Abstract
The HIV Prevention Trials Network (HPTN) is conducting the HPTN 071(PopART) study in 21 communities in Zambia and South Africa with support from a consortium of funders. HPTN 071(PopART) is a community-randomized trial of a combination prevention strategy to reduce HIV incidence in the context of the generalized epidemic of southern Africa. The full PopART intervention strategy is anchored in home-based HIV testing and facilitated linkage of HIV-infected persons to care through community health workers, and universal antiretroviral therapy for seropositive persons regardless of CD4+ cell count or HIV viral load. In order to further reduce risk of HIV acquisition among uninfected individuals, the study aims to expand voluntary medical male circumcision, diagnosis and treatment of sexually transmitted infections, behavioral counseling, and condom distribution. The full PopART intervention strategy also incorporates promotion of other interventions designed to reduce HIV and tuberculosis transmission, including optimization of the prevention of mother to child HIV transmission and enhanced individual and public health tuberculosis services. Success for the PopART strategy depends upon the ability to increase coverage for the study interventions whose uptake is a necessary antecedent to a prevention effect. Processes will be measured to assess the degree of penetration of the interventions into the communities. A randomly sampled population cohort from each community will be used to measure the impact of the PopART strategy on HIV incidence over three years. We describe the strategy being tested and progress to date in the HPTN 071(PopART) study.
Keywords: HIV, prevention, combination prevention, cluster randomized trial, treatment for prevention, antiretroviral therapy, HIV testing, circumcision, South Africa, Zambia
Combining prevention interventions is a familiar approach for public health interventions in low and middle income countries (LMIC). Control of tuberculosis (TB), for example, is recommended through the combination of case finding, contact tracing, isoniazid preventive therapy, optimized therapy, often directly observed, and environmental risk reduction to improve fresh air exchange in airplanes, housing, prisons or health care settings.1-6 The public health challenge is how to implement what we know works to reduce TB transmission. Another example is malaria control that relies on the use of insecticide-treated bednets, environmental control of mosquito breeding sites, indoor residual spraying, seasonal malaria chemoprophylaxis, improved diagnosis and therapy (e.g., artemisinin combination therapy) in the context of expanded primary care access, community education and engagement, and use of mosquito repellents.7,8 A malaria vaccine may join this list of intervention tools within a decade.9 Similar to tuberculosis and malaria, HIV now has a sound public health evidence base from both clinical trials10 and from observational studies to suggest appropriate elements of a strong combination prevention package suitable to target the generalized epidemic of sub-Saharan Africa (Table 1).
Table 1.
Elements of combination HIV prevention that have strong evidence base for decrease risk behavior or HIV incidence from the published literature and whether they are included as a part of the HPTN 071(PopART) trial
| Prevention Element to Reduce HIV Transmission | References |
|---|---|
|
| |
| • Voluntary medical male circumcision (VMMC)* | 11-13 |
| • Treatment for prevention with integrated elements* | 14-16 |
| ○ Expanded HIV testing as an entry point for services, both therapeutic and preventive |
17-20 |
| ○ Linkage to care to ensure that all seropositive persons receive ongoing primary care |
21 |
| ○ Expanded access and earlier use of combination antiretroviral therapy (cART) to benefit the HIV-infected person and reduce his/her infectiousness to others |
22-32 |
| ○ Opt-out routine HIV testing for pregnant women and use of cART for prevention of mother to child transmission of HIV (PMTCT) |
33,34 |
| • Correct and consistent use of male condoms (some evidence, too, to support use of female condoms)* |
35-38 |
| • Behavior change focused on reducing the number of sexual partners, avoidance of concurrent sexual partners, and selection of lower risk partners, with couples counseling when possible* |
39-41 |
| • Clean needle use in the formal and informal health sectors and for persons self-medicating legal or illegal drugs |
42,43 |
| • Improving decisions as to when blood and blood products should be used, and universal screening of transfused products for HIV and other key infectious agents relevant for local conditions (e.g., hepatitis C virus [HCV] and hepatitis B virus [HBV], Human T-lymphotropic virus Type I [HTLV-1], malaria, and others) |
44 |
| • Post-exposure prophylaxis for occupational exposure (e.g., health care workers with a needle stick) or among recently infected infants |
45-48 |
All the listed elements are components of our community and clinical training efforts. The * indicate those that represent a major focus of the PopART intervention package. Other elements of the PopART package are the improved control of sexually transmitted diseases and co-infections like tuberculosis (see text).
There is mixed evidence supporting the benefits of other biomedical interventions (i.e., those not listed in Table 1). A tenofovir-containing vaginal microbicide worked to reduce short-term risk in the CAPRISA 004 trial, as did tenofovir-emtricitabine oral pre-exposure prophylaxis (PrEP) for men who have sex with men (MSM in the iPrEx trial) and discordant couples in Africa (Partners PrEP and TDF-2 trials), while other clinical trials have been disappointing.49-54 Adherence levels have not yet been high enough to take full advantage of the biological potential of the topical or oral PrEP concept. Similarly, tools like the control of sexually transmitted infections (STI)15,55-58 and diagnosis/treatment of co-infections59-66 have demonstrated inconsistent evidence for their utility in HIV control, though they are valuable contributions to the health of individuals and the well-being of the community and may be justified as components of combination prevention in certain epidemic settings. Hence both STI and TB programmatic improvements are being included in the PopART intervention, but oral/topical PrEP are not.
