Abstract
Pure vascular parkinsonism without evidence of nigral Lewy body pathology may occur as a distinct clinicopathological entity, but a much more frequent occurrence is the comorbid presence of age-associated white matter lesions (WMLs) in idiopathic Parkinson disease (PD). WMLs are associated with motor and cognitive symptoms in otherwise normal elderly individuals. Comorbid WMLs are, therefore, expected to contribute to clinical symptoms in PD. Studies of WMLs in PD differ with regard to methods of assessment of WML burden and the patient populations selected for analysis, but converging evidence suggests that postural stability and gait motor functions are predominantly affected. WMLs are described to contribute to dementia in Alzheimer disease, and emerging but inconclusive evidence indicates similar effects in PD. In this article, we review the literature addressing the occurrence and impact of WMLs in PD, and suggest that WMLs may exacerbate or contribute to some motor and cognitive deficits associated with PD. We review existing and emerging methods for studying white matter pathology in vivo, and propose future research directions.
Introduction
White matter lesions (wMLs), also known as leuKoaraiosis, are commonly observed on imaging studies in older adults, and may present as signal hyperintensities on t2-weighted MRI studies.1 age-associated wMLs are related to balance impairment, mobility and cognitive deficits in otherwise healthy elderly individuals.2–5 Given that some of these dysfunctions overlap with features of Parkinson disease (PD), comorbid WMLs are predicted to contribute to clinical symptoms in this condition.
This Review will focus on the comorbid presence of wMLs in patients with idiopathic PD, and its relationship with motor and cognitive symptoms. we briefly review the contribution of wMLs to declining motor and cognitive function in aging, the contribution of vascular pathology to parkinsonism, and established and emerging methods for evaluating wMLs. we then provide a detailed review of studies of wMLs in patients with PD, and a discussion of the probable clinical correlates of wMLs in PD and interactions with neurodegenerative pathology in this condition. we conclude by discussing possible future research on wMLs and PD.
Presence of white matter lesions
Nondisabled elderly individuals
The Leukoaraiosis and Disability (LaDis) study found that the presence of wMLs in nondisabled elderly people was associated with a history of falls, and correlated with mobility impairments, such as decreased walking speed, decreased balance, and reduced physical activity levels.4 wMLs have also been linked with deficits in attention, executive functions and processing speed in the elderly.6,7
In 2010, Murray et al. reported wML burden, cognitive data and motor data from well-characterized nondemented elderly individuals without PD.1 In this cohort, wML burden in several regions of the brain was inversely correlated with executive function but did not correlate with memory, language or visuospatial dysfunction. In terms of motor function, increasing wML burden correlated with reduced gait velocity and worsening Unified PD rating scale (uPDrs) scores for gait, posture and postural instability. In a study involving a large cohort of healthy elderly individuals (n = 429; age range 50–85 years) de Laat et al. described wMLs associated with gait abnormalities—specifically, slower gait speed, reduced stride length, and increased stride width.8
Vascular pathology and parkinsonism
wMLs are thought to occur in the context of cardiovascular disease.9 One neuropathological study found evidence of an association between wMLs and loss of vascular integrity, indicating a vascular origin for these lesions.10 Vascular damage may, in turn, impair blood– brain barrier integrity, thus providing one mechanism though which wMLs could evolve. Mri–pathological correlation studies of periventricular wMLs revealed correlated vascular changes including arteriolar tortuosity, reduced vessel density, and occlusive venous collagenosis associated with venous insufficiency and vasogenic edema.11–13 In addition, activated microglia, oligodendroglial apoptosis, clasmatodendritic astrocytosis, and upregulation of hypoxia-associated markers were found.
Periventricular wMLs are suggested also to result from a reduction in myelin density due to Wallerian degeneration secondary to primary neocortical neuron loss.14 However, wMLs generally spare subcortical U-fibers, which would be predicted to be affected if Wallerian degeneration was a major contributor.12 Other possible mechanisms involved in wML formation include hypotension,15 low-grade inflammation,16 and metabolic factors, such as vitamin B12 deficiency.17
Parkinsonism due to cerebrovascular disease in the absence of Lewy body pathology (vascular parkinsonism) is a distinct clinicopathological entity, and may account for 4–12% of all cases of parkinsonism.18,19 Commonly noted lesions on brain imaging in vascular parkinsonism are lacunes, wMLs and, in rare cases, large-vessel infarcts.18,20 in a consecutive autopsy series of 700 cases with a clinical diagnosis of parkinsonism, 27 brains (3.9%) showed subcortical vascular lesions in the basal ganglia, white matter or brainstem, with no significant nigral lesions.21 In the uK Parkinson's Disease society Brain Bank clinicopathological series, three of 24 cases misdiagnosed by specialist neurologists as idiopathic PD during life exhibited postmortem evidence of a lacunar state without characteristic Lewy body pathology.22 Although a pure vascular etiology exists for a small subset of patients with parkinsonism, comorbid postmortem vascular lesions are much more common, and have been reported in 19–50% of patients with Lewy body-confirmed PD.21,23,24
In vivo imaging studies show wMLs to be present in 30–55% of patients with PD.25–27 some studies also suggest that wMLs are more common in patients with PD than in normal elderly individuals.28–30 Other studies, however, failed to find an increased incidence of wMLs in PD.25,31,32
Marked age-related changes have been observed in the central dopamine system.33 An average person may lose ≈33% of striatal dopaminergic innervation between the ages of 25 and 75 years,34 although this figure is not as severe as in PD, where losses often exceed 50–80%.35 According to estimates, ≈50% of dopaminergic cells must be destroyed, with an 80% reduction in posterior putaminal dopaminergic innervation, before specific clinical signs and symptoms of PD become manifest.35–37 The combined presence of subclinical age-associated nigrostriatal dopaminergic loss and wMLs may, however, cause motor impairments in the elderly. For example, Louis and colleagues described mild parkinsonian features associated significantly with wML burden in 16.7% of community-dwelling elderly individuals.38 wMLs might, conceivably, lower the threshold for developing parkinsonian symptoms in elderly people with age-associated and pathological nigrostriatal dopaminergic losses (Figure 1).
