Opioid analgesic overdose is a preventable and potentially lethal condition that results from prescribing practices, inadequate understanding on the patient's part of the risks of medication misuse, errors in drug administration, and pharmaceutical abuse.1,2 Three features are key to an understanding of opioid analgesic toxicity. First, opioid analgesic overdose can have life-threatening toxic effects in multiple organ systems. Second, normal pharmacokinetic properties are often disrupted during an overdose and can prolong intoxication dramatically.3 Third, the duration of action varies among opioid formulations, and failure to recognize such variations can lead to inappropriate treatment decisions, sometimes with lethal results.2,4
Epidemiology of Overdose
The number of opioid analgesic overdoses is proportional to the number of opioid prescriptions and the dose prescribed.5 Between 1997 and 2007, prescriptions for opioid analgesics in the United States increased by 700%; the number of grams of methadone prescribed over the same period increased by more than 1200%.6 In 2010, the National Poison Data System, which receives case descriptions from offices, hospitals, and emergency departments, reported more than 107,000 exposures to opioid analgesics, which led to more than 27,500 admissions to health care facilities.7 There is considerable overlap between psychiatric disease and chronic pain syndromes; patients with depressive or anxiety disorders are at increased risk for overdose, as compared with patients without these conditions, because they are more likely to receive higher doses of opioids.8 Such patients are also more likely to receive sedative hypnotic agents (e.g., benzodiazepines) that have been strongly associated with death from opioid overdose.9 In addition, data indicate that the frequent prescription of opioid analgesics contributes to overdose-related mortality among children, who may find and ingest agents in the home that were intended for adults.10,11
Pathophysiology of Opioid Analgesics
Opioids increase activity at one or more G-protein–coupled transmembrane molecules, known as the mu, delta, and kappa opioid receptors, that develop operational diversity from splice variants, post-translational modification and scaffolding of gene products, and the formation of receptor heterodimers and homodimers.12 Opioid receptors are activated by endogenous peptides and exogenous ligands; morphine is the prototypical compound of the latter.13 The receptors are widely distributed throughout the human body; those in the anterior and ventrolateral thalamus, the amygdala, and the dorsal-root ganglia mediate nociception.14 With contributions from dopaminergic neurons, brain-stem opioid receptors modulate respiratory responses to hypercarbia and hypoxemia, and receptors in the Edinger–Westphal nucleus of the oculomotor nerve control pupillary constriction.15 Opioid agonists bind to receptors in the gastrointestinal tract to decrease gut motility.
The mu opioid receptor is responsible for the preponderance of clinical effects caused by opioids. Studies in knockout mice confirm that agonism of these receptors mediates both analgesia and opioid dependence.16 Furthermore, the development of tolerance, in which drug doses must be escalated to achieve a desired clinical effect, involves the progressive inability of mu opioid receptors to propagate a signal after opioid binding. Receptor desensitization, a critical event in the development of tolerance, is a highly conserved process that involves the uncoupling of the receptors from G-protein, and their subsequent entry into an intracellular compartment during endocytosis. The receptors may then be returned to the membrane in a process that resensitizes the cell to opioid binding.17 This dynamic process of endocytosis and recycling is postulated to limit the tolerance of mu opioid receptors for endogenous opioid ligands as they undergo phasic secretion and rapid clearance.17 In contrast, opioid analgesics, which are administered repetitively in long-acting formulations, persist in the extracellular matrix and signal through mu opioid receptors for prolonged periods.17 Whereas endogenous native ligands foster dynamic receptor cycling, opioid analgesics facilitate tolerance by persistently binding and desensitizing the receptors as they blunt receptor recycling.17
However, tolerance of the analgesic and respiratory depressive effects of opioids is not solely related to the desensitization of mu opioid receptors. Conditioned tolerance develops when patients learn to associate the reinforcing effect of opioids with environmental signals that reliably predict drug administration.18 Opioid use in the presence of these signals has attenuated effects; conversely, opioid use in the absence of these stimuli or in new environments results in heightened effects.18 Tolerance of respiratory depression appears to develop at a slower rate than analgesic tolerance; over time, this delayed tolerance narrows the therapeutic window, paradoxically placing patients with a long history of opioid use at increased risk for respiratory depression.19-21
Toxicokinetics of Opioid Analgesics
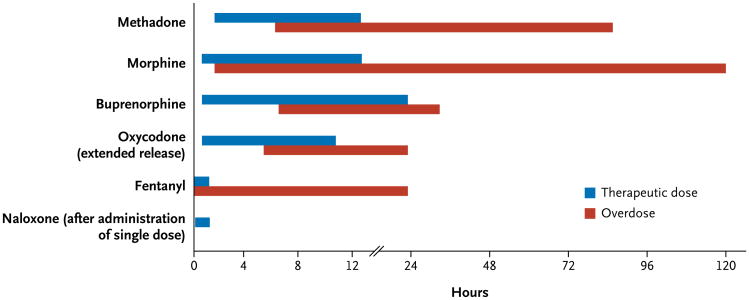
The pharmacokinetics of particular opioid analgesic agents — their absorption, onset of action, clearance, and biologic half-life — are often irrelevant in overdose. For example, bezoars formed after large ingestions of pills may produce erratic rates of drug absorption, and the delayed gastric emptying and diminished gastrointestinal motility caused by opioids may prolong drug absorption.22 Conversely, behaviors associated with drug misuse (e.g., insufflating or injecting ground opioid analgesic tablets, heating fentanyl patches, or applying one or more patches to skin) often increase the rate of absorption, albeit unpredictably. After absorption, most medications, including opioid analgesics, undergo first-order elimination pharmacokinetics, in which a constant fraction of the drug is converted by enzymatic processes per unit of time.3 In the case of an overdose, however, high concentrations of the drug may overwhelm the ability of an enzyme to handle a substrate, a process known as saturation.3 Saturated biologic processes are characterized by a transition from first-order to zero-order elimination kinetics.3 Two phenomena occur in zero-order elimination. First, small increases in the drug dose can lead to disproportionate increases in plasma concentrations and hence to intoxication.23 Second, a constant amount (as opposed to a constant proportion) of drug is eliminated per unit of time.23 Collectively, these toxicokinetic effects converge to produce opioid toxicity that may be severe, delayed in onset, and protracted as compared with the expected therapeutic actions (Fig. 1).24-31
Figure 1. Onset and Duration of Action in Therapeutic Dosing and Overdose of Selected Opioid Analgesic Agents.