As evidence accumulates in the future, other prevention approaches may be considered in combination prevention. HIV vaccines are an obvious choice if products prove efficacious, safe, and are licensed and produced for use.67,68 Future trials may prove both topical and oral PrEP to be more consistently efficacious if adherence can be improved. For example two dapivirine vaginal ring microbicide efficacy trials are underway, one called The Ring Study, sponsored by the International Partnership for Microbicides69 and a sister trial sponsored by the Microbicides Trials Network, called ASPIRE (MTN-020).70,71 The dapivirine microbicide ring delivers drug with only a monthly ring change needed, to potentially mitigate the adherence barrier of event-driven or daily use of oral or topical products.72-74
Rationale for the HPTN trial
In the context of growing evidence of the efficacy of multiple modalities for HIV prevention, the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) leadership determined the need to conduct research to determine the effectiveness of a combination of prevention interventions on HIV incidence at a population level. With support from PEPFAR, the National Institute of Allergy and Infectious Disease (NIAID), the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), as well as the Bill and Melinda Gates Foundation (BMGF), the HPTN 071 (PopART) study, (Population Effects of Antiretroviral Therapy [ART] to Reduce HIV Transmission) was designed to answer this important question. The implementation of the study interventions in South Africa and Zambia is supported through PEPFAR supplements to implementing partners through the U.S. Centers for Disease Control and Prevention (CDC) and the U.S. Agency for International Development (USAID).
Covering greater numbers of persons with such interventions as testing and enhanced linkage to expanded care and VMMC would both help reduce morbidity and mortality among HIV-infected persons receiving combination antiretroviral therapy (cART) and also reduce transmission risk to others. While there are encouraging data from ecological and observational studies supporting the potential for HIV treatment to help with HIV prevention,29-31 none to date have tested the acceptability and operational challenges of delivering a combination universal test and treat and prevention intervention package in SSA.
Testing expansion as an intervention in and of itself was assessed in the NIMH Project Accept (HPTN 043) study which found that although expanded HIV testing was well accepted,20 it did not confer a significant reduction in population level HIV incidence.19 One might speculate that the lack of a substantial impact on HIV transmission from expanded testing alone was the consequence of limited post-testing behavioral change and suboptimal linkage to ART-based care for those found to be HIV-infected. In addition, the balance of benefits versus risks associated with very early and longer-term therapy (currently under trial in the START study),75 and particularly in LMIC settings, is unknown. LMIC with limited health care resources and minimal access to viral load testing might experience a high risk of the emergence of viral resistance from suboptimal adherence in asymptomatic persons, for example.76-79 At a population level, the need for controlled clinical trials in real world field settings is underscored by the challenges of behavioral disinhibition (also termed risk compensation) for persons on cART who may sometimes perceive themselves healthier and/or less infectious to others.80-85 Finally, we do not know the logistical feasibility and cost effectiveness of implementing expanded HIV detection and cART coverage within health care systems struggling to manage high overall disease burdens.86,87
HPTN 071(PopART) Study Design
Of the 21 communities participating in HPTN 071(PopART) study, 14 previously participated in the Zambia-South Africa TB and AIDS Reduction (ZAMSTAR) study, conducted by some of the investigators involved in this study.88-93 Thus, HPTN 071(PopART) study builds on strong relationships established between the investigators and the communities including the presence of active community advisory groups. Continuous consultative feedback from both communities and from government health officials has been essential in forged the details of the trial. The Ministries of Health of South Africa and Zambia and the relevant state, provincial and district health authorities have been fully engaged in ethical vetting, implementation, and planning for dissemination of study results.
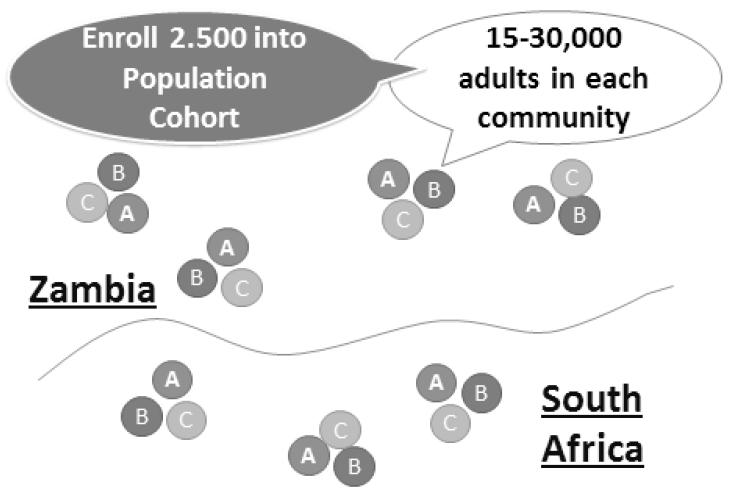
The 21 communities of HPTN 071(PopART) include nine in the Western Cape Province of South Africa and 12 communities in Zambia, and arranged in seven matched triplets, with four triplets in Zambia and three in South Africa. Within each country, communities were matched based on the best available estimates of HIV prevalence and on geographical location and implementing partner for HIV services, with the aim of minimizing the between-community variance in baseline HIV incidence within matched triplets. Restricted randomization was used to ensure overall balance in cluster size, ART uptake and mean HIV prevalence across the study arms.94 In a public randomization ceremony in February 2013, one community from each triplet was randomly assigned to each of the three study arms (Figure 1):
- Arm A: to receive the full PopART combination prevention program consisting of:
-
➢Offering voluntary HIV counseling and testing annually to every household (i.e., home-based testing18 and couples counseling) with expanded HIV testing in health facilities
-
➢Linking those with HIV infection to care at the local health facility
-
➢Offering immediate cART to all HIV-infected persons regardless of CD4+ cell count or viral load
-
➢Initiating cART for those HIV-infected persons already in care
-
➢Promoting voluntary medical male circumcision (VMMC) for men who test HIV seronegative
-
➢Promoting prevention of mother-to-child HIV transmission (PMTCT) services to HIV-infected pregnant women
-
➢Improving the diagnosis and treatment of STI
-
➢Providing risk reduction education and condoms in the community and in the health facilities
-
➢
Arm B: to receive all of the HIV prevention strategies in the PopART combination prevention program, except that cART will not be universal, but will be offered to those who are eligible according to prevailing national guidelines, typically at a threshold of ≤350 CD4+ cells/μL.95
Arm C: to receive the current standard of care. However, special attention will be paid to ensure that there are no drug and laboratory reagent shortages or stock-outs in any of the 21 communities, i.e., in all three study arms.
Figure 1.
HPTN 071/PopART study schema for the 21 community, 3-arm community-randomized clinical trial in Zambia and South Africa.