Figure 1.
Venn diagram showing overlap between idiopathic PD and WMLs of normal aging. Normal aging is accompanied by nigrostriatal degeneration, and the presence of WMLs has been associated with mild parkinsonian symptoms in elderly individuals without PD. Abbreviations: PD, Parkinson disease; WMLs, white matter lesions.
Imaging white matter lesions
wMLs can be recognized on Ct scans, but Mri scans— in particular, t2-weighted or fluid-attenuated inversion recovery (FLair) sequences—are most commonly used for their identification. wMLs appear as punctate or more-confluent hyperintense areas on these sequences, and are often referred to as white matter hyper intensities. Visual rating scales, each with emphases on different aspects of wMLs, have been developed to estimate wML burden.39–42 The most comprehensive scale was developed by scheltens and colleagues.42 In this scale, wML burden is assessed on the basis of on size and quantity of lesions in a given neuroanatomical location, including periventricular and non-periventricular wMLs. Periventricular hyperintensities are further separated into frontal, occipital and lateral regions.
Other rating scales for wMLs are the Brant-Zawadzki et al. scale39 and the Cardiovascular Health study scale,41 both of which place relatively high emphasis on periventricular wMLs. Unlike the Scheltens scale, these two rating scales do not specifically assess the different periventricular regions. Periventricular wML burden is often proportional to the overall burden.43,44 Furthermore, the burden of wMLs around the frontal ventricular horns may be of particular importance, given the potential to disrupt several important subcortical afferent and cortical efferent projections.45
Semiquantitative visual assessments of wML burden are limited by grader dependence: inter-grader variability may affect the reliability of these measures and limit comparison across studies. With increasing magnetic field strengths, white matter abnormalities become more complex and difficult to define by visual inspection. Punctate and confluent white matter areas are observed that appear to be below a threshold to classify easily as hyperintensities—these are sometimes referred to as ‘dirty’ white matter.
Automated routines have also been developed to define wML burden. These methods are typically based on segmentation of white matter Mri series, thresholding of hyperintense white matter voxels, or ‘fuzzy’ neighboring cluster-based voxel analysis. A different approach involves use of a reference region or tissue, such as intensity of normal gray matter46 or the cerebellar white matter, which usually has few age-associated wMLs.47 These methods have the advantage of improved quantitative assessment of wML burden compared with visual grading, and might be particularly advantageous for assessing changes over time.
Diffusion tensor imaging (Dti) is an Mri technique that examines the local microenvironmental characteristics of water diffusion and is used to evaluate the integrity of white matter fiber tracts. Dti measures of the magnitude and direction of water diffusion are termed mean diffusivity and fractional anisotropy, the former being a measure of water diffusivity and the latter being a measure of tract directionality and integrity.48 Dti measures were recently shown to add significantly to assessment of wMLs in studies of healthy elders.8 A Dti study published in 2009 showed evidence of microstructural wMLs in early PD, most prominently in the genu of the corpus callosum and in the superior longitudinal fasciculus, suggesting connectivity changes in the frontal and parietal white matter.49 Another study found decreased fractional anisotropy in the frontal lobes in PD patients without cognitive impairment and with no evidence of brain volume loss.50
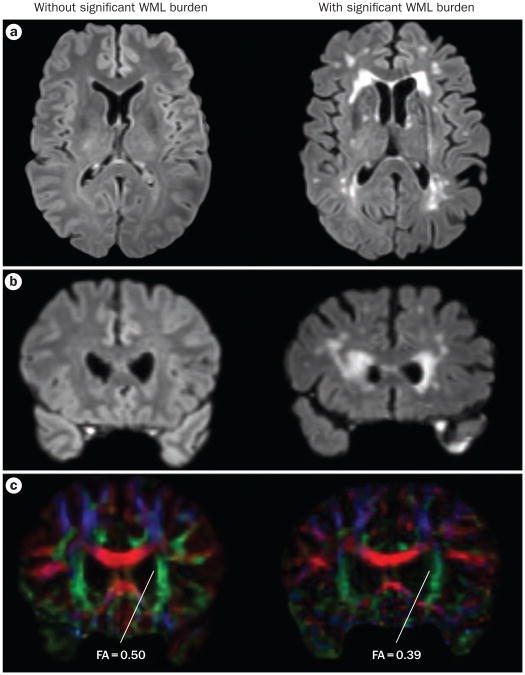
These data suggest that widespread microstructural damage to frontal and parietal white matter occurs in early stages of PD, and may occur before hyperintensities are visually apparent on t2-weighted Mri. Dti could be particularly valuable for studying wMLs in earlier stages of the disease, and Dti of the corpus callosum offers a unique window to study regional disruption in the inter-hemispheric connection during neuro degeneration.51 Figure 2 shows examples of FLair and Dti scans in PD patients without and with significant FLair hyper-intensities. Despite the lack of specific FLair hyper-intensities in the former patient, the fractional anisotropy of the anterior corona radiata suggests mild micro-structural changes in the frontal lobe white matter.
Figure 2.