Information about the toxic effects of opioid analgesic overdose often must be synthesized from case reports, the clinical observations of medical toxicologists, and forensic data.24-31 The difference between the clinical effects of therapeutic use and poisoning for these selected agents arises from the toxicokinetics of overdose, patterns of abuse, and the variation in drug effects in special populations.
Clinical Manifestations of Overdose
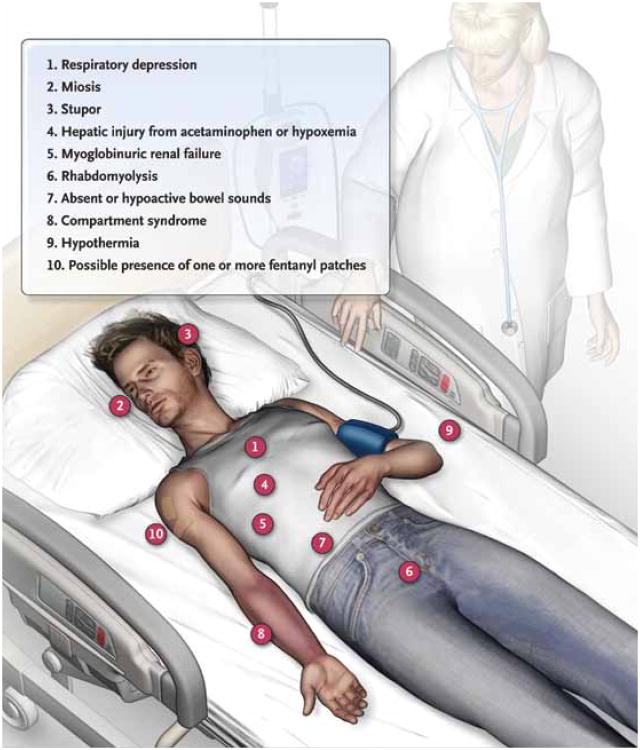
Opioid analgesic overdose encompasses a range of clinical findings (Fig. 2). Although the classic toxidrome of apnea, stupor, and miosis suggests the diagnosis of opioid toxicity, all of these findings are not consistently present.32 The sine qua non of opioid intoxication is respiratory depression. Administration of therapeutic doses of opioids in persons without tolerance to opioids causes a discernible decline in all phases of respiratory activity, with the extent of the decline dependent on the administered dose.33 At the bedside, however, the most easily recognized abnormality in cases of opioid overdose is a decline in respiratory rate culminating in apnea. A respiratory rate of 12 breaths per minute or less in a patient who is not in physiologic sleep strongly suggests acute opioid intoxication, particularly when accompanied by miosis or stupor.34 Miosis alone is insufficient to infer the diagnosis of opioid intoxication. Polysubstance ingestions may produce normally reactive or mydriatic pupils, as can poisoning from meperidine, propoxyphene, or tramadol.35,36 Conversely, overdose from antipsychotic drugs, anticonvulsant agents, ethanol, and other sedative hypnotic agents can cause miosis and coma, but the respiratory depression that defines opioid toxicity is usually absent.37,38
Figure 2. Clinical Findings in Opioid Analgesic Intoxication.
The sine qua non of opioid intoxication is respiratory depression, but miosis and stupor are often observed in poisoned patients. Hypoxemia or ingestion of drugs that are coformulated with acetaminophen can cause hepatic injury; acute renal failure can result from hypoxemia or precipitation of myoglobin due to rhabdomyolysis. Opioid analgesics decrease intestinal peristalsis by binding to opioid receptors in the gut. Patients with stupor who are motionless often have compressed fascia-bounded muscle groups, culminating in the compartment syndrome; they may also have hypothermia as a result of environmental exposure or misguided attempts at reversing intoxication. Since fentanyl can be a source of overdose, patients should be examined for the presence of fentanyl patches.
Failure of oxygenation, defined as an oxygen saturation of less than 90% while the patient is breathing ambient air and with ventilation adequate to achieve normal arterial carbon dioxide tension (partial pressure of carbon dioxide), is often caused by pulmonary edema that becomes apparent later in the clinical course.39,40 There are several potential causes of pulmonary edema. One likely cause is that attempted inspiration against a closed glottis leads to a decrease in intrathoracic pressure, which causes fluid extravasation. Alternatively, acute lung injury may arise from a mechanism similar to that postulated for neurogenic pulmonary edema.41 In this scenario, sympathetic vasoactive responses to stress in a patient who has reawakened after reversal of intoxication culminate in leakage from pulmonary capillaries.