The full population in all 21 communities is estimated to be about 1.2 million persons. To measure the impact of the strategy, a Population Cohort will be selected from the general population consisting of a random sample of 2,500 adults (one per household) aged 18-44 years from each community. Thus, the Population Cohort will have 52,500 persons recruited from the 21 communities (all three study arms). A baseline survey of the cohort will be carried out at the time the intervention is initiated to assess the comparability of the three study arms. Follow-up surveys of the cohort will be carried out at 12, 24, and 36 months to measure HIV incidence, success in coverage of the interventions in the communities, and other outcomes.
The primary study outcome will be HIV incidence over three years in members of the population cohort who are HIV-negative at baseline, and will be compared in the intervention and control clusters to measure the population-level effectiveness of the PopART intervention. HPTN 071 (PopART) is very well powered to detect an effect of 35% or larger in Arm A or Arm B compared with Arm C, and is moderately well powered to detect an effect of 30%. To compare Arms A and B, the study is well powered to detect a difference between effects of 60% and 30%, 55% and 25%, and 50% and 20%. Assumptions are that there is a baseline HIV prevalence of 15% and that there will be losses to follow-up of 25% over three years in the population cohort.
The secondary outcomes will be measured in the population cohort to assess the effect of the intervention on a number of additional factors, including: HIV incidence during each year of follow up; reported sexual risk behavior; ART adherence and toxicity; HIV-related stigma; HIV disease progression; community viral load; ART drug resistance; HSV-2 incidence; and TB case notification rates.
Process variables to be measured in the intervention clusters will include the following: Acceptance of HIV testing and re-testing; uptake of male circumcision among men testing HIV-negative; proportion started on cART within 3 months of HIV diagnosis; and uptake of PMTCT services. In addition, case control studies will be conducted to examine factors related to the following: Uptake of HIV testing during the first round of home-based testing in Arms A and B; uptake of immediate treatment in Arm A; and uptake of HIV testing in the second round of home-based testing in Arms A and B.
Formative research
In order to inform the intervention before it is deployed in the communities, social science research has been undertaken to better understand the communities, their prior and current HIV landscape, as well as attitudes toward different prevention approaches. In addition, further social science research will be carried out throughout the study period to examine the acceptability of the PopART intervention and to document the effects of the interventions on a number of factors, including risk behaviors, social networks, HIV identity and community-level HIV associated stigma. At the end of the testing campaign in each community, random samples of individuals who accept or decline testing will be interviewed to explore the reason for their decision. In addition, interviews will be carried out with randomly selected patients with good or poor adherence to ART.
Economic evaluations and modeling
Economic studies are planned to measure the incremental cost of the intervention packages, to estimate their cost-effectiveness and to measure the burden on local health facilities of implementing the intervention. Hence, we are recording costs of all implementation efforts for such activities as testing, linkage, care, VMMC, expanded laboratory and ART costs, and community-level educational efforts. Mathematical modeling will use these data to assess the magnitude of the expected impact, given the process inputs, as the trial progresses.
Other population-level combination prevention studies
A large population-based combination prevention study is also planned in Botswana with funding from PEPFAR and sponsorship of the CDC.96-99 The study builds on work from an ongoing study of the Botswana-Harvard AIDS Institute Partnership in Mochudi, a community of 40,000 persons in Botswana.100-105 PEPFAR and the BMGF have subsequently sponsored a harmonization effort between the HPTN 071(PopART) study and the Botswana Combination Prevention Project that shares similar goals as those of HPTN 071(PopART), but has a different study design. Laboratory, questionnaire, cost/economic assessments, and design/analytic issues have all been addressed to facilitate future meta-analysis opportunities. Another large combination prevention study is planned by the Agence Nationale de Recherche sur le Sida et les Hépatites Virales (ANRS in France) with the Africa Centre for Health and Population Studies in KwaZulu Natal Province, South Africa.30,106-108 Initial work of the Africa Centre is promising in suggesting the potential impact of increases in cART coverage in patients with advanced HIV disease on HIV incidence.30,109 The findings from the latter study in rural South Africa is encouraging as it provides more rigorous ecological data than hitherto available.31,32,110,111
Conclusion
The opportunity to combine known efficacious interventions for HIV prevention into combination packages allows the examination of potential synergies that may be achieved in control of HIV transmission.10,15,16,112-115 Challenges are daunting given the need to have a high degree of coverage and efficiency in testing coverage, linkage to care, and high adherence in the context of expanded cART coverage.86,87,116,117 The extent to which efforts are successful in deploying needed interventions to the field at the levels needed to interrupt transmission cycles is the critical unknown at present. The engagement of national health authorities and local communities is essential for conduct of the study, dissemination of results, and future scale-up of successful approaches that are discovered. Combining known efficacious prevention approaches is complex to design and test, but their use in a synergistic strategy may open the door to substantial reductions in HIV incidence in some of the world’s most afflicted nations.
Acknowledgments
HPTN 071 (PopART) is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) under Cooperative Agreements UM1-AI068619, UM1-AI068617, and UM1-AI068613, with funding from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). Additional funding is provided through NIAID, the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), and the International Initiative for Impact Evaluation (3ie, Inc.) with support from the Bill & Melinda Gates Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID, NIMH, NIDA, PEPFAR, 3ie, Inc., or the Bill & Melinda Gates Foundation. The authors thank Dr. Wafaa El-Sadr, Ms. Megan Valentine, and Ms. Megan Pask for their help with the manuscript.
Sources of Funding: HPTN 071 (PopART) is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) under Cooperative Agreements UM1-AI068619, UM1-AI068617, and UM1-AI068613, with funding from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). Additional funding is provided through NIAID, the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), and the International Initiative for Impact Evaluation (3ie, Inc.) with support from the Bill & Melinda Gates Foundation.
Footnotes
Conflicts: Vermund: the NIH supports the institution with grants and money for travel.
Beyers: the NIH (through LSHTM)supports the institution with grants and money for travel.