Imaging WMLs. Comparison of DTI and FLAIR MRI of PD patients without (left panels) and with (right panels) significant WML burden. a | Transaxial slices of the FLAIR image sets. Hyperintensities, especially in the frontal ventricular horns, are prominent in the patient on the right, and absent in the patient on the left. b | Coronal slices of the FLAIR image sets, taken near the anterior border of the lateral ventricles. c | Coronal DTI slices, taken at the same level as the coronal FLAIR images. Degradation of the anterior aspect of the corona radiata is apparent in the patient with significant FLAIR hyperintensities. FA is also reduced in this area. FA values are also slightly reduced in the patient without significant FLAIR hyperintensities, indicating decreased white matter microstructural integrity. These findings are consistent with altered diffusion in the frontal lobes, as observed in DTI studies in patients with PD.49,50 Abbreviations: DTI, diffusion tensor imaging; FA, fractional anisotropy; FLAIR, fluid-attenuated inversion recovery; WML, white matter lesion.
White matter lesions in PD
Motor correlates
Although some studies failed to report an association between wMLs and motor impairments in PD,52 evidence is accumulating that wMLs independently contribute to postural and gait disturbances in this condition. Sohn and Kim, for example, found that comorbid wMLs were associated with more-severe gait problems but not with symptoms of tremor, pure bradykinesia or rigidity.25 Subcortical but not periventricular wMLs were related to more-severe gait symptoms and rigidity. Piccini et al. found that patients with PD and comorbid wMLs had more-severe symptoms of bradykinesia, gait disturbance and postural instability than PD patients without wMLs.29 The same authors found that the presence of periventricular but not subcortical wMLs was associated with more-severe clinical symptoms and more-rapid disease progression. in a study of 141 patients with PD, Lee et al. found that wML burden correlated significantly with postural and gait impairments, speech, facial expression, bradykinesia, and—to a lesser degree—rigidity, but not with tremor.27 Comparison of age-matched groups with the postural instability gait difficulty (PiGD) motor and tremor-dominant phenotypes of PD demonstrated significantly increased wML burden in the PiGD group.27 Acharya et al. found that age-related brain Mri changes superimposed on PD correlated positively with the number of steps and walking time to complete a gait task but did not correlate with total parkinsonian motor scores.31
No studies have yet directly compared the presence or severity of wMLs with the degree of nigrostriatal dopaminergic denervation and motor symptoms in PD. We have preliminary data showing that increased wML burden is associated with worsening motor performance in PD independent of the degree of nigrostriatal dopaminergic denervation, as determined by Pet imaging. We also found that comorbid wMLs are a greater determinant of balance impairment than nigrostriatal dopaminergic denervation in PD.53
Cognitive correlates
Cognitive decline is a frequent finding in PD patients, with point prevalence estimated at up to 40% for dementia and at >60% for executive dysfunction54,55 The cumulative prevalence of dementia is very high, and at least 75% of patients with PD who survive for >10 years will develop dementia.56 wMLs are described to contribute to dementia in alzheimer disease,57,58 and evidence—as yet inconclusive—is emerging of similar effects in PD with dementia.59,60 Choi and colleagues, for example, found preliminary evidence that comorbid wMLs increase the risk of dementia more than twofold in patients with PD.24 Beyer and colleagues also explored whether wMLs were associated with dementia in PD.61 35 patients with PD—16 with dementia and 19 without dementia— and 20 control individuals were included in this study. Cerebrovascular risk factors, education, sex ratios and age distributions were similar across groups. Compared with the PD group, the PD with dementia (PDD) group had significantly higher levels of periventricular and deep wMLs. Deep white matter wML burden was the only variable associated significantly with Mini Mental State Examination scores, and explained 38% of the cognitive score variance in a multivariate linear regression analysis.61 These findings suggest that wMLs in the deep white matter may contribute to dementia in PD.
Lee and colleagues used the Scheltens et al. rating scale to assess wMLs in a consecutive series of 71 patients with PD, including some with PDD.62 These authors found that patients with PDD had significantly more supratentorial wMLs—in particular, periventricular wMLs— than the PD group. A linear regression model indicated that wMLs were independently associated with cognitive impairments in PD, regardless of age, sex, duration or severity of PD symptoms, and vascular risk factors.62 More-severe wMLs correlated with more- profound cognitive deficits, including impairments in attention, visuospatial, memory, and executive functions.
Other studies, one of which involved newly diagnosed PD patients without dementia, have failed to find significant disease-specific cognitive correlates of wMLs.26,63 Although these studies reported a significant inverse correlation between wML burden and scores on tests of attention and executive functions, this correlation vanished on age-adjusted multiple regression analysis. Similar results were reported in a study by Dalaker and colleagues.32 The age-uncorrected correlation coefficients between wMLs and cognition, however, were stronger in the PD group than in the age-matched control group. These findings suggest a significant interaction between the effects of age and wMLs in PD. Another possibility is that the cognitive effects of comorbid wMLs may be more easily detectable in advanced stages of neuro-degeneration, such as in PDD.64
A recent study compared the neuropsychological profiles of parkinsonian patients with and without vascular lesions on Mri, all of whom were confirmed to have nigrostriatal dopaminergic denervation on dopamine transporter imaging.65 PD patients with comorbid wMLs performed less well on tests of executive function and attention than did control PD patients.