Hypothermia may arise from a persistently unresponsive state in a cool environment or from misguided attempts by bystanders to reverse opioid intoxication by immersing a patient in cold water.42 In addition, persons who have been lying immobile in an opioid-induced stupor may be subject to rhabdomyolysis, myoglobinuric renal failure, and the compartment syndrome. Other laboratory abnormalities include elevated serum aminotransferase concentrations in association with liver injury caused by acetaminophen or hypoxemia. Seizures have been associated with overdose of tramadol, propoxyphene, and meperidine.43,44
Diagnosis of Overdose
The presence of hypopnea or apnea, miosis, and stupor should lead the clinician to consider the diagnosis of opioid analgesic overdose, which may be inferred from the patient's vital signs, history, and physical examination. In patients with severe respiratory depression, restoration of ventilation and oxygenation takes precedence over obtaining the history of the present illness or performing a physical examination or diagnostic testing.
After the patient's condition is stabilized, the clinician should inquire about the use of all opioid analgesics, acetaminophen (including products co-formulated with acetaminophen), and illicit substances and determine whether the patient has had contact with anyone receiving pharmacologic treatment for chronic pain or opioid dependence.27,45 In performing the physical examination, the clinician should evaluate the size and reactivity of the pupils and the degree of respiratory effort and look for auscultatory findings suggestive of pulmonary edema. The patient should be completely undressed to allow for a thorough search for fentanyl patches. In addition, the clinician should palpate muscle groups; the firmness, swelling, and tenderness that characterize the compartment syndrome (which results when comatose patients lie on a muscle compartment for a long time) warrant direct measurement of compartment pressures. Finally, the acetaminophen concentration should be measured in all patients because of the prevalence of diversion and misuse of acetaminophen-containing opioids. Clinicians often overlook acetaminophen hepatotoxicity.46
Qualitative analyses of urine for drugs of abuse (toxicology screens) rarely affect decisions about patient care and have little role in the immediate evaluation and management of opioid intoxication, for several reasons.47 First, naloxone should never be withheld from a patient with apnea because the results of qualitative tests are unavailable. Second, the management of opioid overdose, irrespective of the causative agent, varies little. Finally, standard toxic screens, which detect methadone, fentanyl, hydromorphone, and other compounds only infrequently, provide little useful clinical information.48 Newer qualitative screens that detect a broader range of opioid analgesics may allow clinicians specializing in pain treatment, mental health, or other areas of medicine to identify patients who have strayed from prescribed treatment regimens; greater analytic precision, however, does not change the management of acute overdose. Quantitative measures of drug concentrations are useless in cases of overdose because patients who have been prescribed elevated doses of opioid analgesics may have therapeutic serum concentrations that greatly exceed laboratory reference ranges.
Management of Overdose
Patients with apnea need a pharmacologic or mechanical stimulus in order to breathe. For patients with stupor who have respiratory rates of 12 breaths per minute or less, ventilation should be provided with a bag-valve mask; chin-lift and jaw-thrust maneuvers should be performed to ensure that anatomical positioning helps to diminish hypercarbia. Although the relationship between the partial pressure of carbon dioxide and acute lung injury is unclear, providing adequate ventilation is a simple response that offers the certain benefits of restoring oxygenation and preventing the postulated sympathetic surge that triggers pulmonary edema after the reversal of apnea, with minimal risk.
Naloxone, the antidote for opioid overdose, is a competitive mu opioid–receptor antagonist that reverses all signs of opioid intoxication. It is active when the parenteral, intranasal, or pulmonary route of administration is used but has negligible bioavailability after oral administration because of extensive first-pass metabolism.49 In patients with opioid dependence, plasma levels of naloxone are initially lower, the volume of distribution is higher, and the elimination half-life is longer than in patients without dependence.50 The onset of action is less than 2 minutes when naloxone for adults is administered intravenously, and its apparent duration of action is 20 to 90 minutes, a much shorter period than that of many opioids (Fig. 1).51,52
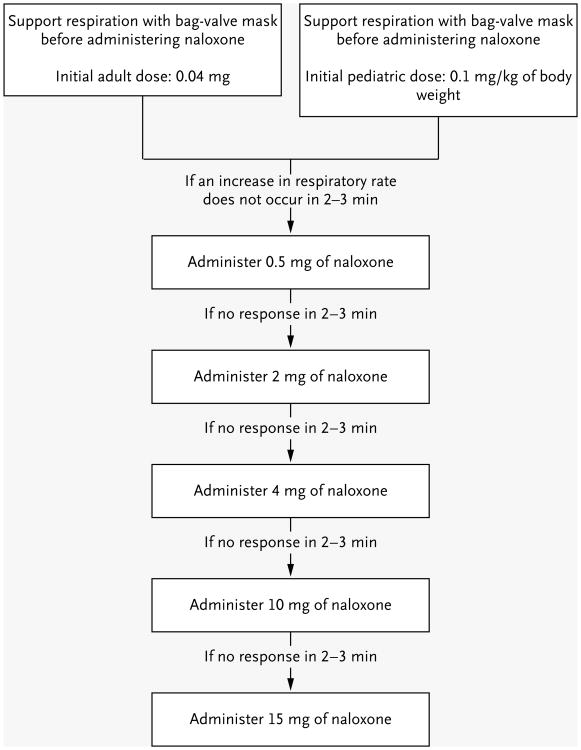
Dosing of naloxone is empirical. The effective dose depends on the amount of opioid analgesic the patient has taken or received, the relative affinity of naloxone for the mu opioid receptor and the opioid to be displaced, the patient's weight, and the degree of penetrance of the opioid analgesic into the central nervous system.25,52 Because most of this information will be unknown, clinicians must rely on the results of therapeutic trials to determine the effective dose of antidote.25 The initial dose of naloxone for adults is 0.04 mg; if there is no response, the dose should be increased every 2 minutes according to the schedule shown in Figure 3, to a maximum of 15 mg. If there is no abatement in respiratory depression after the administration of 15 mg of naloxone, it is unlikely that the cause of the depression is opioid overdose.30,31 Reversal of opioid analgesic toxicity after the administration of single doses of naloxone is often transient; recurrent respiratory depression is an indication for a continuous infusion (see the Supplementary Appendix, available with the full text of this article at NEJM .org) or for orotracheal intubation.53
Figure 3. Naloxone Dosing.