Fidler: money is paid to the institution through grants and in support of travel
Ayles: the NIH supports the institution with grants and money for travel.
Hayes: the NIH supports the institution with grants and money for travel. Insitution also receives money from UK MRC for travel and the author receives payement as member of board of UK MRC. Receives textbook royalties from Chapman and Hall.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Reid SE, Reid CA, Vermund SH. Antiretroviral therapy in sub-Saharan Africa: adherence lessons from tuberculosis and leprosy. International journal of STD & AIDS. 2004 Nov;15(11):713–716. doi: 10.1258/0956462042395195. [DOI] [PubMed] [Google Scholar]
- 2.Raviglione M, Marais B, Floyd K, et al. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet. 2012 May 19;379(9829):1902–1913. doi: 10.1016/S0140-6736(12)60727-2. [DOI] [PubMed] [Google Scholar]
- 3.Lienhardt C, Glaziou P, Uplekar M, Lonnroth K, Getahun H, Raviglione M. Global tuberculosis control: lessons learnt and future prospects. Nature reviews. Microbiology. 2012 Jun;10(6):407–416. doi: 10.1038/nrmicro2797. [DOI] [PubMed] [Google Scholar]
- 4.Pai NP, Pai M. Point-of-care diagnostics for HIV and tuberculosis: landscape, pipeline, and unmet needs. Discovery medicine. 2012 Jan;13(68):35–45. [PubMed] [Google Scholar]
- 5.Uyei J, Coetzee D, Macinko J, Guttmacher S. Integrated delivery of HIV and tuberculosis services in sub-Saharan Africa: a systematic review. The Lancet infectious diseases. 2011 Nov;11(11):855–867. doi: 10.1016/S1473-3099(11)70145-1. [DOI] [PubMed] [Google Scholar]
- 6.Legido-Quigley H, Montgomery CM, Khan P, et al. Integrating tuberculosis and HIV services in low- and middle-income countries: a systematic review. Tropical medicine & international health: TM & IH. 2013 Feb;18(2):199–211. doi: 10.1111/tmi.12029. [DOI] [PubMed] [Google Scholar]
- 7.Breman JG, Brandling-Bennett AD. The challenge of malaria eradication in the twenty-first century: research linked to operations is the key. Vaccine. 2011 Dec 30;29(Suppl 4):D97–103. doi: 10.1016/j.vaccine.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 8.mal ERACGoHS. Operational R. A research agenda for malaria eradication: health systems and operational research. PLoS medicine. 2011;8(1):e1000397. doi: 10.1371/journal.pmed.1000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moorthy V, Newman R, Duclos P, Okwo-Bele J, Smith P. Assessment of the RTS,S/AS01 malaria vaccine. The Lancet infectious diseases. 2013 Feb 28; doi: 10.1016/S1473-3099(13)70047-1. [DOI] [PubMed] [Google Scholar]
- 10.Padian NS, McCoy SI, Karim SS, et al. HIV prevention transformed: the new prevention research agenda. Lancet. 2011 Jul 16;378(9787):269–278. doi: 10.1016/S0140-6736(11)60877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS medicine. 2005 Nov;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007 Feb 24;369(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 13.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007 Feb 24;369(9562):657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 14.Smith MK, Powers KA, Muessig KE, Miller WC, Cohen MS. HIV treatment as prevention: the utility and limitations of ecological observation. PLoS medicine. 2012;9(7):e1001260. doi: 10.1371/journal.pmed.1001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vermund SH, Hayes RJ. Combination Prevention: New Hope for Stopping the Epidemic. Current HIV/AIDS reports. 2013 Mar 1; doi: 10.1007/s11904-013-0155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurth AE, Celum C, Baeten JM, Vermund SH, Wasserheit JN. Combination HIV prevention: significance, challenges, and opportunities. Current HIV/AIDS reports. 2011 Mar;8(1):62–72. doi: 10.1007/s11904-010-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jurgensen M, Sandoy IF, Michelo C, Fylkesnes K, Group ZS. Effects of home-based Voluntary Counselling and Testing on HIV-related stigma: Findings from a cluster-randomized trial in Zambia. Social science & medicine. 2013 Mar;81:18–25. doi: 10.1016/j.socscimed.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Sabapathy K, Van den Bergh R, Fidler S, Hayes R, Ford N. Uptake of home-based voluntary HIV testing in sub-Saharan Africa: a systematic review and meta-analysis. PLoS medicine. 2012;9(12):e1001351. doi: 10.1371/journal.pmed.1001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coates T, Eshleman S, Chariyalertsak S, et al. Community-level Reductions in Estimated HIV Incidence: HIV Prevention Trials Network 043, Project Accept; Paper presented at: 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]
- 20.Sweat M, Morin S, Celentano D, et al. Community-based intervention to increase HIV testing and case detection in people aged 16-32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. The Lancet infectious diseases. 2011 Jul;11(7):525–532. doi: 10.1016/S1473-3099(11)70060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safren SA, O’Cleirigh C, Skeer MR, et al. Demonstration and evaluation of a peer-delivered, individually-tailored, HIV prevention intervention for HIV-infected MSM in their primary care setting. AIDS and behavior. 2011 Jul;15(5):949–958. doi: 10.1007/s10461-010-9807-8. [DOI] [PubMed] [Google Scholar]
- 22.Cohen MS, McCauley M, Gamble TR. HIV treatment as prevention and HPTN 052 Current opinion in HIV and AIDS. 2012 Mar;7(2):99–105. doi: 10.1097/COH.0b013e32834f5cf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNairy ML, Cohen M, El-Sadr WM. Antiretroviral Therapy for Prevention Is a Combination Strategy. Current HIV/AIDS reports. 2013 Jan 31; doi: 10.1007/s11904-013-0152-1. [DOI] [PubMed] [Google Scholar]