Further studies examining the relationship between wMLs and specific cognitive domains are needed. In addition, no published studies have directly compared the effects of nigrostriatal dopaminergic denervation and wMLs in PD. We have, however, obtained preliminary data indicating that increased wML burden is associated with worsening cognitive performance in PD independent of the degree of nigrostriatal dopaminergic denervation.66
Clinical features
Accumulating evidence from studies of the correlates of wMLs in normal elderly individuals and patients with PD suggest that wMLs might cause or exacerbate some features of PD. wMLs could cause or exacerbate motor or cognitive features through several mechanisms. First, any gray matter vascular lesion—the pathogenesis of which is probably driven by the same vascular risk factors that lead to wMLs—involving the substantia nigra or a sufficient volume of striatum can produce ‘parkinsonism’.19,67 Second, wMLs may disrupt the corticostriatal–thalamocortical loops.68 Periventricular wMLs may reflect damage of both periventricular ascending thalamocortical and descending corticospinal fibers, especially those from the medial frontal cortex that project to the lower extremities. These fibers pass close to the lateral ventricles before entering the internal capsule, and wMLs could interfere with long-loop reflexes that are critical for gait and balance. Third, wMLs might disrupt the interhemispheric connections of the corpus callosum, which are critical for complex integrated motor programs, such as bipedal walking. Last, wMLs may disrupt important sub cortical afferents. For example, wMLs underlying the frontal lobes and residing within periventricular regions may destroy projection fibers originating from sub cortical mono aminergic and cholinergic nuclei, including dopaminergic fibers originating from the ventral tegmental area that project to the mesofrontal and limbic cortex,47 and cholinergic projections from the nucleus basalis of Meynert (Figure 3). wMLs of the deep frontal lobe could result in cortical cholinergic deafferentation and related cognitive impairment in elderly individuals without PD.45
Figure 3.
Monoaminergic and cholinergic pathways that can be disrupted by white matter lesions. Sagittal brain MRI slice showing the deep forebrain where subcortical monoaminergic and cholinergic axons pass close to the ventricles before fanning out to the cortex. The dopaminergic mesofrontal and limbic cortical pathways (green) originate from the ventral tegmental area. Noradrenergic cortical pathways (red) originate from the locus coeruleus. Serotonergic cortical pathways originate from the raphe nuclei (blue). Cholinergic projections originating from the nucleus basalis of Meynert are shown in black. Periventricular frontal white matter lesions are strategically located to disrupt these ascending neuromodulator system projections.
More speculatively, wML burden might also be associated with depression, which is another prominent feature of PD. As discussed in a systematic review,69 some studies suggest that wML burden is a risk factor for late-life depression. Frontal lobe wMLs have been implicated in depression in PD, and increased wML burden could be an additional risk factor in this setting.
wMLs of the longitudinal fasciculus may preferentially affect executive cognitive functions.69 wMLs at the level of the brainstem might interfere with sensorimotor afferent and efferent cortical projections (table 1). Taken together, these observations suggest that supratentorial and brainstem wMLs interfere with central processing of sensorimotor signals, leading to impaired postural responses and gait functions.70 Such effects might be more prominent in a hypodopaminergic state, such as PD.
Table 1. Disruption of major tracts by WMLs.
| White matter tracts disrupted | Clinical consequences |
|---|---|
| Periventricular WMLs may reflect damage of both periventricular ascending thalamocortical and descending corticospinal fibers | Impaired postural control and gait |
| Corpus callosum | Impaired bipedal stance and gait functions |
| Longitudinal fasciculus | Impaired cognitive—including executive— functions |
| Striato-thalamocortical connections | Impaired executive cognitive and psychomotor functions |
| Deep frontal forebrain monoaminergic and cholinergic projection | Cortical deafferentation with associated cognitive, mood and motor changes |
| Brainstem long tract pathways | Impaired postural control and gait |
Abbreviation: WMLs, white matter lesions.
Motor correlates of wMLs in idiopathic PD may to some degree overlap with typical vascular parkinsonian symptoms. When compared with idiopathic PD, typical motor symptoms in pure vascular parkinsonism tend to be more symmetrical, affecting the lower limbs more than the arms (‘lower-body parkinsonism’), and resting tremor is usually absent.18,71–73 The gait pattern in patients with subcortical arteriosclerotic encephalopathy (Binswanger disease) has been described as having elements of both parkinsonian gait and ataxia, with the difficulty in using the legs to walk being out of proportion to that of other movements of the lower limbs when lying or seated.74 Postural instability, freezing, gait disturbance, and pyramidal signs have been found to be significantly more prevalent in patients with vascular parkinsonism than in idiopathic PD.18
In view of the prominent nigrostriatal pathology in PD, and because postural instability is a cardinal feature of the condition, the historical assumption was that postural instability is attributable mainly to striatal dopaminergic denervation.75 Balance-related deficits are, however, the features that are least responsive to levodopa treatment,76 and non-dopaminergic mechanisms, including degeneration of the pedunculopontine–thalamic choliner gic system, contribute substantially to impaired balance in PD.75,77 Postural control is not simply a summation of static reflexes but involves complex coordination and integration of visual, somatosensory and vestibular systems. The relative emphases placed on each of these sensory system inputs depend on the goals of the movement task and the environmental context.78 Comorbid wMLs, therefore, are predicted to markedly interfere with postural control systems owing to the widespread and bilateral distribution of these neural systems.
Progression, treatment response and prognosis
One study has reported more-severe clinical features in typical late-onset PD patients with a history of minor stroke, ischemic heart disease, or diabetes mellitus than in PD patients without such histories.79 The presence of vascular disease might affect PD by aggravating symptoms in the same manner as in patients with vascular parkinsonism. Establishing the existence of synergistic detrimental effects of PD and comorbid wMLs on disease progression and mobility impairment may lead to new therapeutic interventions, such as more-aggressive treatment of possible risk factors for wMLs, including the so-called ‘metabolic syndrome’.80 Therapies targeted at secondary prevention of wML progression might reduce axial motor and cognitive impairments in PD.