Empirical trials are needed to determine the effective dose of naloxone. Patients who do not have a response to an initial dose of naloxone should receive escalating doses until respiratory effort is restored. Naloxone, which is frequently dispensed as an injectable solution in doses of 0.4 mg per milliliter and 1 mg per milliliter for adults, is almost devoid of adverse effects. Pediatric patients are defined as children up to the age of about 5 years or with a body weight of up to 20 kg. Pediatric patients with opioid intoxication frequently require larger doses of naloxone to reverse the effects of overdose because of the relatively higher ingested dose per kilogram of body weight.
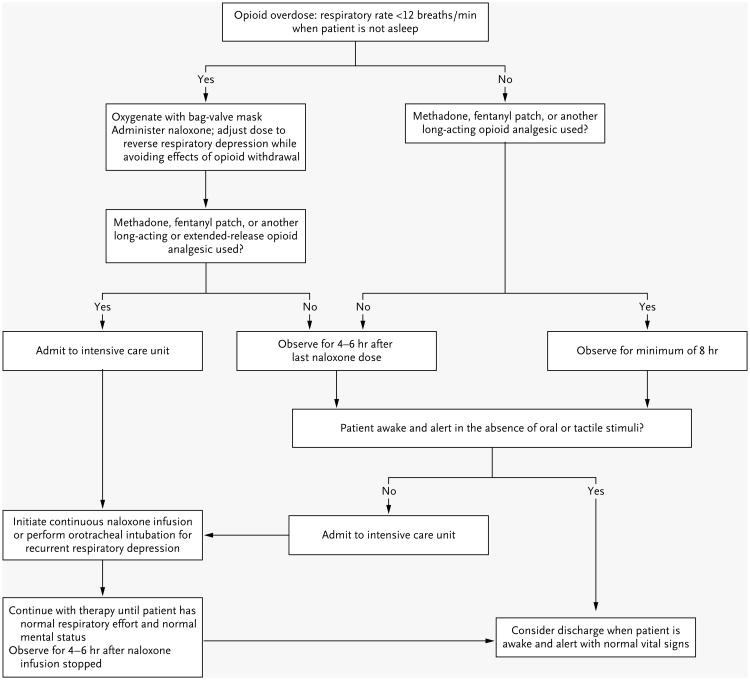
Naloxone can be administered without compunction in any patient, including patients with opioid dependence. Concerns that naloxone will harm patients with opioid dependence are unfounded; all signs of opioid abstinence (e.g., yawning, lacrimation, piloerection, diaphoresis, myalgias, vomiting, and diarrhea) are unpleasant but not life-threatening.25 In addition, patients with opioid tolerance frequently have a response to low doses of naloxone that are sufficient to restore breathing without provoking withdrawal.54 Once the respiratory rate improves after the administration of naloxone, the patient should be observed for 4 to 6 hours before discharge is considered (Fig. 4).57,58
Figure 4. Decision Tree for Managing Opioid Analgesic Overdose in Adults.
Because of the long duration of action of many opioid analgesic formulations, the brief effectiveness of naloxone, and the potential lethality of an opioid analgesic overdose, there should be a low threshold for admitting intoxicated patients to a hospital unit that provides close monitoring, such as an intensive care unit.26,53,54 Published guidelines for the management of opioid intoxication were developed on the basis of data from patients with heroin overdose and should not be applied to patients with opioid analgesic overdose.55,56
An alternative to the administration of naloxone is orotracheal intubation, a procedure that safely ensures oxygenation and ventilation while providing protection against aspiration.30 Gastrointestinal decontamination with activated charcoal should be reserved for patients who present within 1 hour after ingestion; charcoal offers no benefit outside this time frame and complicates visualization of airway anatomy during orotracheal intubation.59 Patients who are intoxicated by long-acting or extended-release opioid formulations, have recurrent respiratory depression, or require a naloxone infusion or orotracheal intubation should be admitted to an intensive care unit.26
Once the patient's condition is stable, the clinician should search the patient for fentanyl patches, even when fentanyl abuse is not suspected.45 Fentanyl patches have been associated with delayed toxicity after cursory physical examination.45 The axillae, perineum, scrotum, and oropharynx, in particular, should be examined; any patches should be removed, and skin decontaminated with soap and cool water.60 A patient who has ingested a fentanyl patch may benefit from whole-bowel irrigation with polyethylene glycol to accelerate elimination of the patch.30
Persistent hypoxemia after the administration of naloxone may signify the presence of negative-pressure pulmonary edema. Mild cases resolve with supportive care, but patients with severe hypoxemia often benefit from orotracheal intubation and positive-pressure ventilation. Resolution of lung injury, if uncomplicated by aspiration of gastric contents, normally occurs within 24 hours. Because the probable cause of lung injury is not fluid overload, reducing the intravascular volume with diuretics is unlikely to be effective and may worsen myoglobinuric renal failure, if present. Naloxone has been mistakenly implicated as a cause of pulmonary edema. However, pulmonary edema is present in nearly all fatal cases of opioid overdose, including those that occurred before the development of naloxone.39,40,61 Moreover, studies have shown that pulmonary edema does not develop in patients who receive large doses of naloxone by means of continuous infusion.62-64 Finally, auscultatory signs of pulmonary edema, which are often obscure in patients with apnea, become apparent only after naloxone restores ventilation.