- 24.Chen YQ, Masse B, Wang L, et al. Statistical considerations for the HPTN 052 Study to evaluate the effectiveness of early versus delayed antiretroviral strategies to prevent the sexual transmission of HIV-1 in serodiscordant couples. Contemporary clinical trials. 2012 Nov;33(6):1280–1286. doi: 10.1016/j.cct.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen MS, McCauley M, Sugarman J. Establishing HIV treatment as prevention in the HIV Prevention Trials Network 052 randomized trial: an ethical odyssey. Clinical trials. 2012 Jun;9(3):340–347. doi: 10.1177/1740774512443594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eshleman SH, Hudelson SE, Redd AD, et al. Analysis of genetic linkage of HIV from couples enrolled in the HIV Prevention Trials Network 052 trial. The Journal of infectious diseases. 2011 Dec 15;204(12):1918–1926. doi: 10.1093/infdis/jir651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010 Jun 12;375(9731):2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia Z, Ruan Y, Li Q, et al. Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003-11): a national observational cohort study. Lancet. 2012 Nov 30; doi: 10.1016/S0140-6736(12)61898-4. [DOI] [PubMed] [Google Scholar]
- 30.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013 Feb 22;339(6122):966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PloS one. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montaner JS. Treatment as prevention--a double hat-trick. Lancet. 2011 Jul 16;378(9787):208–209. doi: 10.1016/S0140-6736(11)60821-0. [DOI] [PubMed] [Google Scholar]
- 33.Chi BH, Adler MR, Bolu O, et al. Progress, challenges, and new opportunities for the prevention of mother-to-child transmission of HIV under the US President’s Emergency Plan for AIDS Relief. Journal of acquired immune deficiency syndromes. 2012 Aug 15;60(Suppl 3):S78–87. doi: 10.1097/QAI.0b013e31825f3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertagnolio S, Penazzato M, Jordan MR, et al. World Health Organization generic protocol to assess drug-resistant HIV among children <18 months of age and newly diagnosed with HIV in resource-limited countries. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012 May;54(Suppl 4):S254–260. doi: 10.1093/cid/cis003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmes KK, Levine R, Weaver M. Effectiveness of condoms in preventing sexually transmitted infections. Bulletin of the World Health Organization. 2004 Jun;82(6):454–461. [PMC free article] [PubMed] [Google Scholar]
- 36.Sweat MD, Denison J, Kennedy C, Tedrow V, O’Reilly K. Effects of condom social marketing on condom use in developing countries: a systematic review and meta-analysis, 1990-2010. Bulletin of the World Health Organization. 2012 Aug 1;90(8):613–622A. doi: 10.2471/BLT.11.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beksinska ME, Smit JA, Mantell JE. Progress and challenges to male and female condom use in South Africa. Sexual health. 2012 Mar;9(1):51–58. doi: 10.1071/SH11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallo MF, Kilbourne-Brook M, Coffey PS. A review of the effectiveness and acceptability of the female condom for dual protection. Sexual health. 2012 Mar;9(1):18–26. doi: 10.1071/SH11037. [DOI] [PubMed] [Google Scholar]
- 39.Stoneburner RL, Low-Beer D. Population-level HIV declines and behavioral risk avoidance in Uganda. Science. 2004 Apr 30;304(5671):714–718. doi: 10.1126/science.1093166. [DOI] [PubMed] [Google Scholar]
- 40.Marum E, Taegtmeyer M, Parekh B, et al. “What took you so long?” The impact of PEPFAR on the expansion of HIV testing and counseling services in Africa. Journal of acquired immune deficiency syndromes. 2012 Aug 15;60(Suppl 3):S63–69. doi: 10.1097/QAI.0b013e31825f313b. [DOI] [PubMed] [Google Scholar]
- 41.Allen S, Serufilira A, Bogaerts J, et al. Confidential HIV testing and condom promotion in Africa. Impact on HIV and gonorrhea rates. JAMA: the journal of the American Medical Association. 1992 Dec 16;268(23):3338–3343. [PubMed] [Google Scholar]
- 42.Needle R, Fu J, Beyrer C, et al. PEPFAR’s evolving HIV prevention approaches for key populations--people who inject drugs, men who have sex with men, and sex workers: progress, challenges, and opportunities. Journal of acquired immune deficiency syndromes. 2012 Aug 15;60(Suppl 3):S145–151. doi: 10.1097/QAI.0b013e31825f315e. [DOI] [PubMed] [Google Scholar]
- 43.Dutta A, Wirtz AL, Baral S, Beyrer C, Cleghorn FR. Key harm reduction interventions and their impact on the reduction of risky behavior and HIV incidence among people who inject drugs in low-income and middle-income countries. Current opinion in HIV and AIDS. 2012 Jul;7(4):362–368. doi: 10.1097/COH.0b013e328354a0b5. [DOI] [PubMed] [Google Scholar]
- 44.van Hulst M, de Wolf JT, Staginnus U, Ruitenberg EJ, Postma MJ. Pharmaco-economics of blood transfusion safety: review of the available evidence. Vox sanguinis. 2002 Aug;83(2):146–155. doi: 10.1046/j.1423-0410.2002.00198.x. [DOI] [PubMed] [Google Scholar]
- 45.Persaud D, Gay H, Ziemniak C, et al. Functional HIV Cure after Very Early ART of an Infected Infant. 20th Conference on Retroviruses and Opportunistic Infections (CROI); Atlanta, GA. 2013. [Google Scholar]
- 46.Cohen MS, Muessig KE, Smith MK, Powers KA, Kashuba AD. Antiviral agents and HIV prevention: controversies, conflicts, and consensus. Aids. 2012 Aug 24;26(13):1585–1598. doi: 10.1097/QAD.0b013e3283543e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rey D. Post-exposure prophylaxis for HIV infection. Expert review of anti-infective therapy. 2011 Apr;9(4):431–442. doi: 10.1586/eri.11.20. [DOI] [PubMed] [Google Scholar]