One study has examined the progression of wMLs in PD. Burton et al. Looked at 1 year wML progression in older individuals clinically diagnosed with PDD or Alzheimer dementia.60 The severity of wMLs at baseline was the best predictor of 1 year progression in wML burden. This result implies that early and aggressive management of vascular risk factors would be necessary to have an impact on the severity of clinical features in PD.79
A suggestion has been made that treating PD patients with dopaminergic medications might increase the risk of endothelial dysfunction and atherosclerosis by raising homocysteine levels.81 At this point, this concern is entirely theoretical, as intervention studies do not support a major role for hyperhomocysteinemia in cerebrovascular disease or cognitive decline.82,83
Future directions
Improved quantification methods
Studies on the relationship between comorbid wMLs and clinical features of PD have yielded variable results, probably at least partly owing to differences in the methods used to assess these lesions. Use of different rating scales and wML quantification algorithms, for example, impedes proper comparison of studies. Another common problem is the nonlinearity of scale units, which limits statistical analysis by linear regression. More-sensitive imaging techniques, such as Dti, are expected to give a better spread of data in people who have lower burdens of typical hyperintensities on t2-weighted or FLair Mri. Some clinical features may be influenced by clinical threshold effects of wMLs before symptoms emerge, thereby limiting correlation analysis with wML ratings.84 In addition, summed or global wML scores might be insensitive for symptoms related to topographically ‘eloquent’ locations of wMLs.
Future research should investigate the effects of strategically located wMLs, in particular those in periventricular frontal regions that could disrupt neuromodulator projection systems and result in monoaminergic and cholinergic cortical deafferentation (Figure 3 and table 1). Comprehensive analysis of brain small-vessel disease effects should not be limited to white matter, but should also include subcortical gray matter and provide regionally specific information. Most studies to date have been cross-sectional in nature, and longitudinal studies are needed to better and more-specifically evaluate the impact of wMLs and clinical symptoms in PD. Such investigations are of particular importance as the presence of the PiGD motor phenotype may be a risk factor for dementia in PD.85
Combined gray and white matter imaging
The presence of wMLs is associated with reduced cortical perfusion,86 and confluent wMLs are associated with up to 20% reductions in global cerebral blood flow.87 Such relationships do not, however, establish that wMLs lead to diminished perfusion. Nevertheless, a combined Mri and glucose metabolic Pet study suggested that wMLs preferentially affect glucose metabolism in the frontal lobes.6
Studies are required that investigate how regional white matter integrity affects gray matter metabolism or perfusion, so as to unravel the complex link between white matter degeneration and cortical function, and to establish whether a synergistically detrimental effect exists.88,89 Gray matter studies should not be limited to the cortex, but should also involve subcortical gray matter, such as the basal ganglia and thalamus.
The amyloid cascade and white matter lesions
Evidence is emerging for the existence of genetic risk factors for wMLs, which, in the case of microbleeds, may involve the amyloid cascade.90 Amyloid pathology is often present when PD is complicated by dementia,91 and the relationship between amyloid angiopathy and amyloidogenesis and occurrence of wMLs clearly needs to be elucidated. For example, cerebral apolipoprotein E leakage induced by small-vessel disease has been associated with amyloid-β deposition in perivascular astrocytes.92 suggestions have been made that metabolic abnormalities that drive vascular risk factors also contribute to amyloidogenesis.57 Even if this proves not to be the case, vascular injury to the brain might still have an additive effect on primary neurodegenerative processes.89,93
Conclusions
The association between comorbid wMLs and PD motor symptoms most consistently manifests in impairment of axial motor symptoms. Correlations between wMLs and symptoms of rigidity and bradykinesia are less consistent. wMLs are likely to disrupt connectivity in widespread neural systems underlying bipedal stance and gait, which include corticostriatal–thalamocortical loops, interhemispheric fibers, striatothalamic–brainstem systems, and proper processing of multimodal sensory information. Evidence—albeit inconclusive— is accumulating that cognitive effects of wMLs tend to preferentially affect executive functions, and may reflect frontal lobe wMLs. Preliminary evidence suggests that the detrimental cognitive effects of wMLs in PD could become more profound with increasing age, with pre-existing cognitive impairment representing a possible threshold effect. New Mri methods are expected to provide more-sensitive imaging tools to better characterize and quantify the wML burden at a micro structural level in PD. wMLs could, plausibly, contribute to the clinical features of PD, raising the possibility that aggressive primary or secondary treatment of vascular or metabolic risk factors may affect the severity and progression of the disease.
Key points.
White matter lesions (WMLs) correlate with motor and cognitive abnormalities in otherwise normal elderly individuals, and some of these abnormalities overlap with features of Parkinson disease (PD)
Comorbid WMLs in PD may contribute to motor symptoms—in particular, balance disturbances—possibly by disrupting subcortical–cortical tracts involved in gait and balance
Most pathobiological emphasis in PD has been on proximal neural systems, but clinical symptom augmentation by comorbid WMLs illustrates the importance of integrity of afferent and efferent subcortical–cortical projections
Diffusion tensor MRI is a novel imaging modality to study microstructural white matter changes; findings of such changes in early PD are consistent with other evidence of widespread neurodegeneration
Early prevention or mitigation of comorbid WMLs, perhaps by reducing cerebrovascular or metabolic risk factors, could result in reduced motor and cognitive disability in PD
Review criteria.
Articles for the Review were selected on the basis of a PubMed database search, using the following search terms: “Parkinson disease”, “white matter”, “leukoaraiosis”, “motor”, “cognitive” and “magnetic resonance imaging”. Papers in the English language published between 1947 and 2010 were selected for review, and full-text versions were obtained. Additional references were identified from the publication lists of identified papers.
Acknowledgments
The authors thank Bryan Benson for his assistance with the MRI figures. The authors gratefully acknowledge research support from the NIH–National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs and the Michael J. Fox Foundation.
Footnotes
Competing interests: The authors declare no competing interests.
Author contributions: Both authors researched data for the article, discussed the content, wrote the text, and reviewed and edited the manuscript before submission.