Rhabdomyolysis (defined as a creatine kinase concentration that is five times as high as the upper end of the normal range) should be treated with fluid resuscitation to prevent myoglobin precipitation in the renal tubules; the addition of bicarbonate does not improve outcomes and should be avoided.65 Patients with the compartment syndrome should receive an emergency surgical consultation for possible fasciotomy. Patients with hypothermia may require immediate rewarming. Elevated aminotransferase concentrations, the presence of acetaminophen in the blood, or both may indicate the need for treatment with N-acetylcysteine.66 Cerebrospinal fluid lavage and the administration of naloxone may be needed in rare instances in which profound toxicity occurs as a result of overfilled or incorrectly programmed intrathecal pumps, which can contain hundreds of times the daily dose of an opioid analgesic.67 Finally, determining the cause of the overdose will identify patients who require referral to psychiatric or drug treatment.
Considerations in Special Populations
Opioid overdose in children is often characterized by a delayed onset of toxicity, unexpectedly severe poisoning, and prolonged toxic effects.24-26 These seemingly paradoxical effects result from ontogeny-related pharmacokinetics: children have rates of drug absorption, distribution into the central nervous system, and metabolism that differ from those in adults.68 Children 3 years of age or younger who have been exposed to any opioid analgesic other than immediate-release opioid formulations (e.g., methadone, fentanyl patches, and extended-release formulations) should be admitted for a 24-hour observation period, even if ingestion of these agents cannot be confirmed.28,29 Similarly, all toddlers exposed to buprenorphine formulations, including buprenorphine–naloxone products, must be admitted for close observation.27,69 The reported “ceiling effect” of buprenor-phine, in which escalating doses do not cause additional respiratory depression, has not been observed in children.70 Children who ingest opioid formulations often ingest a higher dose than adults per kilogram of body weight and therefore require larger doses of naloxone to reverse the effects of overdose (Fig. 3).
Elderly patients also have increased susceptibility to opioid effects and should be watched closely. A coexisting condition (e.g., renal insufficiency, chronic obstructive pulmonary disease, or sleep apnea) may exacerbate the inhibitory effects of opioids on respiration; age-related changes in physiology (e.g., decreased stroke volume, leading to diminished hepatic blood flow) and in body composition (leading to reduced binding of the drug to plasma proteins) may cause unexpected, persistent intoxication.71,72 These pharmacokinetic effects have been implicated in the failure of naloxone to successfully reverse cases of intoxication caused by short-acting opioid analgesics.73
Pitfalls of Overdose Management
Lack of knowledge about several aspects of opioid analgesic toxicity may complicate patient care. First, even clinicians with experience treating heroin overdose may believe that naloxone will prevent the recurrence of opioid analgesic toxicity.55 Naloxone, with its transient duration of action, does not truncate opioid toxicity; in many patients with intoxication from opioid analgesics, naloxone treatment does not forestall recrudescent respiratory depression. Second, clinicians may incorrectly assume that the dose of naloxone that is required to restore respiration correlates with the severity of intoxication. Because patients with opioid dependence frequently require low initial doses of antidote, physicians often provide only a brief period of patient observation, decide not to readminister the antidote, or admit patients to units that cannot perform intensive monitoring. Third, clinicians may associate peak plasma opioid concentrations with the greatest degree of respiratory depression.74 Opioid-induced respiratory depression is unrelated to the peak concentration, the timing of which cannot be reliably determined in cases of overdose.74 Fourth, early acetaminophen toxicity may go unrecognized at the time when intervention is most effective.47,66 Finally, clinicians may believe that pharmacologic responses in children and elderly patients are in keeping with the pharmacokinetic findings in healthy young adults and thus may inappropriately curtail the observation period.75
Prevention of Overdose
Several strategies may limit the harm of opioid analgesics, which are among the most effective drugs used to treat pain. Clinicians who prescribe these agents should understand the basics of safe opioid dosing, screen for mental illness in potential recipients of opioids, perform behavioral testing and urine screens to detect problematic opioid use, and use electronic prescription-drug monitoring programs (see the Supplementary Appendix) to help identify patients who may be receiving opioids inappropriately from multiple prescribers.8,76,77 The manufacturers of opioid analgesics should be assiduously honest in marketing their products, fund the independent development of objective prescribing information, and help prevent opioid exposure in children by distributing child-safety devices and educational materials for prescribers, patients, and families.2 Finally, patients should understand that opioid analgesics are not effective in treating all painful conditions, can engender long-term use, and are highly lethal when used inappropriately.78
Summary
Opioid analgesic overdose is a life-threatening condition, and the antidote naloxone may have limited effectiveness in patients with poisoning from long-acting agents. The unpredictable clinical course of intoxication demands empirical management of this potentially lethal condition.