- 48.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-Treatment HIV-1 Controllers with a Long-Term Virological Remission after the Interruption of Early Initiated Antiretroviral Therapy ANRS VISCONTI Study. PLoS pathogens. 2013 Mar;9(3):e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. The New England journal of medicine. 2012 Aug 2;367(5):411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. The New England journal of medicine. 2012 Aug 2;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010 Dec 30;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 Sep 3;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marrazzo J, Ramjee G, Palanee T, et al. Pre-exposure Prophylaxis for HIV in Women: Daliy Oral Tenofovir/Emtricitabine, or Vaginal Tenofovir Gel in teh VOICE Study (MTN 003); 20th Conference on Retroviruses and Opportunistic Infections (CROI); Atlanta, GA. 2013.Mar 3-6, 2013. [Google Scholar]
- 54.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine. 2012 Aug 2;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 55.Grosskurth H, Gray R, Hayes R, Mabey D, Wawer M. Control of sexually transmitted diseases for HIV-1 prevention: understanding the implications of the Mwanza and Rakai trials. Lancet. 2000 Jun 3;355(9219):1981–1987. doi: 10.1016/S0140-6736(00)02336-9. [DOI] [PubMed] [Google Scholar]
- 56.White RG, Orroth KK, Korenromp EL, et al. Can population differences explain the contrasting results of the Mwanza, Rakai, and Masaka HIV/sexually transmitted disease intervention trials?: A modeling study. Journal of acquired immune deficiency syndromes. 2004 Dec 1;37(4):1500–1513. doi: 10.1097/01.qai.0000127062.94627.31. [DOI] [PubMed] [Google Scholar]
- 57.Korenromp EL, White RG, Orroth KK, et al. Determinants of the impact of sexually transmitted infection treatment on prevention of HIV infection: a synthesis of evidence from the Mwanza, Rakai, and Masaka intervention trials. The Journal of infectious diseases. 2005 Feb 1;191(Suppl 1):S168–178. doi: 10.1086/425274. [DOI] [PubMed] [Google Scholar]
- 58.Hayes R, Watson-Jones D, Celum C, van de Wijgert J, Wasserheit J. Treatment of sexually transmitted infections for HIV prevention: end of the road or new beginning? Aids. 2010 Oct;24(Supply 4):S15–26. doi: 10.1097/01.aids.0000390704.35642.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walson J, Singa B, Sangare L, et al. Empiric deworming to delay HIV disease progression in adults with HIV who are ineligible for initiation of antiretroviral treatment (the HEAT study): a multi-site, randomised trial. The Lancet infectious diseases. 2012 Dec;12(12):925–932. doi: 10.1016/S1473-3099(12)70207-4. [DOI] [PubMed] [Google Scholar]
- 60.Walson JL, Sangare LR, Singa BO, et al. Evaluation of impact of long-lasting insecticide-treated bed nets and point-of-use water filters on HIV-1 disease progression in Kenya. Aids. 2013 Jan 16; doi: 10.1097/QAD.0b013e32835ecba9. [DOI] [PubMed] [Google Scholar]
- 61.Modjarrad K, Vermund SH. Effect of treating co-infections on HIV-1 viral load: a systematic review. The Lancet infectious diseases. 2010 Jul;10(7):455–463. doi: 10.1016/S1473-3099(10)70093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. Aids. 2008 Oct 18;22(16):2179–2185. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Webb EL, Kyosiimire-Lugemwa J, Kizito D, et al. The effect of anthelmintic treatment during pregnancy on HIV plasma viral load: results from a randomized, double-blind, placebo-controlled trial in Uganda. Journal of acquired immune deficiency syndromes. 2012 Jul 1;60(3):307–313. doi: 10.1097/QAI.0b013e3182511e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webb EL, Mawa PA, Ndibazza J, et al. Effect of single-dose anthelmintic treatment during pregnancy on an infant’s response to immunisation and on susceptibility to infectious diseases in infancy: a randomised, double-blind, placebo-controlled trial. Lancet. 2011 Jan 1;377(9759):52–62. doi: 10.1016/S0140-6736(10)61457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ndibazza J, Muhangi L, Akishule D, et al. Effects of deworming during pregnancy on maternal and perinatal outcomes in Entebbe, Uganda: a randomized controlled trial. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010 Feb 15;50(4):531–540. doi: 10.1086/649924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walson JL, Otieno PA, Mbuchi M, et al. Albendazole treatment of HIV-1 and helminth co-infection: a randomized, double-blind, placebo-controlled trial. Aids. 2008 Aug 20;22(13):1601–1609. doi: 10.1097/QAD.0b013e32830a502e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McMichael AJ, Haynes BF. Lessons learned from HIV-1 vaccine trials: new priorities and directions. Nature immunology. 2013 Apr;14(4):413. doi: 10.1038/ni.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liao HX, Lynch R, Zhou T, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013 Apr 3; doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Microbicides IPf. The Ring Study 2011. [Accessed April 28, 2013]. 2013. The Ring Study. http://www.ipmglobal.org/the-ring-study. [Google Scholar]
- 70.(NIAID) NIoAaID . NIH to test dapivirine vaginal ring for HIV prevention in women. National Institutes of Health; 2012. http://www.nih.gov/news/health/jul2012/niaid-24.htm: [Google Scholar]
- 71.(MTN) MTN . ASPIRE – A Study to Prevent Infection with a Ring for Extended Use. Microbicide Trials Network (MTN); 2012. http://www.mtnstopshiv.org/news/studies/mtn020/qa: [Google Scholar]
- 72.Fetherston SM, Malcolm RK, Woolfson AD. Controlled-release vaginal ring drug-delivery systems: a key strategy for the development of effective HIV microbicides. Therapeutic delivery. 2010 Dec;1(6):785–802. doi: 10.4155/tde.10.74. [DOI] [PubMed] [Google Scholar]