References
- 1.Murray ME, et al. Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch Neurol. 2010;67:1379–1385. doi: 10.1001/archneurol.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baloh RW, Yue Q, Socotch TM, Jacobson KM. White matter lesions and disequilibrium in older people. I. Case–control comparison. Arch Neurol. 1995;52:970–974. doi: 10.1001/archneur.1995.00540340062013. [DOI] [PubMed] [Google Scholar]
- 3.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- 4.Baezner H, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70:935–942. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- 5.Novak V, et al. White matter hyperintensities and dynamics of postural control. Magn Reson Imaging. 2009;27:752–759. doi: 10.1016/j.mri.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tullberg M, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prins ND, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 8.de Laat KF, et al. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134:73–83. doi: 10.1093/brain/awq343. [DOI] [PubMed] [Google Scholar]
- 9.Pantoni L, Garcia JH. Pathogenesis of leuKoaraiosis. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 10.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71:804–811. doi: 10.1212/01.wnl.0000319691.50117.54. [DOI] [PubMed] [Google Scholar]
- 11.Black S, Gao F, Bilbao J. Understanding white matter disease: imaging–pathological correlations in vascular cognitive impairment. Stroke. 2009;40(3 Suppl.):S48–S52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 12.van Swieten JC, et al. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain. 1991;114:761–774. doi: 10.1093/brain/114.2.761. [DOI] [PubMed] [Google Scholar]
- 13.Fazekas F, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 14.Leys D, et al. Could Wallerian degeneration contribute to “leuKo-araiosis” in subjects free of any vascular disorder? J Neurol Neurosurg Psychiatry. 1991;54:46–50. doi: 10.1136/jnnp.54.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballard C, et al. Neurocardiovascular instability, hypotensive episodes, and MRI lesions in neurodegenerative dementia. Ann N Y Acad Sci. 2000;903:442–445. doi: 10.1111/j.1749-6632.2000.tb06396.x. [DOI] [PubMed] [Google Scholar]
- 16.Wersching H, et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74:1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- 17.Pieters B, et al. Periventricular white matter lucencies relate to low vitamin B12 levels in patients with small vessel stroke. Stroke. 2009;40:1623–1626. doi: 10.1161/STROKEAHA.108.523431. [DOI] [PubMed] [Google Scholar]
- 18.Demirkiran M, Bozdemir H, Sarica Y. Vascular parkinsonism: a distinct, heterogeneous clinical entity. Acta Neurol Scand. 2001;104:63–67. doi: 10.1034/j.1600-0404.2001.104002063.x. [DOI] [PubMed] [Google Scholar]
- 19.Thanvi B, Lo N, Robinson T. Vascular parkinsonism—an important cause of parkinsonism in older people. Age Ageing. 2005;34:114–119. doi: 10.1093/ageing/afi025. [DOI] [PubMed] [Google Scholar]
- 20.Zijlmans JC, Daniel SE, Hughes AJ, Revesz T, Lees AJ. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord. 2004;19:630–640. doi: 10.1002/mds.20083. [DOI] [PubMed] [Google Scholar]
- 21.Jellinger KA. The pathology of Parkinson's disease. Adv Neurol. 2001;86:55–72. [PubMed] [Google Scholar]
- 22.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinicopathologic study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jellinger KA. Prevalence of cerebrovascular lesions in Parkinson's disease. A postmortem study. Acta Neuropathol. 2003;105:415–419. doi: 10.1007/s00401-003-0676-3. [DOI] [PubMed] [Google Scholar]
- 24.Choi SA, et al. Are there differences in cerebral white matter lesion burdens between Parkinson's disease patients with or without dementia? Acta Neuropathol. 2010;119:147–149. doi: 10.1007/s00401-009-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sohn YH, Kim JS. The influence of white matter hyperintensities on the clinical features of Parkinson's disease. Yonsei Med J. 1998;39:50–55. doi: 10.3349/ymj.1998.39.1.50. [DOI] [PubMed] [Google Scholar]
- 26.Slawek J, et al. The influence of vascular risk factors and white matter hyperintensities on the degree of cognitive impairment in Parkinson's disease. Neurol Neurochir Pol. 2008;42:505–512. [PubMed] [Google Scholar]
- 27.Lee SJ, et al. The severity of leuKoaraiosis correlates with the clinical phenotype of Parkinson's disease. Arch Gerontol Geriatr. 2009;49:255–259. doi: 10.1016/j.archger.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Stern MB, Braffman BH, Skolnick BE, Hurtig HI, Grossman RI. Magnetic resonance imaging in Parkinson's disease and parkinsonian syndromes. Neurology. 1989;39:1524–1526. doi: 10.1212/wnl.39.11.1524. [DOI] [PubMed] [Google Scholar]
- 29.Piccini P, et al. White matter hyperintensities in Parkinson's disease. Clinical correlations. Arch Neurol. 1995;52:191–194. doi: 10.1001/archneur.1995.00540260097023. [DOI] [PubMed] [Google Scholar]
- 30.Van Rossum E, et al. The level of physical activity in patients with Parkinson's disease [abstract P2.096] Parkinsonism Relat Disord. 2008;14(Suppl. 1):S67–S68. [Google Scholar]
- 31.Acharya HJ, Bouchard TP, Emery DJ, Camicioli RM. Axial signs and magnetic resonance imaging correlates in Parkinson's disease. Can J Neurol Sci. 2007;34:56–61. doi: 10.1017/s0317167100005795. [DOI] [PubMed] [Google Scholar]
- 32.Dalaker TO, et al. Brain atrophy and white matter hyperintensities in early Parkinson's disease. Mov Disord. 2009;24:2233–2241. doi: 10.1002/mds.22754. [DOI] [PubMed] [Google Scholar]