Supplementary Material
Footnotes
Dr. Boyer reports reviewing medical malpractice documents for CRICO (Controlled Risk Insurance Company) Vermont, MCIC Vermont, and PMSLIC (Pennsylvania Medical Group Management Association). No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the author are available with the full text of this article at NEJM.org.
References
- 1.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–5. doi: 10.1056/NEJMp1011512. Erratum, N Engl J Med 2011;364:290. [DOI] [PubMed] [Google Scholar]
- 2.Opioid analgesic risk evaluation and mitigation strategies (REMS): July 22-23, 2010 Joint Meeting of the Anesthetic and Life Support Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. Silver Spring, MD: Food and Drug Administration; 2010. [Google Scholar]
- 3.Allen L, Kimura K, MacKichan J, Ritschel W. Committee for Pharmacokinetic Nomenclature of the American College of Clinical Pharmacology. Manual of symbols, equations & definitions in pharmacokinetics. J Clin Pharmacol. 1982;22:1S–23S. [PubMed] [Google Scholar]
- 4.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 5.Paulozzi LJ, Budnitz D, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15:618–27. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- 6.Department of Justice Drug Enforcement Administration. Automation of reports and consolidated orders system (ARCOS) http://www.deadiversion.usdoj.gov/arcos/index.html.
- 7.American Association of Poison Control Centers. National Poison Data System 2010 annual report. http://www.aapcc.org.
- 8.Paulozzi L, Weisler R, Patkar A. A national epidemic of unintentional prescription opioid overdose deaths: how physicians can help control it. J Clin Psychiatry. 2011;72:589–92. doi: 10.4088/JCP.10com06560. [DOI] [PubMed] [Google Scholar]
- 9.Toblin RL, Paulozzi LJ, Logan JE, Hall AJ, Kaplan JA. Mental illness and psychotropic drug use among prescription drug overdose deaths: a medical examiner chart review. J Clin Psychiatry. 2010;71:491–6. doi: 10.4088/JCP.09m05567blu. [DOI] [PubMed] [Google Scholar]
- 10.Bailey JE, Campagna E, Dart RC. The underrecognized toll of prescription opioid abuse on young children. Ann Emerg Med. 2009;53:419–24. doi: 10.1016/j.annemergmed.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Bond GR, Woodward RW, Ho M. The growing impact of pediatric pharmaceutical poisoning. J Pediatr. 2012;160:265–70. doi: 10.1016/j.jpeds.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 12.Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–90. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 13.Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267:495–9. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- 14.Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685–90. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- 15.Lalley PM. Opioidergic and dopaminergic modulation of respiration. Respir Physiol Neurobiol. 2008;164:160–7. doi: 10.1016/j.resp.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattes HW, Maldonado R, Simonin F, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioidreceptor gene. Nature. 1996;383:819–23. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 17.Whistler JL. Examining the role of mu opioid receptor endocytosis in the beneficial and side-effects of prolonged opioid use: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 2012;121:189–204. doi: 10.1016/j.drugalcdep.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrman R, Ternes J, O'Brien CP, McLellan AT. Conditioned tolerance in human opiate addicts. Psychopharmacology (Berl) 1992;108:218–24. doi: 10.1007/BF02245311. [DOI] [PubMed] [Google Scholar]
- 19.White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction. 1999;94:961–72. [PubMed] [Google Scholar]
- 20.Gal TJ, DiFazio CA, Moscicki J. Analgesic and respiratory depressant activity of nalbuphine: a comparison with morphine. Anesthesiology. 1982;57:367–74. doi: 10.1097/00000542-198211000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Etches RC. Respiratory depression associated with patient-controlled anesthesia: a review of eight cases. Can J Anaesth. 1994;41:125–32. doi: 10.1007/BF03009805. [DOI] [PubMed] [Google Scholar]
- 22.Smith SW, Howland MA, Hoffman RS, Nelson LS. Acetaminophen overdose with altered acetaminophen pharmacokinetics and hepatotoxicity associated with premature cessation of intravenous N-acetylcysteine therapy. Ann Pharmacother. 2008;42:1333–9. doi: 10.1345/aph.1K680. [DOI] [PubMed] [Google Scholar]
- 23.Juurlink D, Sivilotti M. Principles of pharmacology. In: Shannon M, Borron S, Burns M, editors. Clinical management of poisoning and drug overdose. 4th. Vol. 2007. Philadelphia: Elsevier; pp. 81–95. [Google Scholar]
- 24.Jefferson County Alabama coroner/medical examiner office case no 2007-0654. 2011 [Google Scholar]
- 25.Tenenbein M. Continuous naloxone infusion for opiate poisoning in infancy. J Pediatr. 1984;105:645–8. doi: 10.1016/s0022-3476(84)80440-0. [DOI] [PubMed] [Google Scholar]
