumor antigens? As Linette et al concluded, improved analyses of the cross-reactivity of engineered TCRs are required, and these will likely use more sophisticated cell culture systems. In addition, prompt treatment of early symptoms may provide a window for targeting the toxic T cells before recipients become irreversibly damaged. Although one of the patients was refractory to immunosuppression with corticosteroids, earlier detection of toxicity or incorporation of a rapid suicide switch8 in the construct may allow ablation of the transferred T cells before the onset of lethal cardiac toxicity.
Footnotes
Conflict-of-interest disclosure: H.E.H.’s center has a collaborative research agreement with Celgene for developing genetically modified T-cell approaches.
REFERENCES
- 1.Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122(6):863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorritsma A, Schotte R, Coccoris M, de Witte MA, Schumacher TN. Prospects and limitations of T cell receptor gene therapy. Curr Gene Ther. 2011;11(4):276–287. doi: 10.2174/156652311796150390. [DOI] [PubMed] [Google Scholar]
- 3.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitt TM, Aggen DH, Stromnes IM, Dossett ML, Richman SA, Kranz DM, Greenberg PD. Enhanced-affinity murine TCRs for tumor/self-antigens can be safe in gene therapy despite surpassing the threshold for thymic selection [published online ahead of print May 14, 2013]. Blood. doi: 10.1182/blood-2013-01-478164. doi:10.1182/blood-2013-01-478164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan RA, Chinnasamy N, Abate-Daga D, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36(2):133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7):917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365(18):1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]