- 33.Joseph JA, Roth GS, Strong R. The striatum, a microcosm for the examination of age-related alterations in the CNS: a selected review. Rev Biol Res Aging. 1990;4:181–199. [Google Scholar]
- 34.Volkow ND, et al. Dopamine transporters decrease with age in healthy subjects. J Nucl Med. 1996;37:554–558. [PubMed] [Google Scholar]
- 35.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 36.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: Substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 37.Bohnen NI, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006;26:1198–1212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- 38.Louis ED, et al. Quantitative brain measurements in community-dwelling elderly persons with mild parkinsonian signs. Arch Neurol. 2008;65:1649–1654. doi: 10.1001/archneurol.2008.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brant-Zawadzki M, et al. MR imaging of the aging brain: patchy white-matter lesions and dementia. Am J Neuroradiol. 1985;6:675–682. [PMC free article] [PubMed] [Google Scholar]
- 40.Fazekas F, ChawluK JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 41.Longstreth WT, Jr, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the cardiovascular health study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 42.Scheltens P, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114:7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- 43.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barkhof F, Scheltens P. Is the whole brain periventricular? J Neurol Neurosurg Psychiatry. 2006;77:143–144. doi: 10.1136/jnnp.2005.075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bohnen NI, Muller ML, Kuwabara H, Constantine GM, Studenski SA. Age-associated leukoaraiosis and cortical cholinergic deafferentation. Neurology. 2009;72:1411–1416. doi: 10.1212/WNL.0b013e3181a187c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Boer R, et al. White matter lesion extension to automatic brain tissue segmentation on MRI. Neuroimage. 2009;45:1151–1161. doi: 10.1016/j.neuroimage.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Bohnen NI, Bogan CW, Müller ML. Frontal and periventricular brain white matter lesions and cortical deafferentation of cholinergic and other neuromodulatory axonal projections. Eur Neurol J. 2009;1:33–40. [PMC free article] [PubMed] [Google Scholar]
- 48.Sugihara S, Kinoshita T, Matsusue E, Fujii S, Ogawa T. Usefulness of diffusion tensor imaging of white matter in Alzheimer disease and vascular dementia. Acta Radiol. 2004;45:658–663. doi: 10.1080/02841850410008388. [DOI] [PubMed] [Google Scholar]
- 49.Gattellaro G, et al. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2009;30:1222–1226. doi: 10.3174/ajnr.A1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karagulle Kendi AT, Lehericy S, Luciana M, Ugurbil K, Tuite P. Altered diffusion in the frontal lobe in Parkinson disease. AJNR Am J Neuroradiol. 2008;29:501–505. doi: 10.3174/ajnr.A0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee DY, et al. Vascular and degenerative processes differentially affect regional interhemispheric connections in normal aging, mild cognitive impairment, and Alzheimer disease. Stroke. 2010;41:1791–1797. doi: 10.1161/STROKEAHA.110.582163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slawek J, et al. Vascular risk factors do not contribute to motor and cognitive impairment in Parkinson's disease. Parkinsonism Relat Disord. 2010;16:73–74. doi: 10.1016/j.parkreldis.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 53.Albin RL, et al. White matter lesions augment motor impairments of nigrostriatal dopaminergic denervation in Parkinson disease [abstract S53.004] Neurology. 2010;74(Suppl. 2):A500. [Google Scholar]
- 54.Mayeux R, et al. An estimate of the incidence of dementia in idiopathic Parkinson's disease. Neurology. 1990;40:1513–1517. doi: 10.1212/wnl.40.10.1513. [DOI] [PubMed] [Google Scholar]
- 55.Marder K, Tang MX, Côté L, Stern Y, Mayeux R. The frequency and associated risk factors for dementia in patients with Parkinson's disease. Arch Neurol. 1995;52:695–701. doi: 10.1001/archneur.1995.00540310069018. [DOI] [PubMed] [Google Scholar]
- 56.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289:18–22. doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 57.Kuczynski B, et al. Cognitive and anatomic contributions of metabolic decline in Alzheimer disease and cerebrovascular disease. Arch Neurol. 2008;65:650–655. doi: 10.1001/archneur.65.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chui HC, et al. Cognitive impact of subcortical vascular and Alzheimer's disease pathology. Ann Neurol. 2006;60:677–687. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marshall GA, Shchelchkov E, Kaufer DI, Ivanco LS, Bohnen NI. White matter hyperintensities and cortical acetylcholinesterase activity in parkinsonian dementia. Acta Neurol Scand. 2006;113:87–91. doi: 10.1111/j.1600-0404.2005.00553.x. [DOI] [PubMed] [Google Scholar]
- 60.Burton EJ, McKeith IG, Burn DJ, Firbank MJ, O'Brien JT. Progression of white matter hyperintensities in Alzheimer disease, dementia with lewy bodies, and Parkinson disease dementia: a comparison with normal aging. Am J Geriatr Psychiatry. 2006;14:842–849. doi: 10.1097/01.JGP.0000236596.56982.1c. [DOI] [PubMed] [Google Scholar]
- 61.Beyer MK, Aarsland D, Greve OJ, Larsen JP. Visual rating of white matter hyperintensities in Parkinson's disease. Mov Disord. 2006;21:223–229. doi: 10.1002/mds.20704. [DOI] [PubMed] [Google Scholar]
- 62.Lee SJ, et al. Influence of white matter hyperintensities on the cognition of patients with Parkinson disease. Alzheimer Dis Assoc Disord. 2010;24:227–233. doi: 10.1097/WAD.0b013e3181d71a13. [DOI] [PubMed] [Google Scholar]