- 26.Nelsen J, Marraffa J, Jones L, Grant W. Management considerations follow overdoses of modified-release morphine preparations. World J Emerg Med. 2010;1:75–6. [PMC free article] [PubMed] [Google Scholar]
- 27.Geib AJ, Babu K, Ewald MB, Boyer EW. Adverse effects in children after unintentional buprenorphine exposure. Pediatrics. 2006;118:1746–51. doi: 10.1542/peds.2006-0948. [DOI] [PubMed] [Google Scholar]
- 28.Boyer EW, McCance-Katz EF, Marcus S. Methadone and buprenorphine toxicity in children. Am J Addict. 2010;19:89–95. doi: 10.1111/j.1521-0391.2009.00002.x. [DOI] [PubMed] [Google Scholar]
- 29.Osterhoudt K. Pediatric emergency medicine: legal briefs. Pediatr Emerg Care. 2008;24:700–4. doi: 10.1097/PEC.0b013e318188fbf4. [DOI] [PubMed] [Google Scholar]
- 30.Prosser JM, Jones BE, Nelson L. Complications of oral exposure to fentanyl transdermal delivery system patches. J Med Toxicol. 2010;6:443–7. doi: 10.1007/s13181-010-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumann H, Erickson T, Thompson TM, Zautcke JL, Denton JS. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila) 2008;46:501–6. doi: 10.1080/15563650701877374. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann RS, Goldfrank LR. The poisoned patient with altered consciousness: controversies in the use of a ‘coma cocktail’. JAMA. 1995;274:562–9. [PubMed] [Google Scholar]
- 33.Gutstein H, Akil H. Opioid analgesics. In: Hardman J, editor. Goodman & Gilman's the pharmacologic basis of therapeutics. 11th. New York: McGraw-Hill; 2006. pp. 547–50. [Google Scholar]
- 34.Hoffmann JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Med. 1991;20:246–52. doi: 10.1016/s0196-0644(05)80933-3. [DOI] [PubMed] [Google Scholar]
- 35.Clark RF, Wei EM, Anderson PO. Meperidine: therapeutic use and toxicity. J Emerg Med. 1995;13:797–802. doi: 10.1016/0736-4679(95)02002-0. [DOI] [PubMed] [Google Scholar]
- 36.Zacny JP. Profiling the subjective, psychomotor, and physiological effects of tramadol in recreational drug users. Drug Alcohol Depend. 2005;80:273–8. doi: 10.1016/j.drugalcdep.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Alberto G, Erickson T, Popiel R, Narayanan M, Hryhorczuk D. Central nervous system manifestations of a valproic acid overdose responsive to naloxone. Ann Emerg Med. 1989;18:889–91. doi: 10.1016/s0196-0644(89)80220-3. [DOI] [PubMed] [Google Scholar]
- 38.O'Malley GF, Seifert S, Heard K, Daly F, Dart RC. Olanzepine overdose mimicking opioid intoxication. Ann Emerg Med. 1999;34:279–81. doi: 10.1016/s0196-0644(99)70249-0. [DOI] [PubMed] [Google Scholar]
- 39.Sporer KA, Dorn E. Heroin-related noncardiogenic pulmonary edema: a case series. Chest. 2001;120:1628–32. doi: 10.1378/chest.120.5.1628. [DOI] [PubMed] [Google Scholar]
- 40.Osler W. Oedema of left lung–morphia poisoning. Montreal Gen Hosp Rep. 1880;1:291–2. [Google Scholar]
- 41.Baumann A, Audibert G, McDonnell J, Mertes PM. Neurogenic pulmonary edema. Acta Anaesthesiol Scand. 2007;51:447–55. doi: 10.1111/j.1399-6576.2007.01276.x. [DOI] [PubMed] [Google Scholar]
- 42.Osterhoudt KC, Perrone J. Induced hypothermia for drug overdose. Acad Emerg Med. 2002;9:962. doi: 10.1197/aemj.9.9.962. [DOI] [PubMed] [Google Scholar]
- 43.Talaie H, Panahandeh R, Fayaznouri M, Asadi Z, Abdollahi M. Dose-independent occurrence of seizure with tramadol. J Med Toxicol. 2009;5:63–7. doi: 10.1007/BF03161089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaiko RF, Foley KM, Grabinski PY, et al. Central nervous system excitatory effects of meperidine in cancer patients. Ann Neurol. 1983;13:180–5. doi: 10.1002/ana.410130213. [DOI] [PubMed] [Google Scholar]
- 45.Moon JM, Chun BJ. Fentanyl intoxication caused by abuse of transdermal fentanyl. J Emerg Med. 2011;40:37–40. doi: 10.1016/j.jemermed.2007.10.075. [DOI] [PubMed] [Google Scholar]
- 46.Khandelwal N, James LP, Sanders C, Larson AM, Lee WM. Unrecognized acetaminophen toxicity as a cause of indeterminate acute liver failure. Hepatology. 2011;53:567–76. doi: 10.1002/hep.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyer EW, Shannon MW. Which drug tests in medical emergencies? Clin Chem. 2003;49:353–4. doi: 10.1373/49.3.353. [DOI] [PubMed] [Google Scholar]
- 48.Shaw L. The clinical toxicological laboratory: contemporary practice of poisoning evaluation. Washington, DC: AACC Press; 2001. [Google Scholar]
- 49.Pond SM, Tozer TN. First-pass elimination: basic concepts and clinical consequences. Clin Pharmacokinet. 1984;9:1–25. doi: 10.2165/00003088-198409010-00001. [DOI] [PubMed] [Google Scholar]
- 50.Fishman J, Roffwarg H, Hellman L. Disposition of naloxone-7,8,3H in normal and narcotic-dependent men. J Pharmacol Exp Ther. 1973;187:575–80. [PubMed] [Google Scholar]
- 51.Evans JM, Hogg MI, Rosen M. Degree and duration of reversal by naloxone of effects of morphine in conscious subjects. BMJ. 1974;2:589–91. doi: 10.1136/bmj.2.5919.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berkowitz BA. The relationship of pharmacokinetics to pharmacological activity: morphine, methadone and naloxone. Clin Pharmacokinet. 1976;1:219–30. doi: 10.2165/00003088-197601030-00004. [DOI] [PubMed] [Google Scholar]