- 73.Malcolm RK, Fetherston SM, McCoy CF, Boyd P, Major I. Vaginal rings for delivery of HIV microbicides. International journal of women’s health. 2012;4:595–605. doi: 10.2147/IJWH.S36282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geonnotti AR, Katz DF. Compartmental transport model of microbicide delivery by an intravaginal ring. Journal of pharmaceutical sciences. 2010 Aug;99(8):3514–3521. doi: 10.1002/jps.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lifson AR, Group IERCW. Belloso WH, et al. Development of diagnostic criteria for serious non-AIDS events in HIV clinical trials. HIV clinical trials. 2010 Jul-Aug;11(4):205–219. doi: 10.1310/hct1104-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manasa J, Katzenstein D, Cassol S, et al. Primary drug resistance in South Africa: data from 10 years of surveys. AIDS research and human retroviruses. 2012 Jun;28(6):558–565. doi: 10.1089/aid.2011.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nichols BE, Boucher CA, van de Vijver DA. HIV testing and antiretroviral treatment strategies for prevention of HIV infection: impact on antiretroviral drug resistance. Journal of internal medicine. 2011 Dec;270(6):532–549. doi: 10.1111/j.1365-2796.2011.02456.x. [DOI] [PubMed] [Google Scholar]
- 78.Sigaloff KC, Calis JC, Geelen SP, van Vugt M, de Wit TF. HIV-1-resistance-associated mutations after failure of first-line antiretroviral treatment among children in resource-poor regions: a systematic review. The Lancet infectious diseases. 2011 Oct;11(10):769–779. doi: 10.1016/S1473-3099(11)70141-4. [DOI] [PubMed] [Google Scholar]
- 79.Obiako OR, Murktar HM, Ogoina D. Antiretroviral drug resistance--implications for HIV/AIDS reduction in sub-Saharan Africa and other developing countries. Nigerian journal of medicine: journal of the National Association of Resident Doctors of Nigeria. 2010 Oct-Dec;19(4):352–360. [PubMed] [Google Scholar]
- 80.DiClemente RJ, Funkhouser E, Wingood G, Fawal H, Holmberg SD, Vermund SH. Protease inhibitor combination therapy and decreased condom use among gay men. Southern medical journal. 2002 Apr;95(4):421–425. [PubMed] [Google Scholar]
- 81.Ostrow DE, Fox KJ, Chmiel JS, et al. Attitudes towards highly active antiretroviral therapy are associated with sexual risk taking among HIV-infected and uninfected homosexual men. Aids. 2002 Mar 29;16(5):775–780. doi: 10.1097/00002030-200203290-00013. [DOI] [PubMed] [Google Scholar]
- 82.Stolte IG, Dukers NH, Geskus RB, Coutinho RA, de Wit JB. Homosexual men change to risky sex when perceiving less threat of HIV/AIDS since availability of highly active antiretroviral therapy: a longitudinal study. Aids. 2004 Jan 23;18(2):303–309. doi: 10.1097/00002030-200401230-00021. [DOI] [PubMed] [Google Scholar]
- 83.Dukers NH, Goudsmit J, de Wit JB, Prins M, Weverling GJ, Coutinho RA. Sexual risk behaviour relates to the virological and immunological improvements during highly active antiretroviral therapy in HIV-1 infection. Aids. 2001 Feb 16;15(3):369–378. doi: 10.1097/00002030-200102160-00010. [DOI] [PubMed] [Google Scholar]
- 84.Tun W, Celentano DD, Vlahov D, Strathdee SA. Attitudes toward HIV treatments influence unsafe sexual and injection practices among injecting drug users. Aids. 2003 Sep 5;17(13):1953–1962. doi: 10.1097/00002030-200309050-00014. [DOI] [PubMed] [Google Scholar]
- 85.MacKellar DA, Hou SI, Whalen CC, et al. HIV/AIDS complacency and HIV infection among young men who have sex with men, and the race-specific influence of underlying HAART beliefs. Sexually transmitted diseases. 2011 Aug;38(8):755–763. doi: 10.1097/OLQ.0b013e31820d5a77. [DOI] [PubMed] [Google Scholar]
- 86.Vermund SH, Sidat M, Weil LF, Tique JA, Moon TD, Ciampa PJ. Transitioning HIV care and treatment programs in southern Africa to full local management. Aids. 2012 Jun 19;26(10):1303–1310. doi: 10.1097/QAD.0b013e3283552185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shelton JD. HIV/AIDS. ARVs as HIV prevention: a tough road to wide impact. Science. 2011 Dec 23;334(6063):1645–1646. doi: 10.1126/science.1212353. [DOI] [PubMed] [Google Scholar]
- 88.Ayles H, Schaap A, Nota A, et al. Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PloS one. 2009;4(5):e5602. doi: 10.1371/journal.pone.0005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ayles HM, Sismanidis C, Beyers N, Hayes RJ, Godfrey-Faussett P. ZAMSTAR, The Zambia South Africa TB and HIV Reduction Study: design of a 2 × 2 factorial community randomized trial. Trials. 2008;9:63. doi: 10.1186/1745-6215-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shanaube K, Sismanidis C, Ayles H, et al. Annual risk of tuberculous infection using different methods in communities with a high prevalence of TB and HIV in Zambia and South Africa. PloS one. 2009;4(11):e7749. doi: 10.1371/journal.pone.0007749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sismanidis C, Moulton LH, Ayles H, et al. Restricted randomization of ZAMSTAR: a 2 × 2 factorial cluster randomized trial. Clinical trials. 2008;5(4):316–327. doi: 10.1177/1740774508094747. [DOI] [PubMed] [Google Scholar]
- 92.Zachary D, Mwenge L, Muyoyeta M, et al. Field comparison of OraQuick ADVANCE Rapid HIV-1/2 antibody test and two blood-based rapid HIV antibody tests in Zambia. BMC infectious diseases. 2012;12:183. doi: 10.1186/1471-2334-12-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Brien J. Using radio to create awareness and educate the community about tuberculosis and HIV in Zambia. Int J Tuberc Lung Dis. 2011;15(11 Suppl 3):S37. [Google Scholar]
- 94.Hayes R, Sabapathy K, Fidler S. Universal testing and treatment as an HIV prevention strategy: research questions and methods. Current HIV research. 2011 Sep;9(6):429–445. doi: 10.2174/157016211798038515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.WHO . In: Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach 2010 revision. HIV/AIDS Programme: Strengthening health services to fight HIV/AIDS. WHO, editor. World Health Organization; Austria: 2010. pp. 1–156. 2010 revision. [PubMed] [Google Scholar]