- 63.Dalaker TO, et al. White matter hyperintensities do not impact cognitive function in patients with newly diagnosed Parkinson's disease. Neuroimage. 2009;47:2083–2089. doi: 10.1016/j.neuroimage.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 64.Meyer JS, Huang J, Chowdhury MH. MRI confirms mild cognitive impairments prodromal for Alzheimer's, vascular and Parkinson–Lewy body dementias. J Neurol Sci. 2007;257:97–104. doi: 10.1016/j.jns.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 65.Santangelo G, et al. Differential neuropsychological profiles in Parkinsonian patients with or without vascular lesions. Mov Disord. 2010;25:50–56. doi: 10.1002/mds.22893. [DOI] [PubMed] [Google Scholar]
- 66.Zarzhevsky N, et al. White matter lesions augment cognitive impairments of dopaminergic denervation of the caudate nucleus in Parkinson disease [abstract OP294] Eur J Nucl Med Mol Imaging. 2010;37(Suppl. 2):S249. [Google Scholar]
- 67.Murrow RW, Schweiger GD, Kepes JJ, Koller WC. Parkinsonism due to a basal ganglia lacunar state: clinicopathologic correlation. Neurology. 1990;40:897–900. doi: 10.1212/wnl.40.6.897. [DOI] [PubMed] [Google Scholar]
- 68.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 69.Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79:619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- 70.Kerr B, Condon SM, McDonald LA. Cognitive spatial processing and the regulation of posture. J Exp Psychol Hum Percept Perform. 1985;11:617–622. doi: 10.1037//0096-1523.11.5.617. [DOI] [PubMed] [Google Scholar]
- 71.Takakusaki K, Tomita N, Yano M. Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. J Neurol. 2008;255(Suppl. 4):19–29. doi: 10.1007/s00415-008-4004-7. [DOI] [PubMed] [Google Scholar]
- 72.Critchley M. Arteriosclerotic parkinsonism. Brain. 1929;52:23–83. [Google Scholar]
- 73.Kalra S, Grosset DG, Benamer HT. Differentiating vascular parkinsonism from idiopathic Parkinson's disease: a systematic review. Mov Disord. 2010;25:149–156. doi: 10.1002/mds.22937. [DOI] [PubMed] [Google Scholar]
- 74.Thompson PD, Marsden CD. Gait disorder of subcortical arteriosclerotic encephalopathy: Binswanger's disease. Mov Disord. 1987;2:1–8. doi: 10.1002/mds.870020101. [DOI] [PubMed] [Google Scholar]
- 75.Grimbergen YA, Langston JW, Roos RA, Bloem BR. Postural instability in Parkinson's disease: the adrenergic hypothesis and the locus coeruleus. Expert Rev Neurother. 2009;9:279–290. doi: 10.1586/14737175.9.2.279. [DOI] [PubMed] [Google Scholar]
- 76.Bloem BR, Steijns JA, Smits-Engelsman BC. An update on falls. Curr Opin Neurol. 2003;16:15–26. doi: 10.1097/01.wco.0000053580.70044.70. [DOI] [PubMed] [Google Scholar]
- 77.Bohnen NI, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73:1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Suppl. 2):ii7–ii11. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 79.PapaPetropoulos S, et al. The effect of vascular disease on late onset Parkinson's disease. Eur J Neurol. 2004;11:231–235. doi: 10.1046/j.1468-1331.2003.00748.x. [DOI] [PubMed] [Google Scholar]
- 80.Park K, et al. Significant association between leukoaraiosis and metabolic syndrome in healthy subjects. Neurology. 2007;69:974–978. doi: 10.1212/01.wnl.0000266562.54684.bf. [DOI] [PubMed] [Google Scholar]
- 81.Nakaso K, et al. Hypertrophy of IMC of carotid artery in Parkinson's disease is associated with L-DOPA, homocysteine, and MTHFR genotype. J Neurol Sci. 2003;207:19–23. doi: 10.1016/s0022-510x(02)00353-2. [DOI] [PubMed] [Google Scholar]
- 82.Aisen PS, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300:1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.VITATOPS Trial Study Group. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol. 2010;9:855–865. doi: 10.1016/S1474-4422(10)70187-3. [DOI] [PubMed] [Google Scholar]
- 84.Wright CB, et al. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke. 2008;39:800–805. doi: 10.1161/STROKEAHA.107.484147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord. 2006;21:1123–1130. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- 86.Brickman AM, et al. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Res. 2009;172:117–120. doi: 10.1016/j.pscychresns.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bastos-Leite AJ, et al. Cerebral blood flow by using pulsed arterial spin-labeling in elderly subjects with white matter hyperintensities. AJNR Am J Neuroradiol. 2008;29:1296–1301. doi: 10.3174/ajnr.A1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuczynski B, et al. White matter integrity and cortical metabolic associations in aging and dementia. Alzheimers Dement. 2010;6:54–62. doi: 10.1016/j.jalz.2009.04.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jagust WJ, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63:72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schuur M, et al. Genetic risk factors for cerebral small-vessel disease in hypertensive patients from a genetically isolated population. J Neurol Neurosurg Psychiatry. 2011;82:41–44. doi: 10.1136/jnnp.2009.176362. [DOI] [PubMed] [Google Scholar]
- 91.Lieberman A, et al. Dementia in Parkinson's disease. Ann Neurol. 1979;6:355–359. doi: 10.1002/ana.410060409. [DOI] [PubMed] [Google Scholar]
- 92.Utter S, et al. Cerebral small vessel disease-induced apolipoprotein E leakage is associated with Alzheimer disease and the accumulation of amyloid beta-protein in perivascular astrocytes. J Neuropathol Exp Neurol. 2008;67:842–856. doi: 10.1097/NEN.0b013e3181836a71. [DOI] [PubMed] [Google Scholar]
- 93.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]