- 53.Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion of intravenous naloxone. Ann Emerg Med. 1986;15:566–70. doi: 10.1016/s0196-0644(86)80994-5. [DOI] [PubMed] [Google Scholar]
- 54.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: adult cancer pain, version 2. 2011 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 55.POISINDEX system [intranet database], version 5.1. Greenwood Village, CO: Thompson-Reuters (Healthcare); 2003. [Google Scholar]
- 56.Christenson J, Etherington J, Grafstein E, et al. Early discharge of patients with presumed opioid overdose: development of a clinical prediction rule. Acad Emerg Med. 2000;7:1110–8. doi: 10.1111/j.1553-2712.2000.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 57.Clarke SF, Dargan PI, Jones AL. Naloxone in opioid poisoning: walking the tightrope. Emerg Med J. 2005;22:612–6. doi: 10.1136/emj.2003.009613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hendra TJ, Gerrish SP, Forrest AR. Fatal methadone overdose. BMJ. 1996;313:481–2. doi: 10.1136/bmj.313.7055.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chyka PA, Seger D. Position statement: single-dose activated charcoal. J Toxicol Clin Toxicol. 1997;37:721–41. doi: 10.3109/15563659709162569. [DOI] [PubMed] [Google Scholar]
- 60.Nelson L, Schwaner R. Transdermal fentanyl: pharmacology and toxicology. J Med Toxicol. 2009;5:230–41. doi: 10.1007/BF03178274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smialek JE, Monforte JR, Aronow R, Spitz WU. Methadone deaths in children: a continuing problem. JAMA. 1977;238:2516–7. [PubMed] [Google Scholar]
- 62.Peters WP, Johnson MW, Friedman PA, Mitch WE. Pressor effect of naloxone in septic shock. Lancet. 1981;1:529–32. doi: 10.1016/s0140-6736(81)92865-8. [DOI] [PubMed] [Google Scholar]
- 63.Groeger JS, Carlon GC, Howland WS. Naloxone in septic shock. Crit Care Med. 1983;11:650–4. doi: 10.1097/00003246-198308000-00013. [DOI] [PubMed] [Google Scholar]
- 64.Olinger CP, Adams HP, Jr, Brott TG, et al. High-dose intravenous naloxone for the treatment of acute ischemic stroke. Stroke. 1990;21:721–5. doi: 10.1161/01.str.21.5.721. [DOI] [PubMed] [Google Scholar]
- 65.Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore) 1982;61:141–52. doi: 10.1097/00005792-198205000-00002. [DOI] [PubMed] [Google Scholar]
- 66.Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359:285–92. doi: 10.1056/NEJMct0708278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsui BC, Malherbe S, Koller J, Aronyk K. Reversal of an unintentional spinal anesthetic by cerebrospinal lavage. Anesth Analg. 2004;98:434–6. doi: 10.1213/01.ANE.0000095152.81728.DC. [DOI] [PubMed] [Google Scholar]
- 68.Kearns GL, Abdel-Rahman SM, Al-ander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology — drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–67. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 69.Pedapati EV, Bateman ST. Toddlers requiring pediatric intensive care unit admission following at-home exposure to buprenorphine/naloxone. Pediatr Crit Care Med. 2011;12(2):e102–e107. doi: 10.1097/PCC.0b013e3181f3a118. [DOI] [PubMed] [Google Scholar]
- 70.Olkkola KT, Leijala MA, Maunuksela EL. Paediatric ventilatory effects of morphine and buprenorphine revisited. Paediatr Anaesth. 1995;5:303–5. doi: 10.1111/j.1460-9592.1995.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 71.Holdsworth MT, Forman WB, Killilea T, et al. Transdermal fentanyl patch disposition in elderly subjects. Gerontology. 1994;40:32–7. doi: 10.1159/000213572. [DOI] [PubMed] [Google Scholar]
- 72.Greenblatt DJ, Sellers EM, Shader RI. Drug disposition in old age. N Engl J Med. 1982;306:1081–8. doi: 10.1056/NEJM198205063061804. [DOI] [PubMed] [Google Scholar]
- 73.Chau DL, Walker V, Pai L, Cho LM. Opiates and elderly: use and side effect. Clin Interv Aging. 2008;3:273–8. doi: 10.2147/cia.s1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rigg JR. Ventilatory effects and plasma concentration of morphine in man. Br J Anaesth. 1978;50:759–65. doi: 10.1093/bja/50.8.759. [DOI] [PubMed] [Google Scholar]
- 75.Hayes BD, Klein-Schwartz W, Doyon S. Toxicity of buprenorphine overdose in children. Pediatrics. 2008;121(4):e782–e786. doi: 10.1542/peds.2007-1774. [DOI] [PubMed] [Google Scholar]
- 76.Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving longterm opioid therapy. Anesth Analg. 2003;97:1097–102. doi: 10.1213/01.ANE.0000080159.83342.B5. [DOI] [PubMed] [Google Scholar]
- 77.Katz N, Houle B, Fernandez KC, et al. Update on prescription monitoring in clinical practice: a survey study of prescription monitoring program administrators. Pain Med. 2008;9:587–94. doi: 10.1111/j.1526-4637.2008.00471.x. [DOI] [PubMed] [Google Scholar]
- 78.Alan A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172:425–30. doi: 10.1001/archinternmed.2011.1827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.