- 96.Jayeoba O, Dryden-Peterson S, Okui L, et al. Acceptability of male circumcision among adolescent boys and their parents, Botswana. AIDS and behavior. 2012 Feb;16(2):340–349. doi: 10.1007/s10461-011-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andersson N, Cockcroft A. Male circumcision, attitudes to HIV prevention and HIV status: a cross-sectional study in Botswana, Namibia and Swaziland. AIDS care. 2012;24(3):301–309. doi: 10.1080/09540121.2011.608793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Njeuhmeli E, Forsythe S, Reed J, et al. Voluntary medical male circumcision: modeling the impact and cost of expanding male circumcision for HIV prevention in eastern and southern Africa. PLoS medicine. 2011 Nov;8(11):e1001132. doi: 10.1371/journal.pmed.1001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Plank RM, Ndubuka NO, Wirth KE, et al. A Randomized Trial of Mogen Clamp versus Plastibell for Neonatal Male Circumcision in Botswana. Journal of acquired immune deficiency syndromes. 2013 Jan 10; doi: 10.1097/QAI.0b013e318285d449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McDonald B, Moyo S, Gabaitiri L, et al. Significant Elevations in Interleukin-6 Levels as a Predictor of All-cause Mortality among Adults Receiving cART in Botswana: Results from a Clinical Trial (Paper #780); 20th Conference on Retroviruses and Opportunistic Infections (CROI); Atlanta, GA. 2013. [Google Scholar]
- 101.Plank R, Wirth K, Ndubuka NK, P., et al. Uptake of neonatal male circumcision as part of HIV prevention efforts in Botswana: maternal motivators and barriers (Paper #1011); 20th Conference on Retroviruses and Opportunistic Infections (CROI); Atlanta, GA. 2013. [Google Scholar]
- 102.Davis R, Dzoro S, Moyo S, et al. Optimizing a dried blood spot-based pooled RT-PCR technique for identification of acute HIV infections in Mochudi, Botswana (TUPDBO203); XIX International AIDS Conference; Washington, DC, USA. 2012. [Google Scholar]
- 103.Rossenkhan R, Novitsky V, Sebunya TK, Musonda R, Gashe BA, Essex M. Viral diversity and diversification of major non-structural genes vif, vpr, vpu, tat exon 1 and rev exon 1 during primary HIV-1 subtype C infection. PloS one. 2012;7(5):e35491. doi: 10.1371/journal.pone.0035491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Novitsky V, Smith UR, Gilbert P, et al. Human immunodeficiency virus type 1 subtype C molecular phylogeny: consensus sequence for an AIDS vaccine design? Journal of virology. 2002 Jun;76(11):5435–5451. doi: 10.1128/JVI.76.11.5435-5451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Novitsky VA, Montano MA, McLane MF, et al. Molecular cloning and phylogenetic analysis of human immunodeficiency virus type 1 subtype C: a set of 23 full-length clones from Botswana. Journal of virology. 1999 May;73(5):4427–4432. doi: 10.1128/jvi.73.5.4427-4432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Granich R, Gupta S, Suthar AB, et al. Antiretroviral therapy in prevention of HIV and TB: update on current research efforts. Current HIV research. 2011 Sep;9(6):446–469. doi: 10.2174/157016211798038597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barnighausen T, Tanser F, Dabis F, Newell ML. Interventions to improve the performance of HIV health systems for treatment-as-prevention in sub-Saharan Africa: the experimental evidence. Current opinion in HIV and AIDS. 2012 Mar;7(2):140–150. doi: 10.1097/COH.0b013e32834fc1df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dabis F, Newell ML, Hirschel B. HIV drugs for treatment, and for prevention. Lancet. 2010 Jun 12;375(9731):2056–2057. doi: 10.1016/S0140-6736(10)60838-0. [DOI] [PubMed] [Google Scholar]
- 109.Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013 Feb 22;339(6122):961–965. doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010 Aug 14;376(9740):532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Granich R, Williams B, Montaner J. Fifteen million people on antiretroviral treatment by 2015: treatment as prevention. Current opinion in HIV and AIDS. 2013 Jan;8(1):41–49. doi: 10.1097/COH.0b013e32835b80dd. [DOI] [PubMed] [Google Scholar]
- 112.Chang LW, Serwadda D, Quinn TC, Wawer MJ, Gray RH, Reynolds SJ. Combination implementation for HIV prevention: moving from clinical trial evidence to population-level effects. The Lancet infectious diseases. 2013 Jan;13(1):65–76. doi: 10.1016/S1473-3099(12)70273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Estill J, Egger M, Blaser N, et al. Cost-effectiveness of point-of-care viral load monitoring of ART in resource-limited settings: Mathematical modelling study. Aids. 2013 Mar 4; doi: 10.1097/QAD.0b013e328360a4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Estill J, Egger M, Johnson LF, et al. Monitoring of Antiretroviral Therapy and Mortality in HIV Programmes in Malawi, South Africa and Zambia: Mathematical Modelling Study. PloS one. 2013;8(2):e57611. doi: 10.1371/journal.pone.0057611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Padian NS, McCoy SI, Manian S, Wilson D, Schwartlander B, Bertozzi SM. Evaluation of large-scale combination HIV prevention programs: essential issues. Journal of acquired immune deficiency syndromes. 2011 Oct 1;58(2):e23–28. doi: 10.1097/QAI.0b013e318227af37. [DOI] [PubMed] [Google Scholar]
- 116.Smith K, Powers KA, Kashuba AD, Cohen MS. HIV-1 treatment as prevention: the good, the bad, and the challenges. Current opinion in HIV and AIDS. 2011 Jul;6(4):315–325. doi: 10.1097/COH.0b013e32834788e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mills EJ, Nachega JB, Ford N. Can we stop AIDS with antiretroviral-based treatment as prevention. Glob Health Sci Pract. 2013;1(1):29–34. doi: 10.9745/GHSP-D-12-00053. [DOI] [PMC free article] [PubMed] [Google Scholar]